Ag-ZnS Embedded Polymeric Receptors for the Recognition of Human Serum Albumin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of Ag-ZnS Microspheres
2.3. Synthesis of HSA-Imprinted Polymer
2.4. Materials Characterization
2.5. Quartz Crystal Microbalance (QCM) Setup
2.6. Sensor Measurements
3. Results and Discussion
3.1. Characterizations
3.2. Optimization of MIP Formulations
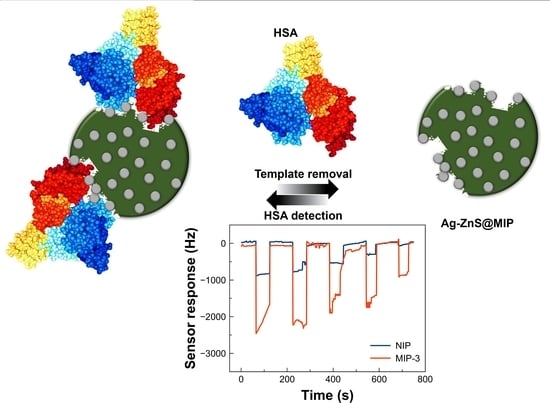
3.3. Sensor Measurements
3.3.1. Sensor Response and Sensitivity
3.3.2. Sensor Selectivity and Stability
3.3.3. Sensor’s Performance Comparison
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, R.E.; Tian, L.; Chang, Y.-H. A Homogeneous Fluorescent Sensor for Human Serum Albumin. J. Pharm. Biomed. Anal. 2012, 63, 165–169. [Google Scholar] [CrossRef] [Green Version]
- Levitt, D.G.; Levitt, M.D. Human Serum Albumin Homeostasis: A New Look at the Roles of Synthesis, Catabolism, Renal and Gastrointestinal Excretion, and the Clinical Value of Serum Albumin Measurements. Int. J. Gen. Med. 2016, 9, 229–255. [Google Scholar] [CrossRef] [Green Version]
- Sugio, S.; Kashima, A.; Mochizuki, S.; Noda, M.; Kobayashi, K. Crystal Structure of Human Serum Albumin at 2.5 Å Resolution. Protein Eng. Des. Sel. 1999, 12, 439–446. [Google Scholar] [CrossRef]
- Curry, S. Beyond Expansion: Structural Studies on the Transport Roles of Human Serum Albumin. Vox Sang. 2002, 83, 315–319. [Google Scholar] [CrossRef]
- Taverna, M.; Marie, A.-L.; Mira, J.-P.; Guidet, B. Specific Antioxidant Properties of Human Serum Albumin. Ann. Intensive Care 2013, 3, 4. [Google Scholar] [CrossRef] [Green Version]
- Rabbani, G.; Ahn, S.N. Review: Roles of Human Serum Albumin in Prediction, Diagnoses and Treatment of COVID-19. Int. J. Biol. Macromol. 2021, 193, 948–955. [Google Scholar] [CrossRef]
- Arques, S. Human Serum Albumin in Cardiovascular Diseases. Eur. J. Intern. Med. 2018, 52, 8–12. [Google Scholar] [CrossRef]
- Cieplak, M.; Szwabinska, K.; Sosnowska, M.; Chandra, B.K.C.; Borowicz, P.; Noworyta, K.; D’Souza, F.; Kutner, W. Selective Electrochemical Sensing of Human Serum Albumin by Semi-Covalent Molecular Imprinting. Biosens. Bioelectron. 2015, 74, 960–966. [Google Scholar] [CrossRef] [Green Version]
- Zheng, D.-J.; Xu, J.; Su, M.-M.; Sun, Z.-G.; Jiao, Q.-C.; Yang, Y.-S.; Zhu, H.-L. A Small, Steady, Rapid and Selective TICT Based Fluorescent HSA Sensor for Pre-Clinical Diagnosis. Sens. Actuators B Chem. 2018, 271, 82–89. [Google Scholar] [CrossRef]
- Ahn, Y.-H.; Lee, J.-S.; Chang, Y.-T. Selective Human Serum Albumin Sensor from the Screening of a Fluorescent Rosamine Library. J. Comb. Chem. 2008, 10, 376–380. [Google Scholar] [CrossRef]
- Ma, X.-T.; He, X.-W.; Li, W.-Y.; Zhang, Y.-K. Epitope Molecularly Imprinted Polymer Coated Quartz Crystal Microbalance Sensor for the Determination of Human Serum Albumin. Sens. Actuators B Chem. 2017, 246, 879–886. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, B.; Zhu, K.; Huang, Y.; Pan, C.; Wang, B.; Wang, L. An Environment-Sensitive Fluorescent Probe for Quantification of Human Serum Albumin: Design, Sensing Mechanism, and Its Application in Clinical Diagnosis of Hypoalbuminemia. Dye. Pigment. 2018, 152, 60–66. [Google Scholar] [CrossRef]
- Yang, R.-J.; Tseng, C.-C.; Ju, W.-J.; Wang, H.-L.; Fu, L.-M. A Rapid Paper-Based Detection System for Determination of Human Serum Albumin Concentration. Chem. Eng. J. 2018, 352, 241–246. [Google Scholar] [CrossRef]
- Song, S.; Wang, L.; Li, J.; Fan, C.; Zhao, J. Aptamer-Based Biosensors. TrAC Trends Anal. Chem. 2008, 27, 108–117. [Google Scholar] [CrossRef]
- Zhou, W.; Jimmy Huang, P.-J.; Ding, J.; Liu, J. Aptamer-Based Biosensors for Biomedical Diagnostics. Analyst 2014, 139, 2627. [Google Scholar] [CrossRef] [Green Version]
- Sakti, S.P.; Hauptmann, P.; Zimmermann, B.; Bühling, F.; Ansorge, S. Disposable HSA QCM-Immunosensor for Practical Measurement in Liquid. Sens. Actuators B Chem. 2001, 78, 257–262. [Google Scholar] [CrossRef]
- Holford, T.R.J.; Davis, F.; Higson, S.P.J. Recent Trends in Antibody Based Sensors. Biosens. Bioelectron. 2012, 34, 12–24. [Google Scholar] [CrossRef]
- Omidfar, K.; Khorsand, F.; Darziani Azizi, M. New Analytical Applications of Gold Nanoparticles as Label in Antibody Based Sensors. Biosens. Bioelectron. 2013, 43, 336–347. [Google Scholar] [CrossRef]
- Bucur, B.; Munteanu, F.-D.; Marty, J.-L.; Vasilescu, A. Advances in Enzyme-Based Biosensors for Pesticide Detection. Biosensors 2018, 8, 27. [Google Scholar] [CrossRef] [Green Version]
- Kurbanoglu, S.; Erkmen, C.; Uslu, B. Frontiers in Electrochemical Enzyme Based Biosensors for Food and Drug Analysis. TrAC Trends Anal. Chem. 2020, 124, 115809. [Google Scholar] [CrossRef]
- Whitcombe, M.J.; Kirsch, N.; Nicholls, I.A. Molecular Imprinting Science and Technology: A Survey of the Literature for the Years 2004–2011. J. Mol. Recognit. 2014, 27, 297–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular Imprinting: Perspectives and Applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef] [PubMed]
- Nasrullah, A.; Afzal, A.; Mujahid, A.; Lieberzeit, P.A.; Bajwa, S.Z.; Mustafa, G.; Latif, U. Imprinted Polymer and Cu2O-Graphene Oxide Nanocomposite for the Detection of Disease Biomarkers. Meas. Sci. Technol. 2021, 32, 105111. [Google Scholar] [CrossRef]
- Nasrullah, A.; Roshan, S.; Latif, U.; Mujahid, A.; Mustafa, G.; Bajwa, S.Z.; Afzal, A. ZnO Nanoparticles and β-Cyclodextrin Containing Molecularly Imprinted Polymers for Gravimetric Sensing of Very-Low-Density Lipoprotein. Meas. Sci. Technol. 2022, 33, 045106. [Google Scholar] [CrossRef]
- Mujahid, A.; Mustafa, G.; Dickert, F. Label-Free Bioanalyte Detection from Nanometer to Micrometer Dimensions—Molecular Imprinting and QCMs †. Biosensors 2018, 8, 52. [Google Scholar] [CrossRef] [Green Version]
- Omidvar, A.H.; Amanati Shahri, A.; Serrano, A.L.C.; Gruber, J.; Pamplona Rehder, G. A Highly Sensitive Molecularly Imprinted Polymer (MIP)-Coated Microwave Glucose Sensor. Sensors 2022, 22, 8648. [Google Scholar] [CrossRef]
- Bajaber, M.A.; Kamel, A.H. All-Solid State Potentiometric Sensors for Desvenlafaxine Detection Using Biomimetic Imprinted Polymers as Recognition Receptors. Polymers 2022, 14, 4814. [Google Scholar] [CrossRef]
- Olivares Moreno, C.A.; Altintas, Z. Bioselective PES Membranes Based on Chitosan Functionalization and Virus-Imprinted NanoMIPs for Highly Efficient Separation of Human Pathogenic Viruses from Water. Membranes 2022, 12, 1117. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, H.; Sun, J.; Xu, J.; Yang, M.; Yu, J. Hollow-Channel Paper Analytical Devices Supported Biofuel Cell-Based Self-Powered Molecularly Imprinted Polymer Sensor for Pesticide Detection. Biosensors 2022, 12, 974. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, M.; Pan, J. Composite Hydrogel Microspheres Encapsulating Hollow Mesoporous Imprinted Nanoparticles for Selective Capture and Separation of 2′-Deoxyadenosine. Molecules 2022, 27, 7444. [Google Scholar] [CrossRef]
- Nanda, J.; Sapra, S.; Sarma, D.D.; Chandrasekharan, N.; Hodes, G. Size-Selected Zinc Sulfide Nanocrystallites: Synthesis, Structure, and Optical Studies. Chem. Mater. 2000, 12, 1018–1024. [Google Scholar] [CrossRef]
- Zhang, F.; Li, C.; Li, X.; Wang, X.; Wan, Q.; Xian, Y.; Jin, L.; Yamamoto, K. ZnS Quantum Dots Derived a Reagentless Uric Acid Biosensor. Talanta 2006, 68, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Karki, B.; Uniyal, A.; Chauhan, B.; Pal, A. Sensitivity Enhancement of a Graphene, Zinc Sulfide-Based Surface Plasmon Resonance Biosensor with an Ag Metal Configuration in the Visible Region. J. Comput. Electron. 2022, 21, 445–452. [Google Scholar] [CrossRef]
- Cheng, Q.; Wang, Y.; Su, L.; Wang, H.; Zhu, G.; Yu, W. Wide-Spectrum Manipulation of Triboelectrification-Induced Electroluminescence by Long Afterglow Phosphors in Elastomeric Zinc Sulfide Composites. J. Mater. Chem. C 2019, 7, 4567–4572. [Google Scholar] [CrossRef]
- Lee, W.; Lyu, H.-K.; Cho, H.-S.; Lee, S.E.; Choi, B. Brightness Enhancement of a Direct-Current-Driven Electroluminescent Device Prepared with Zinc-Sulfide Powder. J. Lumin. 2020, 220, 117015. [Google Scholar] [CrossRef]
- Baniasadi, E.; Dincer, I.; Naterer, G.F. Hybrid Photocatalytic Water Splitting for an Expanded Range of the Solar Spectrum with Cadmium Sulfide and Zinc Sulfide Catalysts. Appl. Catal. A Gen. 2013, 455, 25–31. [Google Scholar] [CrossRef]
- Azarang, M.; Sookhakian, M.; Aliahmad, M.; Dorraj, M.; Basirun, W.J.; Goh, B.T.; Alias, Y. Nitrogen-Doped Graphene-Supported Zinc Sulfide Nanorods as Efficient Pt-Free for Visible-Light Photocatalytic Hydrogen Production. Int. J. Hydrog. Energy 2018, 43, 14905–14914. [Google Scholar] [CrossRef]
- Chen, Z.-G.; Cheng, L.; Zou, J.; Yao, X.; (Max) Lu, G.Q.; Cheng, H.-M. Zinc Sulfide Nanowire Arrays on Silicon Wafers for Field Emitters. Nanotechnology 2010, 21, 065701. [Google Scholar] [CrossRef]
- Khalil, A.A.I.; Abd El-Gawad, A.-S.H.M.; Gadallah, A.-S. Impact of Silver Dopants on Structural, Morphological, Optical, and Electrical Properties of Copper-Zinc Sulfide Thin Films Prepared via Sol-Gel Spin Coating Method. Opt. Mater. 2020, 109, 110250. [Google Scholar] [CrossRef]
- Abd El-Gawad, A.H.M.; Khalil, A.A.I.; Gadallah, A.-S. Influence of Preparation Conditions on the Properties of Silver Doped Copper-Zinc Sulfide Thin Films Prepared via Sol-Gel Spin Coating Technique. Optik 2020, 223, 165561. [Google Scholar] [CrossRef]
- Buglak, A.A.; Kononov, A.I. Comparative Study of Gold and Silver Interactions with Amino Acids and Nucleobases. RSC Adv. 2020, 10, 34149–34160. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.I.; Lee, S.; Kim, S.K.; Kim, Y.-I.; Cho, D.W.; Khan, M.M.; Sohn, Y. Fabrication of ZnO, ZnS, Ag-ZnS, and Au-ZnS Microspheres for Photocatalytic Activities, CO Oxidation and 2-Hydroxyterephthalic Acid Synthesis. J. Alloys Compd. 2016, 675, 46–56. [Google Scholar] [CrossRef]
- Mazhar, S.; Qazi, U.Y.; Nadeem, N.; Zahid, M.; Jalil, A.; Khan, F.; Ul-Hasan, I.; Shahid, I. Photocatalytic Degradation of Methylene Blue Using Polyaniline-Based Silver-Doped Zinc Sulfide (PANI-Ag/ZnS) Composites. Environ. Sci. Pollut. Res. 2022, 29, 9203–9217. [Google Scholar] [CrossRef]
- Patel, K.; Deshpande, M.P.; Chaki, S.H. Effect of Ag on Structural, Optical and Luminescence Properties of ZnS Nanoparticles Synthesized by Microwave-Assisted Chemical Route. Appl. Phys. A 2017, 123, 367. [Google Scholar] [CrossRef]
- Muraleedharan, K.; Rajan, V.K.; Abdul Mujeeb, V.M. Green Synthesis of Pure and Doped Semiconductor Nanoparticles of ZnS and CdS. Trans. Nonferrous Met. Soc. China 2015, 25, 3265–3270. [Google Scholar] [CrossRef]
- Xie, M.; Chen, Z.; Zhao, F.; Lin, Y.; Zheng, S.; Han, S. Correction: Xie et al. Selection and Application of SsDNA Aptamers for Fluorescence Biosensing Detection of Malachite Green. Foods 2022, 11, 801. Foods 2022, 11, 1933. [Google Scholar] [CrossRef]
- Arshad, U.; Mujahid, A.; Lieberzeit, P.; Afzal, A.; Zafar Bajwa, S.; Iqbal, N.; Roshan, S. Molecularly Imprinted Polymeric Coatings for Sensitive and Selective Gravimetric Detection of Artemether. RSC Adv. 2020, 10, 34355–34363. [Google Scholar] [CrossRef]
- Wang, B.; Lin, Y.; Tan, H.; Luo, M.; Dai, S.; Lu, H.; Huang, Z. One-Pot Synthesis of N-Doped Carbon Dots by Pyrolyzing the Gel Composed of Ethanolamine and 1-Carboxyethyl-3-Methylimidazolium Chloride and Their Selective Fluorescence Sensing for Cr(VI) Ions. Analyst 2018, 143, 1906–1915. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Song, J.-F. Interface Potential Sensing from Adsorption of Human Serum Albumin (HSA) on Carbon Nanotube (CNT) Monitored by Zero Current Potentiometry for HSA Determination. Biosens. Bioelectron. 2015, 72, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Gui, W.; Chen, X.; Ma, Q. A Novel Detection Method of Human Serum Albumin Based on CuInZnS Quantum Dots-Co2+ Sensing System. Anal. Bioanal. Chem. 2017, 409, 3871–3876. [Google Scholar] [CrossRef]
- Li, J.; Wu, J.; Cui, F.; Zhao, X.; Li, Y.; Lin, Y.; Li, Y.; Hu, J.; Ju, Y. A Dual Functional Fluorescent Sensor for Human Serum Albumin and Chitosan. Sens. Actuators B Chem. 2017, 243, 831–837. [Google Scholar] [CrossRef]







| Formulations | MAA (µL) | EGDMA (µL) | HAS 1 (µL) | Ag-ZnS 2 (µL) |
|---|---|---|---|---|
| MIP-1 | 50 | 25 | 14 | 100 |
| MIP-2 | 38.5 | 38.5 | 14 | 100 |
| MIP-3 | 45 | 30 | 14 | 100 |
| MIP-4 | 15 | 60 | 14 | 100 |
| Sensing Element | Transducer | Range | LOD | Ref. |
|---|---|---|---|---|
| Thiophene–based conducting polymers | DPV | 800–20,000 ng/mL | 16.6 ng/mL | [8] |
| EIS | 4000–80,000 ng/mL | 800 ng/mL | ||
| Epitope–imprinted polymer (zinc acrylate–co–EGDMA) | QCM | 50–500 ng/mL | 26 ng/mL | [11] |
| Multiwalled carbon nanotubes (MWCNTs) | Potentiometry | 2.8 × 10−8–3.4 × 10−7 M | 2 × 10−8 M | [49] |
| CuInZnS quantum dots | Photoluminescence | 7.5 × 10–8 to 1.0 × 10–4 M | 4.5 × 10–8 M | [50] |
| Aggregation–induced emission based on salicylidene | Fluorescence | 0–100,000 ng/mL | 6110 ng/mL | [51] |
| Ag–ZnS/poly(MAA–co–EGDMA) | QCM | 5–200 ng/mL | 0.456 ng/mL | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasrullah, A.; Zahid, M.; Ali, A.; Ahmad, M.N.; Mujahid, A.; Hussain, T.; Latif, U.; Din, M.I.; Afzal, A. Ag-ZnS Embedded Polymeric Receptors for the Recognition of Human Serum Albumin. Chemosensors 2023, 11, 240. https://doi.org/10.3390/chemosensors11040240
Nasrullah A, Zahid M, Ali A, Ahmad MN, Mujahid A, Hussain T, Latif U, Din MI, Afzal A. Ag-ZnS Embedded Polymeric Receptors for the Recognition of Human Serum Albumin. Chemosensors. 2023; 11(4):240. https://doi.org/10.3390/chemosensors11040240
Chicago/Turabian StyleNasrullah, Amara, Muhammad Zahid, Asghar Ali, Mirza Nadeem Ahmad, Adnan Mujahid, Tajamal Hussain, Usman Latif, Muhammad Imran Din, and Adeel Afzal. 2023. "Ag-ZnS Embedded Polymeric Receptors for the Recognition of Human Serum Albumin" Chemosensors 11, no. 4: 240. https://doi.org/10.3390/chemosensors11040240
APA StyleNasrullah, A., Zahid, M., Ali, A., Ahmad, M. N., Mujahid, A., Hussain, T., Latif, U., Din, M. I., & Afzal, A. (2023). Ag-ZnS Embedded Polymeric Receptors for the Recognition of Human Serum Albumin. Chemosensors, 11(4), 240. https://doi.org/10.3390/chemosensors11040240