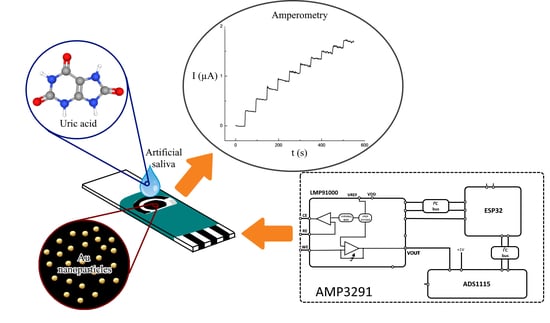
Determination of Uric Acid in Artificial Saliva with Compact AMP3291 Reader and Au Nanoparticles Modified Electrode
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electronic Components
2.2. Reagents and Chemicals
2.3. Apparatus and Equipment
2.4. Preparation of Electrodes
2.5. Design of AMP3291 Instrument
2.6. Electrochemical Measurements
3. Results
3.1. SPE-AuNps Sensor Characterization
3.2. Evaluation of AMP3291 for UA Detection in PBS
3.3. Detection Performance of UA in Artificial Saliva
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El Ridi, R.; Tallima, H. Physiological functions and pathogenic potential of uric acid: A review. J. Adv. Res. 2017, 8, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wen, X.; Kong, J. Recent Progress on Uric Acid Detection: A Review. Crit. Rev. Anal. Chem. 2019, 0, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Shakarami, A.; Ghafarzadeh, M.; Yari, F.; Fathi, L. Association between maternal serum uric acid and preeclampsia. Arch. Physiol. Biochem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Esra, P.; Kılıc, E. A review of enzymatic uric acid biosensors based on amperometric detection. Talanta 2013, 107, 312–323. [Google Scholar] [CrossRef]
- Riis, J.L.; Bryce, C.I.; Matin, M.J.; Stebbins, J.L.; Kornienko, O.; Van Huisstede, L.; Granger, D.A. The validity, stability, and utility of measuring uric acid in saliva. Biomark. Med. 2018, 12, 583–596. [Google Scholar] [CrossRef]
- Heikenfeld, J. Let them see you sweat. IEEE Spectr. 2014, 51, 46–63. [Google Scholar] [CrossRef]
- Kim, J.; Imani, S.; de Araujo, W.R.; Warchall, J.; Valdés-Ramírez, G.; Paixão, T.R.L.C.; Mercier, P.P.; Wang, J. Wearable salivary uric acid mouthguard biosensor with integrated wireless electronics. Biosens. Bioelectron. 2015, 74, 1061–1068. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Song, Y.; Bo, X.; Min, J.; Pak, O.S.; Zhu, L.; Wang, M.; Tu, J.; Kogan, A.; Zhang, H.; et al. A laser-engraved wearable sensor for sensitive detection of uric acid and tyrosine in sweat. Nat. Biotechnol. 2020, 38, 217–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrivastava, S.; Trung, T.Q.; Lee, N.E. Recent progress, challenges, and prospects of fully integrated mobile and wearable point-of-care testing systems for self-testing. Chem. Soc. Rev. 2020, 49, 1812–1866. [Google Scholar] [CrossRef]
- Kosack, C.S.; Page, A.-L.; Klatser, P.R. A guide to aid the selection of diagnostic tests. Bull World Heal. Organ 2017. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.D.; Linder, V.; Sia, S.K. Commercialization of microfluidic point-of-care diagnostic devices. Lab Chip 2012, 12, 2118. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, H.; Gao, Y.; Huang, L.; Pan, H.; Du, M. Miniaturised electrochemical analyser for glucose determination based on Chitosan/GOD/Electroreduced graphene oxide sensor. Int. J. Electrochem. Sci. 2020, 15, 2458–2469. [Google Scholar] [CrossRef]
- Saygili, E.; Orakci, B.; Koprulu, M.; Demirhan, A.; Ilhan-Ayisigi, E.; Kilic, Y.; Yesil-Celiktas, O. Quantitative determination of H2O2 for detection of alanine aminotransferase using thin film electrodes. Anal. Biochem. 2020, 591. [Google Scholar] [CrossRef]
- Muralidharan, R.; Chandrashekhar, V.; Butler, D.; Ebrahimi, A. A Smartphone-Interfaced, Flexible Electrochemical Biosensor Based on Graphene Ink for Selective Detection of Dopamine. IEEE Sens. J. 2020, 20, 13204–13211. [Google Scholar] [CrossRef]
- Serafín, V.; Martínez-García, G.; Aznar-Poveda, J.; Lopez-Pastor, J.A.; Garcia-Sanchez, A.J.; Garcia-Haro, J.; Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Determination of progesterone in saliva using an electrochemical immunosensor and a COTS-based portable potentiostat. Anal. Chim. Acta 2019, 1049, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.F.D.; Norena, N.; Kaushik, A.; Bhansali, S. A low-cost miniaturized potentiostat for point-of-care diagnosis. Biosens. Bioelectron. 2014, 62, 249–254. [Google Scholar] [CrossRef]
- Nikolaev, K.G.; Kalmykov, E.V.; Shavronskaya, D.O.; Nikitina, A.A.; Stekolshchikova, A.A.; Kosareva, E.A.; Zenkin, A.A.; Pantiukhin, I.S.; Orlova, O.Y.; Skalny, A.V.; et al. ElectroSens Platform with a Polyelectrolyte-Based Carbon Fiber Sensor for Point-of-Care Analysis of Zn in Blood and Urine. ACS Omega 2020, 5, 18987–18994. [Google Scholar] [CrossRef]
- Giannetto, M.; Bianchi, V.; Gentili, S.; Fortunati, S.; De Munari, I.; Careri, M. An integrated IoT-Wi-Fi board for remote data acquisition and sharing from innovative immunosensors. Case of study: Diagnosis of celiac disease. Sensors Actuators B Chem. 2018, 273, 1395–1403. [Google Scholar] [CrossRef]
- Irving, P.; Cecil, R.; Yates, M.Z. MYSTAT: A compact potentiostat/galvanostat for general electrochemistry measurements. HardwareX 2021, 9, e00163. [Google Scholar] [CrossRef]
- Adams, S.D.; Doeven, E.H.; Quayle, K.; Kouzani, A.Z. MiniStat: Development and Evaluation of a Mini-Potentiostat for Electrochemical Measurements. IEEE Access 2019, 7, 31903–31912. [Google Scholar] [CrossRef]
- Shen, X.; Ju, F.; Li, G.; Ma, L. Smartphone-based electrochemical potentiostat detection system using pedot: Pss/chitosan/graphene modified screen-printed electrodes for dopamine detection. Sensors 2020, 20, 2781. [Google Scholar] [CrossRef]
- Ji, D.; Liu, L.; Li, S.; Chen, C.; Lu, Y.; Wu, J.; Liu, Q. Smartphone-based cyclic voltammetry system with graphene modified screen printed electrodes for glucose detection. Biosens. Bioelectron. 2017, 98, 449–456. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.; Li, L.; Mu, X.; Genov, R.; Mason, A.J. CMOS electrochemical instrumentation for biosensor microsystems: A review. Sensors 2017, 17, 74. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, A.; Corrigan, D.K.; Ward, A.C. Electrochemical detection of oxacillin resistance with SimpleStat: a low cost integrated potentiostat and sensor platform. Anal. Methods 2019, 11, 1958–1965. [Google Scholar] [CrossRef] [Green Version]
- Glasscott, M.W.; Verber, M.D.; Hall, J.R.; Pendergast, A.D.; McKinney, C.J.; Dick, J.E. SweepStat: A Build-It-Yourself, Two-Electrode Potentiostat for Macroelectrode and Ultramicroelectrode Studies. J. Chem. Educ. 2020, 97, 265–270. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Wu, G.; Cai, Z.; Oyama, M.; Chen, X. Advances in enzyme-free electrochemical sensors for hydrogen peroxide, glucose, and uric acid. Microchim. Acta 2014, 181, 689–705. [Google Scholar] [CrossRef]
- Lv, J.; Li, C.; Feng, S.; Chen, S.-M.; Ding, Y.; Chen, C.; Hao, Q.; Yang, T.-H.; Lei, W. A novel electrochemical sensor for uric acid detection based on PCN/MWCNT. Ionics 2019. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, M.-q.; Zou, H.-q.; Liu, J.-s.; Wang, D.-z.; Wang, J.; Wang, L.-d. ding Non-enzymatic electrochemical detection of uric acid with electrodeposited Nafion film. J. Electroanal. Chem. 2019, 841, 129–134. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, J.; Li, S.; Yan, B.; Xu, H.; Zhang, K.; Du, Y. The Enhanced Photo-Electrochemical Detection of Uric Acid on Au Nanoparticles Modified Glassy Carbon Electrode. Nanoscale Res. Lett. 2017, 12, 455. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Chao, J.; Zuo, X.; Su, S.; Liu, X.; Yuwen, L.; Fan, C.; Wang, L. Gold nanoparticle-decorated MoS2 nanosheets for simultaneous detection of ascorbic acid, dopamine and uric acid. RSC Adv. 2014, 4, 27625–27629. [Google Scholar] [CrossRef]
- Antuña-Jiménez, D.; González-García, M.B.; Hernández-Santos, D.; Fanjul-Bolado, P. Screen-printed electrodes modified with metal nanoparticles for small molecule sensing. Biosensors 2020, 10, 9. [Google Scholar] [CrossRef] [Green Version]
- Saldan, I.; Dobrovetska, O.; Sus, L.; Makota, O.; Pereviznyk, O.; Kuntyi, O.; Reshetnyak, O. Electrochemical synthesis and properties of gold nanomaterials. J. Solid State Electrochem. 2018, 22, 637–656. [Google Scholar] [CrossRef]
- Machado-López, M.M.; Faure, J.; Espinosa-Medina, M.A.; Espitia-Cabrera, M.I.; Contreras-García, M.E. Enhanced Corrosion Resistance in Artificial Saliva of Ti6Al4V with ZrO 2 Nanostructured Coating. J. Electrochem. Soc. 2015, 162, D3090–D3100. [Google Scholar] [CrossRef]
- Huang, D.; Cheng, Y.; Xu, H.; Zhang, H.; Sheng, L.; Xu, H.; Liu, Z.; Wu, H.; Fan, S. The determination of uric acid in human body fluid samples using glassy carbon electrode activated by a simple electrochemical method. J. Solid State Electrochem. 2015, 19, 435–443. [Google Scholar] [CrossRef]
- Rana, A.; Baig, N.; Saleh, T.A. Electrochemically pretreated carbon electrodes and their electroanalytical applications – A review. J. Electroanal. Chem. 2019, 833, 313–332. [Google Scholar] [CrossRef]
- González-Sánchez, M.I.; Gómez-Monedero, B.; Agrisuelas, J.; Iniesta, J.; Valero, E. Highly activated screen-printed carbon electrodes by electrochemical treatment with hydrogen peroxide. Electrochem. commun. 2018, 91, 36–40. [Google Scholar] [CrossRef]
- Liao, C.; Mak, C.; Zhang, M.; Chan, H.L.W.; Yan, F. Flexible Organic Electrochemical Transistors for Highly Selective Enzyme Biosensors and Used for Saliva Testing. Adv. Mater. 2015, 27, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Sha, R.; Vishnu, N.; Badhulika, S. MoS2 based ultra-low-cost, flexible, non-enzymatic and non-invasive electrochemical sensor for highly selective detection of Uric acid in human urine samples. Sens. Actuators B Chem. 2019, 279, 53–60. [Google Scholar] [CrossRef]
- Sha, R.; Vishnu, N.; Badhulika, S. FeS2 Grown Pencil Graphite as an In-expensive and Non-enzymatic Sensor for Sensitive Detection of Uric Acid in Non-invasive Samples. Electroanalysis 2019, 31, 2397–2403. [Google Scholar] [CrossRef]







| System | Response Time [s] | Linear Range [μM] | Sensitivity * [μA/μM] | R2 | LOD ** [μM] | RSD [n = 6] |
|---|---|---|---|---|---|---|
| 910 PSTAT mini-SPE | 3.35 | 20–200 | 0.014 | 0.999 | 18.63 | 10.53% |
| 910 PSTAT mini SPE-AuNps | 4.05 | 20–200 | 0.02 | 0.999 | 12.47 | 18.70% |
| AMP3291-SPE | 4.1 | 20–200 | 0.017 | 0.996 | 16.39 | 12.94% |
| AMP3291-SPE-AuNps | 3.5 | 20–200 | 0.022 | 0.993 | 11.91 | 1.53 % |
| System Model | Unit Price (USD) | Manufactured by |
|---|---|---|
| 910 PSTAT mini | 4904 | Metrohm, Netherlands |
| C-SENSIT-SM | 930.63 | PalmSens, Netherlands |
| AMP3291 | 76.39 | CINVESTAV, Mexico |
| System | Linear Range [μM] | Sensitivity [μA/μM] | LOD [μM] | Equipment | Technique | Sample | Ref. |
|---|---|---|---|---|---|---|---|
| SPE/PB/PPD/Uox | 0–1000 | 1.08 | -- | Custom | AMP | saliva | [7] |
| GCE/PCN/MWCNT | 0.2–20 | 0.6638 | 0.139 | CHI660D | DPV | serum | [27] |
| SPCEs/Nafion | 62.5–5000 | 9.366 | 20.8 | Reference 600+ | DPV | PBS | [28] |
| GCE/AuNps | 2.8–57.5 | 0.121 | 2.8 | CHI760D | DPV | PBS | [29] |
| GCE/AuNps@MoS2 | 50–4000 | 0.1611 | 10 | PGSTAT 32 | DPV | PBS | [30] |
| Pt/Graphene–Nafion/PANI/Uox-GO | 0.01–500 | -- | 0.01 | PARSTAT 2273 | AMP | saliva | [37] |
| flexible sensor/MoS2 | 10–400 | 98.3 | 1.16 | CHI660E | AMP | urine | [38] |
| PGE/FeS2 | 10–725 | 370 | 6.7 | CHI660E | DPV | urine | [39] |
| SPE-AuNps | 20–200 | 0.034 | 14.64 | AMP3291 | AMP | artificial saliva | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piedras, J.; Dominguez, R.B.; Gutiérrez, J.M. Determination of Uric Acid in Artificial Saliva with Compact AMP3291 Reader and Au Nanoparticles Modified Electrode. Chemosensors 2021, 9, 73. https://doi.org/10.3390/chemosensors9040073
Piedras J, Dominguez RB, Gutiérrez JM. Determination of Uric Acid in Artificial Saliva with Compact AMP3291 Reader and Au Nanoparticles Modified Electrode. Chemosensors. 2021; 9(4):73. https://doi.org/10.3390/chemosensors9040073
Chicago/Turabian StylePiedras, Jessica, Rocio B. Dominguez, and Juan Manuel Gutiérrez. 2021. "Determination of Uric Acid in Artificial Saliva with Compact AMP3291 Reader and Au Nanoparticles Modified Electrode" Chemosensors 9, no. 4: 73. https://doi.org/10.3390/chemosensors9040073
APA StylePiedras, J., Dominguez, R. B., & Gutiérrez, J. M. (2021). Determination of Uric Acid in Artificial Saliva with Compact AMP3291 Reader and Au Nanoparticles Modified Electrode. Chemosensors, 9(4), 73. https://doi.org/10.3390/chemosensors9040073