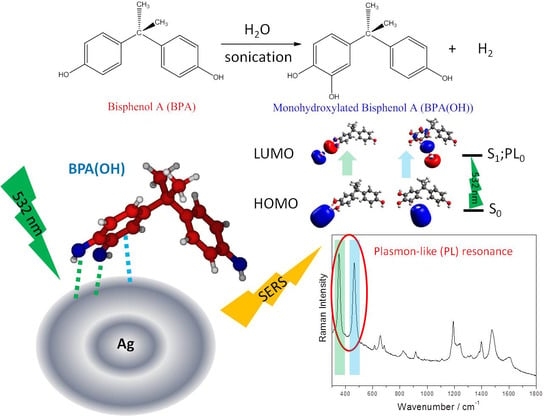
Sensing Bisphenol A by Means of Surface-Enhanced Raman Spectroscopy and DFT Calculations to Elucidate the Enhancement Mechanism That Dominates the Spectrum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Colloidal AgNPs Experiments
2.2. Electrochemical Experiments
2.3. Instrumentation
2.4. Computational Details. Theoretical Resonance Raman Spectra
3. Results and Discussion
3.1. Standard AgNPs as a Chemical Sensor of BPA in a Sonicated Aqueous Solution
3.2. SERS Spectra of a Sonicated Aqueous Solution of BPA on Roughed Silver Electrode
3.3. DFT Calculations on Silver Coordination and Complexation Energy of the Ag2-BPA and Ag2-BPA(OH) Complexes
3.4. Insights into the Nature of SERS Enhancement by Analyzing TD-DFT Resonance Raman Spectra of Ag2-BPA and Ag2-BPA(OH) Complexes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vasiljevic, T.; Harner, T. Bisphenol A and Its Analogues in Outdoor and Indoor Air: Properties, Sources and Global Levels. Sci. Total Environ. 2021, 789, 148013. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.; Chakraborty, P. A Review on Sources and Health Impacts of Bisphenol A. Rev. Environ. Health 2020, 35, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Hengstler, J.G.; Foth, H.; Gebel, T.; Kramer, P.J.; Lilienblum, W.; Schweinfurth, H.; Völkel, W.; Wollin, K.M.; Gundert-Remy, U. Critical Evaluation of Key Evidence on the Human Health Hazards of Exposure to Bisphenol A. Crit. Rev. Toxicol. 2011, 41, 263–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization; FAO. Joint FAO/WHO Expert Meeting to Review Toxicological and Health Aspects of Bisphenol A. Final Report, Including Report of Stakeholder Meeting on Bisphenol A, 1–5 November 2010, Ottawa, Canada; World Health Organization: Geneva, Switzerland, 2011; Available online: https://apps.who.int/iris/handle/10665/44624 (accessed on 15 November 2022).
- Ragavan, K.V.; Rastogi, N.K.; Thakur, M.S. Sensors and Biosensors for Analysis of Bisphenol-A. TrAC Trends Analyt. Chem. 2013, 52, 248–260. [Google Scholar] [CrossRef]
- Sheng, W.; Duan, W.; Shi, Y.; Chang, Q.; Zhang, Y.; Lu, Y.; Wang, S. Sensitive Detection of Bisphenol A in Drinking Water and River Water Using an Upconversion Nanoparticles-Based Fluorescence Immunoassay in Combination with Magnetic Separation. Anal. Methods 2018, 10, 5313–5320. [Google Scholar] [CrossRef]
- Feng, Y.; Ning, B.; Su, P.; Wang, H.; Wang, C.; Chen, F.; Gao, Z. An Immunoassay for Bisphenol A Based on Direct Hapten Conjugation to the Polystyrene Surface of Microtiter Plates. Talanta 2009, 80, 803–808. [Google Scholar] [CrossRef]
- Ballesteros-Gómez, A.; Rubio, S.; Pérez-Bendito, D. Analytical Methods for the Determination of Bisphenol A in Food. J. Chromatogr. A 2009, 1216, 449–469. [Google Scholar] [CrossRef]
- Sun, F.; Kang, L.; Xiang, X.; Li, H.; Luo, X.; Luo, R.; Lu, C.; Peng, X. Recent Advances and Progress in the Detection of Bisphenol A. Anal. Bioanal. Chem. 2016, 408, 6913–6927. [Google Scholar] [CrossRef]
- Martín-Pozo, L.; Martín-Bueno, J.; Moscoso-Ruiz, I.; Zafra-Gómez, A. Methods of Bisphenol A Detection by Gas Chromatography and Mass Spectrometry (GC-Ms) in Human Breast Milk and Foodstuff. In Emerging Contaminants in the Environment: Challenges and Sustainable Practices; Sarma, H., Dominguez, D.C., Lee, W.Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Chapter 18; pp. 465–493. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, D.; Huang, C.; Sun, Y.; Lee, Y.I. Sensitive Detection of Bisphenol A in Complex Samples by In-Column Molecularly Imprinted Solid-Phase Extraction Coupled with Capillary Electrophoresis. Microchem. J. 2015, 121, 1–5. [Google Scholar] [CrossRef]
- Yahaya, N.; Huang, Z.; Yan, B.; Chen, D.D.Y. Capillary Electrophoresis–Mass Spectrometry Analysis of Bisphenol A and Its Analogues in Bottled Tea Beverages with Dynamic PH Focusing. Food Chem. 2022, 372, 131220. [Google Scholar] [CrossRef]
- Shareef, A.; Angove, M.J.; Wells, J.D.; Johnson, B.B. Aqueous Solubilities of Estrone, 17β-Estradiol, 17α-Ethynylestradiol, and Bisphenol A. J. Chem. Eng. Data 2006, 51, 879–881. [Google Scholar] [CrossRef]
- Aroca, R. Surface-Enhanced Vibrational Spectroscopy; John Wiley & Sons Ltd.: Chichester, UK, 2006. [Google Scholar]
- Pilot, R. SERS Detection of Food Contaminants by Means of Portable Raman Instruments. J. Raman Spectrosc. 2018, 49, 954–981. [Google Scholar] [CrossRef]
- Furini, L.N.; Constantino, C.J.L.; Sanchez-Cortes, S.; Otero, J.C.; López-Tocón, I. Adsorption of Carbendazim Pesticide on Plasmonic Nanoparticles Studied by Surface-Enhanced Raman Scattering. J. Colloid. Interface Sci. 2016, 465, 183–189. [Google Scholar] [CrossRef] [Green Version]
- López-Tocón, I.; Otero, J.C.; Arenas, J.F.; García-Ramos, J.V.; Sánchez-Cortés, S. Trace Detection of Triphenylene by Surface Enhanced Raman Spectroscopy Using Functionalized Silver Nanoparticles with Bis-Acridinium Lucigenine. Langmuir 2010, 26, 6977–6981. [Google Scholar] [CrossRef]
- Moskovits, M. Surface-Enhanced Raman Spectroscopy: A Brief Perspective. In Surface-Enhanced Raman Scattering. Topics in Applied Physics; Kneipp, K., Moskovits, M., Kneipp, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 103, pp. 1–17. [Google Scholar] [CrossRef]
- López-Tocón, I.; Otero, J.C.; Arenas, J.F.; Garcia-Ramos, J.V.; Sanchez-Cortes, S. Multicomponent Direct Detection of Polycyclic Aromatic Hydrocarbons by Surface-Enhanced Raman Spectroscopy Using Silver Nanoparticles Functionalized with the Viologen Host Lucigenin. Anal. Chem. 2011, 83, 2518–2525. [Google Scholar] [CrossRef]
- de Bleye, C.; Dumont, E.; Hubert, C.; Sacré, P.Y.; Netchacovitch, L.; Chavez, P.F.; Hubert, P.; Ziemons, E. A Simple Approach for Ultrasensitive Detection of Bisphenols by Multiplexed Surface-Enhanced Raman Scattering. Anal. Chim. Acta 2015, 888, 118–125. [Google Scholar] [CrossRef]
- Roschi, E.; Gellini, C.; Ricci, M.; Sanchez-Cortes, S.; Focardi, C.; Neri, B.; Otero, J.C.; López-Tocón, I.; Smulevich, G.; Becucci, M. Surface-Enhanced Raman Spectroscopy for Bisphenols Detection: Toward a Better Understanding of the Analyte–Nanosystem Interactions. Nanomaterials 2021, 11, 881. [Google Scholar] [CrossRef]
- Wang, C.Y.; Zeng, Y.; Shen, A.G.; Hu, J.M. A Highly Sensitive SERS Probe for Bisphenol A Detection Based on Functionalized Au@Ag Nanoparticles. Anal. Methods 2018, 10, 5622–5628. [Google Scholar] [CrossRef]
- Lee, E.H.; Lee, S.K.; Kim, M.J.; Lee, S.W. Simple and Rapid Detection of Bisphenol A Using a Gold Nanoparticle-Based Colorimetric Aptasensor. Food Chem. 2019, 287, 205–213. [Google Scholar] [CrossRef]
- Liu, S.; Fu, Y.; Xiong, C.; Liu, Z.; Zheng, L.; Yan, F. Detection of Bisphenol A Using DNA-Functionalized Graphene Field Effect Transistors Integrated in Microfluidic Systems. ACS Appl. Mater. Interfaces 2018, 10, 23522–23528. [Google Scholar] [CrossRef]
- Guerrini, L.; Garcia-Ramos, J.V.; Domingo, C.; Sanchez-Cortes, S. Sensing Polycyclic Aromatic Hydrocarbons with Dithiocarbamate-Functionalized Ag Nanoparticles by Surface-Enhanced Raman Scattering. Anal. Chem. 2009, 81, 953–960. [Google Scholar] [CrossRef]
- Inoue, M.; Masuda, Y.; Okada, F.; Sakurai, A.; Takahashi, I.; Sakakibara, M. Degradation of Bisphenol A Using Sonochemical Reactions. Water Res. 2008, 42, 1379–1386. [Google Scholar] [CrossRef]
- Gültekin, I.; Ince, N.H. Ultrasonic Destruction of Bisphenol-A: The Operating Parameters. Ultrason. Sonochem. 2008, 15, 524–529. [Google Scholar] [CrossRef]
- Ye, X.; Zhou, X.; Needham, L.L.; Calafat, A.M. In-Vitro Oxidation of Bisphenol A: Is Bisphenol A Catechol a Suitable Biomarker for Human Exposure to Bisphenol A? Anal. Bioanal. Chem. 2011, 399, 1071–1079. [Google Scholar] [CrossRef]
- Guo, Z.; Feng, R. Ultrasonic Irradiation-Induced Degradation of Low-Concentration Bisphenol A in Aqueous Solution. J. Hazard. Mater. 2009, 163, 855–860. [Google Scholar] [CrossRef]
- López-Tocón, I.; Valdivia, S.; Soto, J.; Otero, J.C.; Muniz-Miranda, F.; Menziani, M.C.; Muniz-Miranda, M. A DFT Approach to the Surface-Enhanced Raman Scattering of 4-Cyanopyridine Adsorbed on Silver Nanoparticles. Nanomaterials 2019, 9, 1211. [Google Scholar] [CrossRef] [Green Version]
- López-Tocón, I.; Imbarack, E.; Soto, J.; Sanchez-Cortes, S.; Leyton, P.; Otero, J.C. Intramolecular and Metal-to-Molecule Charge Transfer Electronic Resonances in the Surface-Enhanced Raman Scattering of 1,4-Bis((E)-2-(Pyridin-4-Yl)Vinyl)Naphthalene. Molecules 2019, 24, 4622. [Google Scholar] [CrossRef] [Green Version]
- Avila, F.; Ruano, C.; Lopez-Tocon, I.; Arenas, J.F.; Soto, J.; Otero, J.C. How the Electrode Potential Controls the Selection Rules of the Charge Transfer Mechanism of SERS. Chem. Commun. 2011, 47, 4213–4215. [Google Scholar] [CrossRef]
- Román-Pérez, J.; López-Tocón, I.; Castro, J.L.; Arenas, J.F.; Soto, J.; Otero, J.C. The Electronic Structure of Metal–Molecule Hybrids in Charged Interfaces: Surface-Enhanced Raman Selection Rules Derived from Plasmon-like Resonances. Phys. Chem. Chem. Phys. 2014, 17, 2326–2329. [Google Scholar] [CrossRef]
- Arenas, J.F.; López Tocón, I.; Otero, J.C.; Marcos, J.I. Charge Transfer Processes in Surface-Enhanced Raman Scattering. Franck−Condon Active Vibrations of Pyridine. J. Phys. Chem. 1996, 100, 9254–9261. [Google Scholar] [CrossRef]
- Yao, G.; Zhai, Z.; Zhong, J.; Huang, Q. DFT and SERS Study of 15N Full-Labeled Adenine Adsorption on Silver and Gold Surfaces. J. Phys. Chem. C 2017, 121, 9869–9878. [Google Scholar] [CrossRef]
- Yao, G.; Huang, Q. DFT and SERS Study of l-Cysteine Adsorption on the Surface of Gold Nanoparticles. J. Phys. Chem. C 2018, 122, 15241–15251. [Google Scholar] [CrossRef]
- de Souza, M.L.; Otero, J.C.; López-Tocón, I. Comparative Performance of Citrate, Borohydride, Hydroxylamine and β-Cyclodextrin Silver Sols for Detecting Ibuprofen and Caffeine Pollutants by Means of Surface-Enhanced Raman Spectroscopy. Nanomaterials 2020, 10, 2339. [Google Scholar] [CrossRef] [PubMed]
- Cañamares, M.V.; Garcia-Ramos, J.V.; Gómez-Varga, J.D.; Domingo, C.; Sanchez-Cortes, S. Comparative Study of the Morphology, Aggregation, Adherence to Glass, and Surface-Enhanced Raman Scattering Activity of Silver Nanoparticles Prepared by Chemical Reduction of Ag+ Using Citrate and Hydroxylamine. Langmuir 2005, 21, 8546–8553. [Google Scholar] [CrossRef] [PubMed]
- Leopold, N.; Lendl, B. A New Method for Fast Preparation of Highly Surface-Enhanced Raman Scattering (SERS) Active Silver Colloids at Room Temperature by Reduction of Silver Nitrate with Hydroxylamine Hydrochloride. J. Phys. Chem. B 2003, 107, 5723–5727. [Google Scholar] [CrossRef]
- Pande, S.; Ghosh, S.K.; Praharaj, S.; Panigrahi, S.; Basu, S.; Jana, S.; Pal, A.; Tsukuda, T.; Pal, T. Synthesis of Normal and Inverted Gold−Silver Core−Shell Architectures in β-Cyclodextrin and Their Applications in SERS. J. Phys. Chem. C 2007, 111, 10806–10813. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A New Hybrid Exchange–Correlation Functional Using the Coulomb-Attenuating Method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Weigend, F. Accurate Coulomb-Fitting Basis Sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Soto, J.; Imbarack, E.; López-Tocón, I.; Sánchez-Cortés, S.; Otero, J.C.; Leyton, P. Application of Surface-Enhanced Resonance Raman Scattering (SERS) to the Study of Organic Functional Materials: Electronic Structure and Charge Transfer Properties of 9,10-Bis((E)-2-(Pyridin-4-Yl)Vinyl)Anthracene. RSC Adv. 2019, 9, 14511–14519. [Google Scholar] [CrossRef] [Green Version]
- Valdivia, S.; Avila, F.J.; Otero, J.C.; López-Tocón, I. Voltage Selection of Physisorbed or Chemisorbed 4-Cyanobenzoate on a Nanostructured Silver Electrode and the Dual Electronic Structure of Charged Metal–Molecule Hybrids. Appl. Surf. Sci. 2022, 579, 152071. [Google Scholar] [CrossRef]
- Santoro, F.; Cerezo, J. FCclasses 3.0, A Code for Vibronic Calculations. 2021. Available online: http://www.iccom.cnr.it/en/fcclasses (accessed on 25 April 2022).
- Santoro, F.; Improta, R.; Lami, A.; Bloino, J.; Barone, V. Effective method tocompute Frank_condon integrals for optical spectra of large molecules in solution. J. Chem. Phys. 2007, 126, 084509. [Google Scholar] [CrossRef] [PubMed]
- Dirac, P.A.M. The quantum theory of dispersion. Proc. Math. Phys. Eng. 1927, 114, 710–728. [Google Scholar] [CrossRef]
- Albretch, A.C. On the theory of Raman intensities. J. Chem. Phys. 1961, 34, 1476–1484. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H. Gaussian 16; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Schaftenaar, G.; Noordik, J.H. Molden: A Pre- and Post-Processing Program for Molecular and Electronic Structures. J. Comput. Aided Mol. Des. 2000, 14, 123–134. [Google Scholar] [CrossRef]
- Geens, T.; Aerts, D.; Berthot, C.; Bourguignon, J.P.; Goeyens, L.; Lecomte, P.; Maghuin-Rogister, G.; Pironnet, A.M.; Pussemier, L.; Scippo, M.L.; et al. A Review of Dietary and Non-Dietary Exposure to Bisphenol-A. Food Chem. Toxicol. 2012, 50, 3725–3740. [Google Scholar] [CrossRef]
- EFSA. Bisphenol A: EFSA Draft Opinion Proposes Lowering the Tolerable Daily Intake; EFSA: Parma, Italy, 2021; Available online: https://www.efsa.europa.eu/en/news/bisphenol-efsa-draft-opinion-proposes-lowering-tolerable-daily-intake (accessed on 8 January 2023).
- Boys, S.F.; Bernardi, F. The Calculation of Small Molecular Interactions by the Differences of Separate Total Energies. Some Procedures with Reduced Errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]





| Coordination | |||
|---|---|---|---|
| Oxygen Atoms | Internal Face | External Face | |
| Ag2-BPA |  |  |  |
| (Kcal/mol) | −6.40 | −7.27 | −6.71 |
| Ag2-BPA(OH) |  |  |  |
| (Kcal/mol) | −8.41 | −7.08 | −7.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Souza, M.L.; Valdivia, S.; Otero, J.C.; López-Tocón, I. Sensing Bisphenol A by Means of Surface-Enhanced Raman Spectroscopy and DFT Calculations to Elucidate the Enhancement Mechanism That Dominates the Spectrum. Chemosensors 2023, 11, 78. https://doi.org/10.3390/chemosensors11020078
De Souza ML, Valdivia S, Otero JC, López-Tocón I. Sensing Bisphenol A by Means of Surface-Enhanced Raman Spectroscopy and DFT Calculations to Elucidate the Enhancement Mechanism That Dominates the Spectrum. Chemosensors. 2023; 11(2):78. https://doi.org/10.3390/chemosensors11020078
Chicago/Turabian StyleDe Souza, Michele Lemos, Samuel Valdivia, Juan Carlos Otero, and Isabel López-Tocón. 2023. "Sensing Bisphenol A by Means of Surface-Enhanced Raman Spectroscopy and DFT Calculations to Elucidate the Enhancement Mechanism That Dominates the Spectrum" Chemosensors 11, no. 2: 78. https://doi.org/10.3390/chemosensors11020078
APA StyleDe Souza, M. L., Valdivia, S., Otero, J. C., & López-Tocón, I. (2023). Sensing Bisphenol A by Means of Surface-Enhanced Raman Spectroscopy and DFT Calculations to Elucidate the Enhancement Mechanism That Dominates the Spectrum. Chemosensors, 11(2), 78. https://doi.org/10.3390/chemosensors11020078