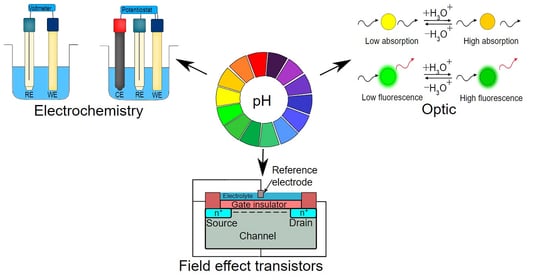
Recent Advances in Optical, Electrochemical and Field Effect pH Sensors
Abstract
:1. Introduction
2. Optical pH Sensors
3. Potentiometric pH Sensors
4. Voltammetric pH Sensors
5. pH Sensors Based on Field Effect Transistors
6. Conclusions and Outlook
Funding
Conflicts of Interest
References
- Sarkawi, S. An Overview on pH Measurement Technique and Application in Biomedical and Industrial Process. In Proceedings of the 2015 2nd International Conference on Biomedical Engineering (ICoBE), Penang, Malaysia, 30–31 March 2015. [Google Scholar]
- Jin, Q.; Kirk, M.F. pH as a Primary Control in Environmental Microbiology: 1. Thermodynamic Perspective. Front. Environ. Sci. 2018, 6, 21. [Google Scholar] [CrossRef]
- Allen, L.; Ansel, H.C. Ansel’s Pharmaceutical Dosage Forms and Drug Delivery Systems; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; ISBN 1451188765. [Google Scholar]
- Salvo, P.; Dini, V.; Di Francesco, F.; Romanelli, M. The role of biomedical sensors in wound healing. Wound Med. 2015, 8, 15–18. [Google Scholar] [CrossRef]
- Salvo, P.; Calisi, N.; Melai, B.; Dini, V.; Paoletti, C.; Lomonaco, T.; Pucci, A.; Di Francesco, F.; Piaggesi, A.; Romanelli, M. Temperature-and pH-sensitive wearable materials for monitoring foot ulcers. Int. J. Nanomed. 2017, 12, 949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Lin, Y.; Gillies, R.J. Tumor pH and its measurement. J. Nucl. Med. 2010, 51, 1167–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Lima, T.M.; Kazama, C.M.; Koczulla, A.R.; Hiemstra, P.S.; Macchione, M.; Godoy Fernandes, A.L.; de Paula Santos, U.; Bueno-Garcia, M.L.; Zanetta, D.M.; Saldiva de André, C.D.; et al. PH in exhaled breath condensate and nasal lavage as a biomarker of air pollution-related inflammation in street traffic-controllers and office-workers. Clinics 2013, 68, 1488–1494. [Google Scholar] [CrossRef]
- Cremer, M. Über die Ursache der elektromotorischen Eigenschaften der Gewebe, zugleich ein Beitrag zur Lehre von polyphasischen Elektrolytketten. Z. Biol. 1906, 47, 562–608. [Google Scholar]
- Leiner, M.J.P.; Hartmann, P. Theory and practice in optical pH sensing. Sens. Actuators B. Chem. 1993. [Google Scholar] [CrossRef]
- Richter, A.; Paschew, G.; Klatt, S.; Lienig, J.; Arndt, K.F.; Adler, H.J.P. Review on hydrogel-based pH sensors and microsensors. Sensors 2008, 8, 561–581. [Google Scholar] [CrossRef] [Green Version]
- Weston, M.; Kuchel, R.P.; Ciftci, M.; Boyer, C.; Chandrawati, R. A polydiacetylene-based colorimetric sensor as an active use-by date indicator for milk. J. Colloid Interface Sci. 2020, 572, 31–38. [Google Scholar] [CrossRef]
- Yuqing, M.; Jianrong, C.; Keming, F. New technology for the detection of pH. J. Biochem. Biophys. Methods 2005, 63, 1–9. [Google Scholar] [CrossRef]
- Salvo, P.; Melai, B.; Calisi, N.; Paoletti, C.; Bellagambi, F.; Kirchhain, A.; Trivella, M.G.; Fuoco, R.; Di Francesco, F. Graphene-based devices for measuring pH. Sens. Actuators B Chem. 2018, 256, 976–991. [Google Scholar] [CrossRef]
- Wencel, D.; Abel, T.; McDonagh, C. Optical chemical pH sensors. Anal. Chem. 2014, 86, 15–29. [Google Scholar] [CrossRef]
- Baldini, F. Critical review of pH sensing with optical fibres. In Chemical, Biochemical, and Environmental Fiber Sensors X; International Society for Optics and Photonics: Bellingham, WA, USA, 1999; Volume 3540, pp. 2–9. [Google Scholar]
- Butler, T.M.; MacCraith, B.D.; McDonagh, C. Leaching in sol-gel-derived silica films for optical pH sensing. J. Non. Cryst. Solids 1998, 224, 249–258. [Google Scholar] [CrossRef]
- Lev, O.; Tsionsky, M.; Rabinovich, L.; Glezer, V.; Sampath, S.; Pankratov, I.; Gun, J. Organically Modified Sol-Gel Sensors. Anal. Chem. 1995, 67, 22A–30A. [Google Scholar] [CrossRef]
- Plaschke, M.; Czolk, R.; Ache, H.J. Fluorimetric determination of mercury with a water-soluble porphyrin and porphyrin-doped sol-gel films. Anal. Chim. Acta 1995, 304, 107–113. [Google Scholar] [CrossRef]
- Zhu, Q.; Aller, R.C. Planar fluorescence sensors for two-dimensional measurements of H2S distributions and dynamics in sedimentary deposits. Mar. Chem. 2013. [Google Scholar] [CrossRef]
- Zhu, Q.; Aller, R.C.; Fan, Y. High-performance planar pH fluorosensor for two-dimensional pH measurements in marine sediment and water. Environ. Sci. Technol. 2005, 39, 8906–8911. [Google Scholar] [CrossRef] [PubMed]
- Stahl, H.; Glud, A.; Schröder, C.R.; Klimant, I.; Tengberg, A.; Glud, R.N. Time-resolved pH imaging in marine sediments with a luminescent planar optode. Limnol. Oceanogr. Methods 2006, 4, 336–345. [Google Scholar] [CrossRef]
- Jiang, Z.; Yu, X.; Hao, Y. Design and fabrication of a ratiometric planar optode for simultaneous imaging of pH and oxygen. Sensors 2017, 17, 1316. [Google Scholar] [CrossRef] [Green Version]
- Jin, Z.; Su, Y.; Duan, Y. An improved optical pH sensor based on polyaniline. Sens. Actuators B Chem. 2000, 71, 118–122. [Google Scholar] [CrossRef]
- Kermis, H.R.; Kostov, Y.; Rao, G. Rapid method for the preparation of a robust optical pH sensor. Analyst 2003, 128, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- Gotor, R.; Ashokkumar, P.; Hecht, M.; Keil, K.; Rurack, K. Optical pH Sensor Covering the Range from pH 0-14 Compatible with Mobile-Device Readout and Based on a Set of Rationally Designed Indicator Dyes. Anal. Chem. 2017, 89, 8437–8444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raoufi, N.; Surre, F.; Sun, T.; Rajarajan, M.; Grattan, K.T.V. Wavelength dependent pH optical sensor using the layer-by-layer technique. Sens. Actuators, B Chem. 2012, 169, 374–381. [Google Scholar] [CrossRef]
- Abu-Thabit, N.; Umar, Y.; Ratemi, E.; Ahmad, A.; Abuilaiwi, F.A. A flexible optical pH sensor based on polysulfone membranes coated with pH-responsive polyaniline nanofibers. Sensors 2016, 16, 986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safavi, A.; Bagheri, M. Novel optical pH sensor for high and low pH values. Sens. Actuators B Chem. 2003, 90, 143–150. [Google Scholar] [CrossRef]
- Hashemi, P.; Abolghasemi, M.M. Preparation of a novel optical sensor for low pH values using agarose membranes as support. Sens. Actuators B Chem. 2006, 115, 49–53. [Google Scholar] [CrossRef]
- Taweetanavanich, T.; Wanno, B.; Tuntulani, T.; Pulpoka, B.; Kaewtong, C. A pH optical and fluorescent sensor based on rhodamine modified on activated cellulose paper. J. Chinese Chem. Soc. 2019, 66, 493–499. [Google Scholar] [CrossRef]
- Li, W.; Cheng, H.; Xia, M.; Yang, K. An experimental study of pH optical sensor using a section of no-core fiber. Sens. Actuators A Phys. 2013, 199, 260–264. [Google Scholar] [CrossRef]
- Jung, Y.; Kim, S.; Lee, D.; Oh, K. Compact three segmented multimode fibre modal interferometer for high sensitivity refractive-index measurement. Meas. Sci. Technol. 2006, 17, 1129. [Google Scholar] [CrossRef] [Green Version]
- Schyrr, B.; Pasche, S.; Scolan, E.; Ischer, R.; Ferrario, D.; Porchet, J.A.; Voirin, G. Development of a polymer optical fiber pH sensor for on-body monitoring application. Sens. Actuators B Chem. 2014, 194, 238–248. [Google Scholar] [CrossRef]
- Sørensen, T.J.; Rosenberg, M.; Frankær, C.G.; Laursen, B.W. An Optical pH Sensor Based on Diazaoxatriangulenium and Isopropyl-Bridged Diazatriangulenium Covalently Bound in a Composite Sol–Gel. Adv. Mater. Technol. 2019. [Google Scholar] [CrossRef]
- Jeon, D.; Yoo, W.J.; Seo, J.K.; Shin, S.H.; Han, K.T.; Kim, S.G.; Park, J.Y.; Lee, B. Fiber-optic pH sensor based on sol-gel film immobilized with neutral red. Opt. Rev. 2013, 20, 209–213. [Google Scholar] [CrossRef]
- Mahboubeh, V.; Rounaghi, G.H.; Es’haghi, Z.; Moradi, Z. Design and Application of an Optical pH Sensor Based on Thionine Doped Modified Sol–Gel Film. Russ. J. Phys. Chem. A 2019, 93, 1389–1393. [Google Scholar] [CrossRef]
- Wencel, D.; Kaworek, A.; Abel, T.; Efremov, V.; Bradford, A.; Carthy, D.; Coady, G.; McMorrow, R.C.N.; McDonagh, C. Optical Sensor for Real-Time pH Monitoring in Human Tissue. Small 2018, 14, 1–8. [Google Scholar] [CrossRef]
- Gong, J.; Tanner, M.G.; Venkateswaran, S.; Stone, J.M.; Zhang, Y.; Bradley, M. A hydrogel-based optical fibre fluorescent pH sensor for observing lung tumor tissue acidity. Anal. Chim. Acta 2020, 1134, 136–143. [Google Scholar] [CrossRef]
- Zhao, Y.; Lei, M.; Liu, S.X.; Zhao, Q. Smart hydrogel-based optical fiber SPR sensor for pH measurements. Sens. Actuators, B Chem. 2018, 261, 226–232. [Google Scholar] [CrossRef]
- Moradi, V.; Akbari, M.; Wild, P. A fluorescence-based pH sensor with microfluidic mixing and fiber optic detection for wide range pH measurements. Sens. Actuators A Phys. 2019, 297, 111507. [Google Scholar] [CrossRef]
- Crespo, G.A.; Gugsa, D.; MacHo, S.; Rius, F.X. Solid-contact pH-selective electrode using multi-walled carbon nanotubes. Anal. Bioanal. Chem. 2009, 395, 2371–2376. [Google Scholar] [CrossRef]
- Gill, E.; Arshak, K.; Arshak, A.; Korostynska, O. Mixed metal oxide films as pH sensing materials. Microsyst. Technol. 2008, 14, 499–507. [Google Scholar] [CrossRef]
- Fog, A.; Buck, R.P. Electronic semiconducting oxides as pH sensors. Sens. Actuators 1984, 5, 137–146. [Google Scholar] [CrossRef]
- Manjakkal, L.; Szwagierczak, D.; Dahiya, R. Metal oxides based electrochemical pH sensors: Current progress and future perspectives. Prog. Mater. Sci. 2020, 109, 100635. [Google Scholar] [CrossRef]
- Wei, A.; Pan, L.; Huang, W. Recent progress in the ZnO nanostructure-based sensors. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2011, 176, 1409–1421. [Google Scholar] [CrossRef]
- Huang, X.J.; Choi, Y.K. Chemical sensors based on nanostructured materials. Sens. Actuators B Chem. 2007, 122, 659–671. [Google Scholar] [CrossRef]
- Głáb, S.; Hulanicki, A.; Edwall, G.; Folke, F.; Ingman, I.; Koch, W.F. Metal-Metal Oxide and Metal Oxide Electrodes as pH Sensors. Crit. Rev. Anal. Chem. 1989, 21, 29–47. [Google Scholar] [CrossRef]
- Semwal, V.; Gupta, B.D. Highly sensitive surface plasmon resonance based fiber optic pH sensor utilizing rGO-Pani nanocomposite prepared by in situ method. Sens. Actuators B Chem. 2019, 283, 632–642. [Google Scholar] [CrossRef]
- Shahamirifard, S.A.; Ghaedi, M.; Montazerozohori, M. Application of nanostructure ZnLI2 complex in construction of optical pH sensor. Appl. Organomet. Chem. 2018, 32, 1–11. [Google Scholar] [CrossRef]
- Bobacka, J.; Ivaska, A.; Lewenstam, A. Potentiometric ion sensors. Chem. Rev. 2008, 108, 329–351. [Google Scholar] [CrossRef] [PubMed]
- Lakard, B.; Herlem, G.; Lakard, S.; Guyetant, R.; Fahys, B. Potentiometric pH sensors based on electrodeposited polymers. Polymer 2005, 46, 12233–12239. [Google Scholar] [CrossRef]
- Lakard, B.; Segut, O.; Lakard, S.; Herlem, G.; Gharbi, T. Potentiometric miniaturized pH sensors based on polypyrrole films. Sens. Actuators, B Chem. 2007, 122, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Li, H.; Zhang, J.; Xu, Z. A novel pH potentiometric sensor based on electrochemically synthesized polybisphenol A films at an ITO electrode. Sens. Actuators B Chem. 2011, 155, 730–736. [Google Scholar] [CrossRef]
- Guinovart, T.; Valdés-Ramírez, G.; Windmiller, J.R.; Andrade, F.J.; Wang, J. Bandage-Based Wearable Potentiometric Sensor for Monitoring Wound pH. Electroanalysis 2014, 26, 1345–1353. [Google Scholar] [CrossRef]
- Park, H.J.; Yoon, J.H.; Lee, K.G.; Choi, B.G. Potentiometric performance of flexible pH sensor based on polyaniline nanofiber arrays. Nano Converg. 2019, 6. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Mao, Y.; Xiao, C.; Xu, X.; Li, X. Flexible pH sensor based on a conductive PANI membrane for pH monitoring. RSC Adv. 2019, 10, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Eftekhari, A. pH sensor based on deposited film of lead oxide on aluminum substrate electrode. Sens. Actuators B Chem. 2003, 88, 234–238. [Google Scholar] [CrossRef]
- Manjakkal, L.; Zaraska, K.; Cvejin, K.; Kulawik, J.; Szwagierczak, D. Potentiometric RuO2-Ta2O5 pH sensors fabricated using thick film and LTCC technologies. Talanta 2016, 147, 233–240. [Google Scholar] [CrossRef]
- Khalil, M.; Liu, N.; Lee, R.L. Super-Nernstian potentiometric pH sensor based on the electrodeposition of iridium oxide nanoparticles. Int. J. Technol. 2018, 57, 446–454. [Google Scholar] [CrossRef] [Green Version]
- Tanumihardja, E.; Olthuis, W.; van den Berg, A. Ruthenium oxide nanorods as potentiometric ph sensor for organs-on-chip purposes. Sensors 2018, 18, 2901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.J.; Savagatrup, S.; Kim, Y.; Lang, J.H.; Swager, T.M. Precision pH Sensor Based on WO3 Nanofiber-Polymer Composites and Differential Amplification. ACS Sens. 2019, 4, 2593–2598. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.E.; Totti, S.; Velliou, E.; Campagnolo, P.; Hingley-Wilson, S.M.; Ward, N.I.; Varcoe, J.R.; Crean, C. Development of a novel highly conductive and flexible cotton yarn for wearable pH sensor technology. Sens. Actuators B Chem. 2019, 287, 338–345. [Google Scholar] [CrossRef]
- Salvo, P.; Calisi, N.; Melai, B.; Cortigiani, B.; Mannini, M.; Caneschi, A.; Lorenzetti, G.; Paoletti, C.; Lomonaco, T.; Paolicchi, A.; et al. Temperature and pH sensors based on graphenic materials. Biosens. Bioelectron. 2017, 91, 870–877. [Google Scholar] [CrossRef]
- Salvo, P.; Dini, V.; Kirchhain, A.; Janowska, A.; Oranges, T.; Chiricozzi, A.; Lomonaco, T.; Di Francesco, F.; Romanelli, M. Sensors and biosensors for C-reactive protein, temperature and pH, and their applications for monitoring wound healing: A review. Sensors 2017, 17, 2952. [Google Scholar] [CrossRef] [Green Version]
- Melai, B.; Salvo, P.; Calisi, N.; Moni, L.; Bonini, A.; Paoletti, C.; Lomonaco, T.; Mollica, V.; Fuoco, R.; Di Francesco, F. A graphene oxide pH sensor for wound monitoring. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 1898–1901. [Google Scholar]
- Rahimi, R.; Ochoa, M.; Tamayol, A.; Khalili, S.; Khademhosseini, A.; Ziaie, B. Highly Stretchable Potentiometric pH Sensor Fabricated via Laser Carbonization and Machining of Carbon−Polyaniline Composite. ACS Appl. Mater. Interfaces 2017, 9, 9015–9023. [Google Scholar] [CrossRef]
- Poma, N.; Vivaldi, F.; Bonini, A.; Carbonaro, N.; Di Rienzo, F.; Melai, B.; Kirchhain, A.; Salvo, P.; Tognetti, A.; Di Francesco, F. Remote monitoring of seawater temperature and pH by low cost sensors. Microchem. J. 2019, 148, 248–252. [Google Scholar] [CrossRef]
- Bonini, A.; Di Francesco, F.; Salvo, P.; Vivaldi, F.; Herrera, E.; Melai, B.; Kirchhain, A.; Poma, N.; Mattonai, M.; Caprioli, R.; et al. A Graphenic Biosensor for Real-Time Monitoring of Urea during Dialysis. IEEE Sens. J. 2020. [Google Scholar] [CrossRef]
- Herrera, E.G.; Bonini, A.; Vivaldi, F.; Melai, B.; Salvo, P.; Poma, N.; Santalucia, D.; Kirchhain, A.; Di Francesco, F. A Biosensor for the Detection of Acetylcholine and Diazinon. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. EMBS 2019, 1159–1162. [Google Scholar] [CrossRef]
- Poma, N.; Vivaldi, F.; Bonini, A.; Salvo, P.; Kirchhain, A.; Melai, B.; Bottai, D.; Tavanti, A.; Di Francesco, F. A graphenic and potentiometric sensor for monitoring the growth of bacterial biofilms. Sens. Actuators B Chem. 2020. [Google Scholar] [CrossRef]
- Vivaldi, F.; Bonini, A.; Melai, B.; Poma, N.; Kirchhain, A.; Santalucia, D.; Salvo, P.; Di Francesco, F. A graphene-based pH sensor on paper for human plasma and seawater. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 1563–1566. [Google Scholar] [CrossRef]
- Manjakkal, L.; Dang, W.; Yogeswaran, N.; Dahiya, R. Textile-based potentiometric electrochemical PH sensor for wearable applications. Biosensors 2019, 9, 14. [Google Scholar] [CrossRef] [Green Version]
- Marxer, S.M.; Schoenfisch, M.H. Sol-gel derived potentiometric pH sensors. Anal. Chem. 2005, 77, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Zuaznabar-Gardona, J.C.; Fragoso, A. A wide-range solid state potentiometric pH sensor based on poly-dopamine coated carbon nano-onion electrodes. Sens. Actuators B Chem. 2018, 273, 664–671. [Google Scholar] [CrossRef]
- Sulka, G.D.; Hnida, K.; Agnieszka Brzózka, A. PH sensors based on polypyrrole nanowire arrays. Electrochim. Acta 2013, 104, 536–541. [Google Scholar] [CrossRef]
- Yoon, J.H.; Kim, S.M.; Park, H.J.; Kim, Y.K.; Oh, D.X.; Cho, H.W.; Lee, K.G.; Hwang, S.Y.; Park, J.; Choi, B.G. Highly self-healable and flexible cable-type pH sensors for real-time monitoring of human fluids. Biosens. Bioelectron. 2020, 150, 111946. [Google Scholar] [CrossRef] [PubMed]
- Stred’Anský, M.; Pizzariello, A.; Stred’Anská, S.; Miertuš, S. Amperometric pH-sensing biosensors for urea, penicillin, and oxalacetate. Anal. Chim. Acta 2000, 415, 151–157. [Google Scholar] [CrossRef]
- Wildgoose, G.G.; Pandurangappa, M.; Lawrence, N.S.; Jiang, L.; Jones, T.G.J.; Compton, R.G. Anthraquinone-derivatised carbon powder: Reagentless voltammetric pH electrodes. Talanta 2003, 60, 887–893. [Google Scholar] [CrossRef]
- Makos, M.A.; Omiatek, D.M.; Ewing, A.G.; Heien, M.L. Development and characterization of a voltammetric carbon-fiber microelectrode pH sensor. Langmuir 2010, 26, 10386–10391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amiri, M.; Amali, E.; Nematollahzadeh, A.; Salehniya, H. Poly-dopamine films: Voltammetric sensor for pH monitoring. Sens. Actuators B Chem. 2016, 228, 53–58. [Google Scholar] [CrossRef]
- Chaisiwamongkhol, K.; Batchelor-Mcauley, C.; Compton, R.G. Amperometric micro pH measurements in oxygenated saliva. Analyst 2017, 142, 2828–2835. [Google Scholar] [CrossRef]
- Vivaldi, F.; Santalucia, D.; Poma, N.; Bonini, A.; Salvo, P.; Del Noce, L.; Melai, B.; Kirchhain, A.; Kolivoška, V.; Sokolová, R.; et al. A voltammetric pH sensor for food and biological matrices. Sens. Actuators B Chem. 2020. [Google Scholar] [CrossRef]
- Chaisiwamongkhol, K.; Batchelor-Mcauley, C.; Compton, R.G. Optimising amperometric pH sensing in blood samples: An iridium oxide electrode for blood pH sensing. Analyst 2019, 144, 1386–1393. [Google Scholar] [CrossRef]
- Tham, G.X.; Fisher, A.C.; Webster, R.D. A vitamin-based voltammetric pH sensor that functions in buffered and unbuffered media. Sens. Actuators B Chem. 2019, 283, 495–503. [Google Scholar] [CrossRef]
- Zhu, H.; Hassan, T.; Kabir, H.; May, J.; Hamal, K.; Lopez, R.; Smith, H.J.; Nicholas, N.W.; Sankaran, P.; McIlroy, D.N.; et al. Voltammetric pH sensor based on electrochemically modified pseudo-graphite. Analyst 2020, 145, 7252–7259. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Li, N.; Zhang, Y.; Li, H. A novel pH sensor with application to milk based on electrochemical oxidative quinone-functionalization of tryptophan residues. J. Electroanal. Chem. 2020, 859, 113871. [Google Scholar] [CrossRef]
- Gao, W.; Song, J. Polyaniline film based amperometric pH sensor using a novel electrochemical measurement system. Electroanalysis 2009, 21, 973–978. [Google Scholar] [CrossRef]
- Sha, R.; Komori, K.; Badhulika, S. Amperometric pH Sensor Based on Graphene-Polyaniline Composite. IEEE Sens. J. 2017, 17, 5038–5043. [Google Scholar] [CrossRef]
- Bergveld, P. Development of an Ion-Sensitive Solid-State Device for Neurophysiological Measurements. IEEE Trans. Biomed. Eng. 1970, BME-17, 70–71. [Google Scholar] [CrossRef]
- Chin, Y.L.; Chou, J.C.; Sun, T.P.; Liao, H.K.; Chung, W.Y.; Hsiung, S.K. A novel SnO2/Al discrete gate ISFET pH sensor with CMOS standard process. Sens. Actuators B Chem. 2001, 75, 36–42. [Google Scholar] [CrossRef]
- Shitashima, K.; Kyo, M.; Koike, Y.; Henmi, H. Development of in situ pH sensor using ISFET Environmental Science Department. In Proceedings of the 2002 Interntional Symposium on Underwater Technology (Cat. No.02EX556), Tokyo, Japan, 19 April 2002; pp. 106–108. [Google Scholar] [CrossRef]
- Fukuba, T.; Tamai, Y.; Kyo, M.; Shitashima, K.; Koike, Y.; Fujii, T. Development and field evaluation of ISFET pH sensor integrated with self-calibration device for deep-sea oceanography applications. In Proceedings of the 12th International Conference on Miniaturized Systems for Life Sciences, San Diego, CA, USA, 12–16 October 2008; pp. 1983–1985. [Google Scholar]
- Rani, R.A.; Syono, M.I.; Ramli, A.S. Multi-finger gate ISFET (Mf-ISFET) for pH sensor application. In Proceedings of the 2008 IEEE International Conference on Semiconductor Electronics, Johor Bahru, Malaysia, 25–27 November 2008; pp. 350–353. [Google Scholar] [CrossRef]
- Parizi, K.B.; Yeh, A.J.; Poon, A.S.Y.; Wong, H.S.P. Exceeding Nernst limit (59mV/pH): CMOS-based pH sensor for autonomous applications. In Proceedings of the 2012 International Electron Devices Meeting, San Francisco, CA, USA, 10–13 December 2012. [Google Scholar] [CrossRef]
- Das, A.; Ko, D.H.; Chen, C.H.; Chang, L.B.; Lai, C.S.; Chu, F.C.; Chow, L.; Lin, R.M. Highly sensitive palladium oxide thin film extended gate FETs as pH sensor. Sens. Actuators B Chem. 2014, 205, 199–205. [Google Scholar] [CrossRef]
- Cho, W.J.; Lim, C.M. Sensing properties of separative paper-based extended-gate ion-sensitive field-effect transistor for cost effective pH sensor applications. Solid. State. Electron. 2018, 140, 96–99. [Google Scholar] [CrossRef]
- Kang, T.; Lee, I.; Oh, S.; Jang, T.; Kim, Y.; Ahn, H.; Kim, G.; Shin, S.U.; Jeong, S.; Sylvester, D.; et al. A 1.74.12 mm3 Fully Integrated pH Sensor for Implantable Applications using Differential Sensing and Drift-Compensation. In Proceedings of the 2019 Symposium on VLSI Circuits, Kyoto, Japan, 9–14 June 2019. [Google Scholar] [CrossRef]
- Sharma, P.; Gupta, S.; Singh, R.; Ray, K.; Kothari, S.L.; Sinha, S.; Sharma, R.; Mukhiya, R.; Awasthi, K.; Kumar, M. Hydrogen ion sensing characteristics of Na3BiO4–Bi2O3 mixed oxide nanostructures based EGFET pH sensor. Int. J. Hydrogen Energy 2020, 45, 18743–18751. [Google Scholar] [CrossRef]
- Jung, S.H.; Seo, Y.M.; Gu, T.; Jang, W.; Kang, S.G.; Hyeon, Y.; Hyun, S.H.; Lee, J.H.; Whang, D. Super-Nernstian pH Sensor Based on Anomalous Charge Transfer Doping of Defect-Engineered Graphene. Nano Lett. 2020, 21, 34–42. [Google Scholar] [CrossRef] [PubMed]





| Transduction | Reproducibility | Precision | Sensitivity | Hysteresis | Interferents | Working Range | Matrix | Response Time | Life Time | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| Optical | - | - | - | - | - | 2–8 | - | 1 s | 1 month | [23] |
| Optical | <0.1 pH units | - | - | - | - | 6–9 | - | 180 s | - | [24] |
| Optical | - | - | - | - | - | 7.3–9.3 | Marine sediments | 200 s | >3 days | [21] |
| Optical | - | - | - | - | - | 6–8 | Marine sediments | 16 s | 1 week | [22] |
| Optical | <0.20 pH units | 0.16 pH units | - | - | - | 0–14 | - | - | - | [25] |
| Optical | - | - | - | - | - | 6.80–9.00 | - | - | - | [26] |
| Optical | - | - | - | - | - | 4–12 | Detergent | <5 s | >1 month | [27] |
| Optical | - | <2% | - | - | - | 1–14 with Neural network | - | 54 s | - | [28] |
| Optical | - | <0.3% | - | - | - | 0.5–5 | - | 180 s | >3 months | [29] |
| Optical | - | - | - | - | Na+, K+, Ag+, Mg2+, Ca2+, Pb2+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+, Hg2+, Al3+, Cr3+, Fe3+, Au3+, Pt2+, Ru2+ | 1–8 | - | - | - | [30] |
| Optical | - | <1% | 6.6 dBm/pH | - | - | 1–13 | - | 40 s | - | [31] |
| Optical | - | ±0.20 pH units | - | - | Ionic Strength | 5–8 | Human serum | 1200 s | >2 months | [33] |
| Optical | - | <0.1 pH units | - | - | - | 4.7–7.7 | - | - | - | [34] |
| Optical | - | - | - | - | - | 6–9 | - | 20 s | - | [35] |
| Optical | - | - | - | - | - | 11–13 | - | 50 s | >7 months | [36] |
| Optical | - | - | - | - | - | 6–8.5 | Subcutaneous tissue | <120 s | 28 days | [37] |
| Optical | - | 0.1 pH units | - | - | - | 5.5–8 | Ovine lung tissue | 30 s | - | [38] |
| Optical | 13 nm/pH | 0.5% | - | 1–12 | - | 20 s | 10 days | [39] | ||
| Optical | - | - | 6–42 mV/pH | - | - | 2.5–9 | - | 10 s | - | [40] |
| Optical | - | - | 24.93–11.35 nm/pH | - | - | 2.4–11.35 | - | - | - | [48] |
| Optical | 4.06% | 1.14% | - | - | Ionic Strength | 4–8 5–8 | - | 240 s | >2 months | [49] |
| Potentiometric | - | - | 34–52 mV/pH | - | - | 2–11 | - | - | - | [51] |
| Potentiometric | - | - | 40–50 mV/pH | - | - | 2–11 | - | - | >1 month | [52] |
| Potentiometric | 2.4–2.9% | - | 56.7–58.6 mV/pH | - | Na+, K+, Cl−, SO42− | 1–15 | Milk, orange juice | <20 s | - | [53] |
| Potentiometric | 0.66% | 1.9% | 54–56 mV/pH | - | Na+, K+, Cl−, SO42− | 5.5–8 | - | <20 s | - | [54] |
| Potentiometric | - | - | 62.4 mV/pH | 5.6 mV | Li+, Na+, K+, Mg2+, Ca2+, NH4+ | 4–10 | milk | 12.8 s | - | [55] |
| Potentiometric | 2.39% | 8% | 58.57 mV/pH | 12% | - | 5.45–8.62 | - | 45 s | - | [56] |
| Potentiometric | 2.7% | - | 58 mV/pH | - | Li+, Na+, Mg2+, Ca2+, NH4+, HCO3− | 1–12 | Cola | - | >1 months | [57] |
| Potentiometric | ±1 mV/pH | - | 56 mV/pH | ±3 mV acid region, ±8 mV basic region | Li+, Na+, K+ | 2–10 | River water, lemon juice | <15 s | - | [58] |
| Potentiometric | - | - | 73 mV/pH | - | - | 2–12 | - | <10 s | - | [59] |
| Potentiometric | - | - | 58 mV/pH | - | Li+, Ca2+, Cl−, SO42− | 2–10 | - | <2 s | - | [60] |
| Potentiometric | - | - | 377.5 mV/pH | - | - | 6.90–8.94 | Artificial sea water | - | - | [61] |
| Potentiometric | - | ±0.4 mV/pH | 58 mV/pH | - | Li+, Na+,K+, Mg2+, Ca2+, NH4+ | 2.89–9.90 | - | 10 s | - | [41] |
| Potentiometric | ±2 mV/pH | - | 61 mV/pH | - | Na+, K+, Mg2+, Ca2+, NH4+ | 2–12 | Artificial sweat | 60 ± 20 s | - | [62] |
| Potentiometric | - | - | 31.8 mV/pH | - | - | 4–10 | Wound exudate | - | >4 days | [65] |
| Potentiometric | - | - | 53 mV/pH | - | - | 4–10 | - | - | - | [66] |
| Potentiometric | 5% | 5% | 45 mV/pH | - | - | 4–10 | sea water | - | >7 days | [67] |
| Potentiometric | - | - | 4 mV/pH | 0.5 mV | Glucose, Urea | 6–9 | - | 5 s | - | [72] |
| Potentiometric | - | - | 44–55 mV/pH | - | Na+, K+ | 3–8 | - | <3 s | >2 weeks | [73] |
| Potentiometric | - | - | 58.3–60.1 mV/pH | - | Li+, Na+, K+ | 2–10 | Milk, sea water, pineapple juice, vinegar | - | - | [74] |
| Potentiometric | - | - | 46–49 mV/pH | - | - | 2–12 | - | - | >2 months | [75] |
| Potentiometric | - | - | 58.7 mV/pH | 5.6 | K+, Na+, Ca+, NH4+ | 4–10 | Urine, Saliva, Sweat, Tears | 5 s | [76] | |
| Voltammetric | - | - | 58 mV/pH | - | - | 1–9 | - | - | - | [78] |
| Voltammetric | - | - | 38 mV/pH | - | Mg+, Ca+, K+ | 6.5–8 | Bacteria broth | 1.6 s | - | [79] |
| Voltammetric | <0.8% | ±0.09 mV/pH | 58 mV/pH | - | - | 1–12 | - | - | - | [80] |
| Voltammetric | - | - | 65 mV/pH | - | - | 2–8 | Synthetic saliva and saliva | - | - | [81] |
| Voltammetric | 5% | 7% | 56 mV/pH | - | Li+, Na+ | 2–12 | Biological and food matrix | - | >2 months | [82] |
| Voltammetric | <2% | - | 62.7 mV/pH | - | - | 5–9 | Animal blood | - | - | [83] |
| Voltammetric | - | - | 50 mV/pH | - | - | 1–12 | - | - | - | [84] |
| Voltammetric | - | - | 63.3 mV/pH | - | Na+, K+, Dissolved oxygen | 0–11 | Apple cider vinegar | - | - | [85] |
| Voltammetric and potentiometric | - | - | 52 mV/pH | - | - | 1–12 | Cola, Milk | - | 16 days | [86] |
| Voltammetric | - | 0.5% | 32,4 mA/pH 15.9 mA/pH | - | K+, Na+, Li+ | 2–5.5 5.5–10 | - | <8 s | - | [87] |
| Voltammetric | <3.4% | - | −50.14 µA/pH cm2 ‒139.2 µA/pH cm2 | - | - | 1–5, 7–11 | - | - | - | [88] |
| Transistor | - | - | 58 mV/pH | - | - | 2–10 | - | - | - | [90] |
| Transistor | - | - | 45.1 mV/pH | 24 mV 12 mV | - | - | - | 600 s | - | [92] |
| Transistor | - | - | 130 mV/pH | - | - | 4–10 | - | - | - | [93] |
| Transistor | - | - | 62 mV/pH | 7.4 mV | - | 2–12 | - | - | - | [94] |
| Transistor | - | - | 57 mV/pH | 25 mV | - | 4–10 | - | - | - | [95] |
| Transitor | - | - | 49.63 mV/pH | - | - | 7–12 | - | - | - | [98] |
| Transistor | - | - | 140 mV/pH | - | - | 6–7.6 | - | - | - | [99] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vivaldi, F.; Salvo, P.; Poma, N.; Bonini, A.; Biagini, D.; Del Noce, L.; Melai, B.; Lisi, F.; Francesco, F.D. Recent Advances in Optical, Electrochemical and Field Effect pH Sensors. Chemosensors 2021, 9, 33. https://doi.org/10.3390/chemosensors9020033
Vivaldi F, Salvo P, Poma N, Bonini A, Biagini D, Del Noce L, Melai B, Lisi F, Francesco FD. Recent Advances in Optical, Electrochemical and Field Effect pH Sensors. Chemosensors. 2021; 9(2):33. https://doi.org/10.3390/chemosensors9020033
Chicago/Turabian StyleVivaldi, Federico, Pietro Salvo, Noemi Poma, Andrea Bonini, Denise Biagini, Lorenzo Del Noce, Bernardo Melai, Fabio Lisi, and Fabio Di Francesco. 2021. "Recent Advances in Optical, Electrochemical and Field Effect pH Sensors" Chemosensors 9, no. 2: 33. https://doi.org/10.3390/chemosensors9020033
APA StyleVivaldi, F., Salvo, P., Poma, N., Bonini, A., Biagini, D., Del Noce, L., Melai, B., Lisi, F., & Francesco, F. D. (2021). Recent Advances in Optical, Electrochemical and Field Effect pH Sensors. Chemosensors, 9(2), 33. https://doi.org/10.3390/chemosensors9020033