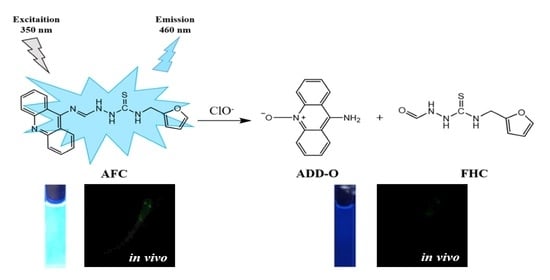
A Novel Thiosemicarbazide-Based Fluorescent Chemosensor for Hypochlorite in Near-Perfect Aqueous Solution and Zebrafish
Abstract
:1. Introduction
2. Experiments
2.1. Materials and Equipment
2.2. Synthesis of FHC (2-Formyl-N-(Furan-2-Ylmethyl)Hydrazine-1-Carbothioamide)
2.3. Synthesis of AFC ((E)-2-((Acridin-9-Ylimino)Methyl)-N-(Furan-2-Ylmethyl)Hydrazine-1-Carbothioamide)
2.4. General Procedures
2.5. Imaging in Zebrafish
3. Results and Discussion
3.1. Spectroscopic Investigations of Chemosensor AFC to ClO−
3.2. In Vivo Imaging in Zebrafish
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ji, R.; Qin, K.; Liu, A.; Zhu, Y.; Ge, Y. A simple and fast-response fluorescent probe for hypochlorite in living cells. Tetrahedron Lett. 2018, 59, 2372–2375. [Google Scholar] [CrossRef]
- Jiang, Y.; Zheng, G.; Duan, Q.; Yang, L.; Zhang, J.; Zhang, H.; He, J.; Sun, H.; Ho, D. Ultra-sensitive fluorescent probes for hypochlorite acid detection and exogenous/endogenous imaging of living cells. Chem. Commun. 2018, 54, 7967–7970. [Google Scholar] [CrossRef] [PubMed]
- Yun, D.; Chae, J.B.; Kim, C. An imine-based colorimetric chemodosimeter for the detection of hypochlorite (ClO−) in aqueous media: Its application in test strips and real water samples. J. Chem. Sci. 2019, 131, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Lou, X.; Zhang, Y.; Qin, J.; Li, Z. Colorimetric hypochlorite detection using an azobenzene acid in pure aqueous solutions and real application in tap water. Sens. Actuators B Chem. 2012, 161, 229–234. [Google Scholar] [CrossRef]
- Shen, S.L.; Zhang, X.F.; Ge, Y.Q.; Zhu, Y.; Cao, X.Q. A mitochondria-targeting ratiometric fluorescent probe for the detection of hypochlorite based on the FRET strategy. RSC Adv. 2017, 7, 55296–55300. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Qian, Y. A novel (3,6-di-tert-butylcarbazol-9-yl) triphenylamine-BODIPY-tricyanofuran conjugated dye: Synthesis and rapid naked-eye detection of hypochlorite. New J. Chem. 2017, 41, 9607–9612. [Google Scholar] [CrossRef]
- Yi, S.; Lu, Z.; Lin, Y.; Wang, J.; Qiao, Z.; Shen, R.; Zhang, J.; Hou, L. A novel mitochondria-targeted phosphorescence probe for hypochlorite ions detection in living cells. Talanta 2020, 209, 120516. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Kim, C. Naphthalimide-based probe for the detection of hypochlorite in a near-perfect aqueous solution. Anal. Sci. 2019, 35, 1189–1193. [Google Scholar] [CrossRef] [Green Version]
- Goswami, S.; Paul, S.; Manna, A. Carbazole based hemicyanine dye for both “naked eye” and ‘NIR’ fluorescence detection of CN− in aqueous solution: From molecules to low cost devices (TLC plate sticks). Dalton Trans. 2013, 42, 10682–10686. [Google Scholar] [CrossRef]
- Lee, S.C.; Kim, C. Naphthol-naphthalimide based ‘turn-on’ fluorescent sensor for ClO− in aqueous media and test kit. Inorg. Chem. Commun. 2019, 108, 107545. [Google Scholar] [CrossRef]
- Sasikumar, T.; Ilanchelian, M. Colorimetric detection of hypochlorite based on the morphological changes of silver nanoprisms to spherical nanoparticles. Anal. Methods 2017, 9, 3151–3158. [Google Scholar] [CrossRef]
- Chen, W.C.; Venkatesan, P.; Wu, S.P. A highly selective turn-on fluorescent probe for hypochlorous acid based on hypochlorous acid-induced oxidative intramolecular cyclization of boron dipyrromethene-hydrazone. Anal. Chim. Acta 2015, 882, 68–75. [Google Scholar] [CrossRef]
- Duan, R.; Li, C.; Liu, S.; Liu, Z.; Li, Y.; Zhu, J.; Hu, X. A selective fluorescence quenching method for the determination of trace hypochlorite in water samples with nile blue A. J. Taiwan Inst. Chem. Eng. 2015, 50, 43–48. [Google Scholar] [CrossRef]
- Lin, X.; Qin, W.; Chen, Y.; Bao, L.; Li, N.; Wang, S.; Liu, K.; Kong, F.; Yi, T. Construction of a multi-signal near-infrared fluorescent probe for sensing of hypochlorite concentration fluctuation in living animals. Sens. Actuators B Chem. 2020, 324. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Fang, H.; Zhu, W.; He, J.X.; Yao, H.; Wei, T.B.; Lin, Q.; Qu, W.J. Ratiometric fluorescent sensor based oxazolo-phenazine derivatives for detect hypochlorite via oxidation reaction and its application in environmental samples. Dyes Pigm. 2020, 172, 107765. [Google Scholar] [CrossRef]
- Chang, C.; Wang, F.; Qiang, J.; Zhang, Z.; Chen, Y.; Zhang, W.; Wang, Y.; Chen, X. Benzothiazole-based fluorescent sensor for hypochlorite detection and its application for biological imaging. Sens. Actuators B Chem. 2017, 243, 22–28. [Google Scholar] [CrossRef]
- Starzak, K.; Matwijczuk, A.; Creaven, B.; Matwijczuk, A.; Wybraniec, S.; Karcz, D. Fluorescence quenching-based mechanism for determination of hypochlorite by coumarin-derived sensors. Int. J. Mol. Sci. 2019, 20, 281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Ning, J.Y.; Liu, J.T.; Miao, J.Y.; Zhao, B.X. A mitochondria-targeted ratiometric fluorescence sensor for the detection of hypochlorite in living cells. Dyes Pigm. 2019, 171, 107708. [Google Scholar] [CrossRef]
- Naha, S.; Varalakshmi, A.; Velmathi, S. Nanomolar colorimetric hypochlorite sensor in water. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 220, 117123. [Google Scholar] [CrossRef]
- Hwang, S.M.; Yun, D.; Kim, C. An Imidazo[1,5-α]Pyridine-Based Fluorometric Chemodosimeter for the Highly Selective Detection of Hypochlorite in Aqueous Media. J. Fluoresc. 2019, 29, 451–459. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, H.; Hong, Y.; Yu, M.; Zeng, R.; Long, Y.; Chen, J. Selective visualization of endogenous hypochlorous acid in zebrafish during lipopolysaccharide-induced acute liver injury using a polymer micelles-based ratiometric fluorescent probe. Biosens. Bioelectron. 2018, 99, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yin, C.; Liu, T.; Wen, Y.; Huo, F. A new mechanism-based fluorescent probe for the detection of ClO− by UV–vis and fluorescent spectra and its applications. Sens. Actuators B Chem. 2017, 252, 1112–1117. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, G.; Shi, J.; Wang, Y.; Wang, M.; Peng, Q.; Zhang, D. A highly selective fluorescence turn-on detection of ClO- with 1-methyl-1,2-dihydropyridine-2-thione unit modified tetraphenylethylene. Chem. Commun. 2017, 53, 11654–11657. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.M.; Kim, A.; Kim, C. A simple hydrazine-based probe bearing anthracene moiety for the highly selective detection of hypochlorite. Inorg. Chem. Commun. 2019, 101, 1–5. [Google Scholar] [CrossRef]
- Jeong, H.Y.; Lee, S.Y.; Kim, C. Furan and Julolidine-Based “Turn-on” Fluorescence Chemosensor for Detection of F− in a Near-Perfect Aqueous Solution. J. Fluoresc. 2017, 27, 1457–1466. [Google Scholar] [CrossRef]
- Yang, Q.; Zhong, X.; Chen, Y.; Yang, J.; Jin, C.; Jiang, Y. A mitochondria-targeted fluorescent probe for hypochlorite sensing and its application in bioimaging. Analyst 2020, 145, 3100–3105. [Google Scholar] [CrossRef]
- Feng, A.; Liu, P.; Liang, Q.; Zhang, X.; Huang, L.; Jia, Y.; Xie, M.; Yan, Q.; Li, C.; Wang, S. A new carbazole-based colormetric and ratiometric fluorescent probe for hypochlorite sensing in living cells and zebrafishes. Dyes Pigm. 2020, 180, 108492. [Google Scholar] [CrossRef]
- Ma, Z.; Chen, X.; Wang, C.; Lv, Q. A novel ratiometric fluorescence probe for hypochlorite detection and its application in cell imaging. J. Mol. Struct. 2020, 1221, 128812. [Google Scholar] [CrossRef]
- Ning, Y.; Cui, J.; Lu, Y.; Wang, X.; Xiao, C.; Wu, S.; Li, J.; Zhang, Y. De novo design and synthesis of a novel colorimetric fluorescent probe based on naphthalenone scaffold for selective detection of hypochlorite and its application in living cells. Sens. Actuators B Chem. 2018, 269, 322–330. [Google Scholar] [CrossRef]
- Zhou, J.; Li, L.; Shi, W.; Gao, X.; Li, X.; Ma, H. HOCl can appear in the mitochondria of macrophages during bacterial infection as revealed by a sensitive mitochondrial-targeting fluorescent probe. Chem. Sci. 2015, 6, 4884–4888. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.; Xu, K.; Li, Q.; Wang, C.; Liu, X.; Wang, P. Acridine-based complex as amino acid anion fluorescent sensor in aqueous solution. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 157, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Jia, Y.; Chen, W.; Chui, P.; Yang, Z. A reaction-based fluorescent probe for rapid detection of hypochlorite in tap water, serum, and living cells. Sens. Actuators B Chem. 2016, 232, 300–305. [Google Scholar] [CrossRef]
- Wang, B.; Chen, D.; Kambam, S.; Wang, F.; Wang, Y.; Zhang, W.; Yin, J.; Chen, H.; Chen, X. A highly specific fluorescent probe for hypochlorite based on fluorescein derivative and its endogenous imaging in living cells. Dyes Pigm. 2015, 120, 22–29. [Google Scholar] [CrossRef]
- Wang, C.; Wang, P.; Liu, X.; Fu, J.; Xue, K.; Xu, K. Novel enantioselective fluorescent sensors for tartrate anion based on acridinezswsxa. Luminescence 2017, 32, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Chemate, S.; Erande, Y.; Mohbiya, D.; Sekar, N. Acridine derivative as a “turn on” probe for selective detection of picric acid: Via PET deterrence. RSC Adv. 2016, 6, 84319–84325. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, S.Y.; Jung, J.M.; Kim, C. A new Schiff-base chemosensor for selective detection of Cu2+ and Co2+ and its copper complex for colorimetric sensing of S2- in aqueous solution. Photochem. Photobiol. Sci. 2017, 16, 1677–1689. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Ghosh, S.; Makhal, S.C.; Guchhait, N. Hydrazine bridged coumarin-pyrimidine conjugate as a highly selective and sensitive Zn2+ sensor: Spectroscopic unraveling of sensing mechanism with practical application. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 183, 306–311. [Google Scholar] [CrossRef]
- Ashraf, A.; Khizar, M.; Islam, M.; Hameed, A.; Tarique Moin, S.; Yaqub, M.; Rauf, W.; Moazzam Naseer, M.; Tayyeb Ahsan, M.; Shafiq, Z.; et al. Synthesis of sensitive novel dual Signaling pyridopyrimidine-based fluorescent “turn off” chemosensors for anions determination. Meas. J. Int. Meas. Confed. 2019, 151, 107267. [Google Scholar] [CrossRef]
- Swamy, K.M.K.; Eom, S.; Liu, Y.; Kim, G.; Lee, D.; Yoon, J. Rhodamine derivatives bearing thiourea groups serve as fluorescent probes for selective detection of ATP in mitochondria and lysosomes. Sens. Actuators B Chem. 2019, 281, 350–358. [Google Scholar] [CrossRef]
- So, H.; Lee, M.; Kim, C. A Unique Thiosemicarbazide-Based Colorimetric Chemosensor for Fe2+ in Pure Aqueous Solution with the Lowest Detection Limit. ChemistrySelect 2020, 5, 10521–10525. [Google Scholar] [CrossRef]
- Shen, B.X.; Qian, Y.; Qi, Z.Q.; Lu, C.G.; Sun, Q.; Xia, X.; Cui, Y.P. Near-infrared BODIPY-based two-photon ClO- probe based on thiosemicarbazide desulfurization reaction: Naked-eye detection and mitochondrial imaging. J. Mater. Chem. B 2017, 5, 5854–5861. [Google Scholar] [CrossRef]
- Zeng, Y.N.; Zheng, H.Q.; He, X.H.; Cao, G.J.; Wang, B.; Wu, K.; Lin, Z.J. Dual-emissive metal-organic framework: A novel turn-on and ratiometric fluorescent sensor for highly efficient and specific detection of hypochlorite. Dalton Trans. 2020, 49, 9680–9687. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yin, P.; Li, T.; Wei, T.; Hu, T.; Chen, J.; Qin, X.; Niu, Q. Rapid and sensitive detection of hypochlorite in ~100% aqueous solution using a bithiophene-based fluorescent sensor: Application to water analysis and live-cell imaging. J. Mol. Liq. 2020, 320, 114396. [Google Scholar] [CrossRef]
- Cai, W.; Wu, J.; Liu, W.; Xie, Y.; Liu, Y.; Zhang, S.; Xu, W.; Tang, L.; Wang, J.; Zhao, G. Systematic structure-activity relationship (SAR) exploration of diarylmethane backbone and discovery of a highly potent novel uric acid transporter 1 (URAT1) inhibitor. Molecules 2018, 23, 252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.S.; Yun, D.; Chae, J.B.; So, H.; Lee, H.; Kim, K.; Kim, M.; Lim, M.H.; Kim, C. A Novel Thiophene-Based Fluorescent Chemosensor for the Detection of Zn2+ and CN−: Imaging Applications in Live Cells and Zebrafish. Sensors 2019, 19, 5458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; He, X.; Yu, Y.; Qian, Y.; Shen, J.; Zhao, S. Execution of aggregation-induced emission as nano-sensors for hypochlorite detection and application for bioimaging in living cells and zebrafish. Talanta 2020, 214, 120842. [Google Scholar] [CrossRef]
- So, H.; Lee, H.; Lee, G.D.; Kim, M.; Lim, M.H.; Kim, K.T.; Kim, C. A thiourea-based fluorescent chemosensor for bioimaging hypochlorite. J. Ind. Eng. Chem. 2020, 89, 436–441. [Google Scholar] [CrossRef]
- Zhu, Y.; Ma, Y.; Liu, Y.; Liu, Z.; Ma, S.; Xing, M.; Cao, D.; Lin, W. Fluorescence response of a fluorescein derivative for hypochlorite ion and its application for biological imaging in wounded zebrafish and living mice. Sens. Actuators B Chem. 2021, 327, 128848. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, Y.; Yang, T.; Gou, Z.; Wang, X.; Lin, W. Novel fluorescent probe with a bridged Si-O-Si bond for the reversible detection of hypochlorous acid and biothiol amino acids in live cells and zebrafish. Analyst 2019, 144, 5075–5080. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Chen, K.; Xu, J.; Zhu, X.; Cao, W.; Wang, Z.; Ye, Y.; Zhao, Y. Mitochondria-targeted fluorescent probes for oxidative stress imaging. Sens. Actuators B Chem. 2019, 299, 126938. [Google Scholar] [CrossRef]
- Lee, S.C.; Park, S.; So, H.; Lee, G.; Kim, K.T.; Kim, C. An acridine-based fluorescent sensor for monitoring clo− in water samples and zebrafish. Sensors 2020, 20, 4764. [Google Scholar] [CrossRef] [PubMed]









| Sample | ClO− Added (µM) | ClO− Found (µM) | Recovery (%) | RSD (n = 3) (%) |
|---|---|---|---|---|
| Drinking water | 0.0 | 0.0 | ||
| 40.0 b | 39.7 | 99.15 | 0.24 | |
| Tap water | 0.0 | 0.00 | ||
| 40.0 c | 38.3 | 95.64 | 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.; Choe, D.; Park, S.; Kim, H.; Jeong, S.; Kim, K.-T.; Kim, C. A Novel Thiosemicarbazide-Based Fluorescent Chemosensor for Hypochlorite in Near-Perfect Aqueous Solution and Zebrafish. Chemosensors 2021, 9, 65. https://doi.org/10.3390/chemosensors9040065
Lee M, Choe D, Park S, Kim H, Jeong S, Kim K-T, Kim C. A Novel Thiosemicarbazide-Based Fluorescent Chemosensor for Hypochlorite in Near-Perfect Aqueous Solution and Zebrafish. Chemosensors. 2021; 9(4):65. https://doi.org/10.3390/chemosensors9040065
Chicago/Turabian StyleLee, Minji, Donghwan Choe, Soyoung Park, Hyeongjin Kim, Soomin Jeong, Ki-Tae Kim, and Cheal Kim. 2021. "A Novel Thiosemicarbazide-Based Fluorescent Chemosensor for Hypochlorite in Near-Perfect Aqueous Solution and Zebrafish" Chemosensors 9, no. 4: 65. https://doi.org/10.3390/chemosensors9040065
APA StyleLee, M., Choe, D., Park, S., Kim, H., Jeong, S., Kim, K. -T., & Kim, C. (2021). A Novel Thiosemicarbazide-Based Fluorescent Chemosensor for Hypochlorite in Near-Perfect Aqueous Solution and Zebrafish. Chemosensors, 9(4), 65. https://doi.org/10.3390/chemosensors9040065