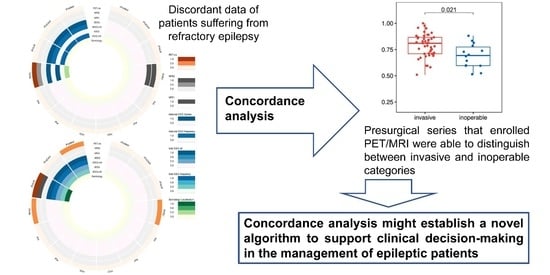
PET/MRI in the Presurgical Evaluation of Patients with Epilepsy: A Concordance Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Patient Preparation
2.3. PET/MRI Acquisition
2.4. Image Processing
2.5. Clinical Data
2.6. Statistical Comparison of Electroclinical and Image Processing Data
2.7. Concordance of the Clinical Data
3. Results
3.1. Quantitative PET and MRI Analysis
3.2. Concordance Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Collaborators, G.B.D.E. Global, regional, and national burden of epilepsy, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 357–375. [Google Scholar] [CrossRef] [Green Version]
- Duncan, J.S.; Winston, G.P.; Koepp, M.J.; Ourselin, S. Brain imaging in the assessment for epilepsy surgery. Lancet Neurol. 2016, 15, 420–433. [Google Scholar] [CrossRef]
- Wang, G.-B.; Long, W.; Li, X.-D.; Xu, G.-Y.; Lu, J.-X. Dynamic Contrast-Enhanced Magnetic Resonance Imaging (DCE-MRI) Combined with Positron Emission Tomography-Computed Tomography (PET-CT) and Video-Electroencephalography (VEEG) Have Excellent Diagnostic Value in Preoperative Localization of Epileptic Foci in Children with Epilepsy. Med. Sci. Monit. 2017, 23, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kogias, E.; Klingler, J.-H.; Urbach, H.; Scheiwe, C.; Schmeiser, B.; Doostkam, S.; Zentner, J.; Altenmüller, D.-M. 3 Tesla MRI-negative focal epilepsies: Presurgical evaluation, postoperative outcome and predictive factors. Clin. Neurol. Neurosurg. 2017, 163, 116–120. [Google Scholar] [CrossRef]
- Ahmed, R.; Rubinger, L.; Go, C.; Drake, J.M.; Rutka, J.T.; Snead, O.C.; Widjaja, E. Utility of additional dedicated high-resolution 3T MRI in children with medically refractory focal epilepsy. Epilepsy Res. 2018, 143, 113–119. [Google Scholar] [CrossRef]
- McGrath, H.; Mandel, M.; Sandhu, M.R.S.; Lamsam, L.; Adenu-Mensah, N.; Farooque, P.; Spencer, D.D.; Damisah, E.C. Optimizing the surgical management of MRI-negative epilepsy in the neuromodulation era. Epilepsia Open 2022, 7, 151–159. [Google Scholar] [CrossRef]
- Engel, J., Jr. Surgery for Seizures. N. Engl. J. Med. 1996, 334, 647–653. [Google Scholar] [CrossRef]
- Wiebe, S.; Blume, W.T.; Girvin, J.P.; Eliasziw, M.; Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group. A Randomized, Controlled Trial of Surgery for Temporal-Lobe Epilepsy. N. Engl. J. Med. 2001, 345, 311–318. [Google Scholar] [CrossRef]
- Téllez-Zenteno, J.F.; Ronquillo, L.H.; Moien-Afshari, F.; Wiebe, S. Surgical outcomes in lesional and non-lesional epilepsy: A systematic review and meta-analysis. Epilepsy Res. 2010, 89, 310–318. [Google Scholar] [CrossRef]
- Taussig, D.; Montavont, A.; Isnard, J. Invasive EEG explorations. Neurophysiol. Clin. 2015, 45, 113–119. [Google Scholar] [CrossRef]
- Desarnaud, S.; Mellerio, C.; Semah, F.; Laurent, A.; Landre, E.; Devaux, B.; Chiron, C.; Lebon, V.; Chassoux, F. 18F-FDG PET in drug-resistant epilepsy due to focal cortical dysplasia type 2: Additional value of electroclinical data and coregistration with MRI. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1449–1460. [Google Scholar] [CrossRef] [PubMed]
- Rathore, C.; Dickson, J.C.; Teotónio, R.; Ell, P.; Duncan, J.S. The utility of 18F-fluorodeoxyglucose PET (FDG PET) in epilepsy surgery. Epilepsy Res. 2014, 108, 1306–1314. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Hu, J.; Pan, L.; Shi, J.; Qiu, C.; Lang, L.; Gu, X.; Guo, J. Surgical Treatment of MRI-Negative Temporal Lobe Epilepsy Based on PET: A Retrospective Cohort Study. Ster. Funct. Neurosurg. 2014, 92, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Chapman, K.; Wyllie, E.; Najm, I.; Ruggieri, P.; Bingaman, W.; Lüders, J.; Kotagal, P.; Lachhwani, D.; Dinner, D.; O Lüders, H. Seizure outcome after epilepsy surgery in patients with normal preoperative MRI. J. Neurol. Neurosurg. Psychiatry 2005, 76, 710–713. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.K.; Lee, D.S.; Lee, S.K.; Chung, C.K.; Chung, J.K.; Lee, M.C. (18)F-FDG PET in localization of frontal lobe epilepsy: Comparison of visual and SPM analysis. J. Nucl. Med. 2002, 43, 1167–1174. [Google Scholar]
- Sebastiano, D.R.; Tassi, L.; Duran, D.; Visani, E.; Gozzo, F.; Cardinale, F.; Nobili, L.; Del Sole, A.; Rubino, A.; Dotta, S.; et al. Identifying the epileptogenic zone by four non-invasive imaging techniques versus stereo-EEG in MRI-negative pre-surgery epilepsy patients. Clin. Neurophysiol. 2020, 131, 1815–1823. [Google Scholar] [CrossRef]
- Ding, Y.; Zhu, Y.; Jiang, B.; Zhou, Y.; Jin, B.; Hou, H.; Wu, S.; Zhu, J.; Wang, Z.I.; Wong, C.H.; et al. 18F-FDG PET and high-resolution MRI co-registration for pre-surgical evaluation of patients with conventional MRI-negative refractory extra-temporal lobe epilepsy. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1567–1572. [Google Scholar] [CrossRef]
- Fernández, S.; Donaire, A.; Serès, E.; Setoain, X.; Bargalló, N.; Falcón, C.; Sanmartí, F.; Maestro, I.; Rumià, J.; Pintor, L.; et al. PET/MRI and PET/MRI/SISCOM coregistration in the presurgical evaluation of refractory focal epilepsy. Epilepsy Res. 2015, 111, 1–9. [Google Scholar] [CrossRef]
- Paldino, M.J.; Yang, E.; Jones, J.Y.; Mahmood, N.; Sher, A.; Zhang, W.; Hayatghaibi, S.; Krishnamurthy, R.; Seghers, V. Comparison of the diagnostic accuracy of PET/MRI to PET/CT-acquired FDG brain exams for seizure focus detection: A prospective study. Pediatr. Radiol. 2017, 47, 1500–1507. [Google Scholar] [CrossRef]
- Grouiller, F.; Delattre, B.M.A.; Pittau, F.; Heinzer, S.; Lazeyras, F.; Spinelli, L.; Iannotti, G.R.; Seeck, M.; Ratib, O.; Vargas, M.I.; et al. All-in-one interictal presurgical imaging in patients with epilepsy: Single-session EEG/PET/(f)MRI. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1133–1143. [Google Scholar] [CrossRef]
- Oldan, J.D.; Shin, H.W.; Khandani, A.H.; Zamora, C.; Benefield, T.; Jewells, V. Subsequent experience in hybrid PET-MRI for evaluation of refractory focal onset epilepsy. Seizure 2018, 61, 128–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, H.W.; Jewells, V.; Sheikh, A.; Zhang, J.; Zhu, H.; An, H.; Gao, W.; Shen, D.; Hadar, E.; Lin, W. Initial experience in hybrid PET-MRI for evaluation of refractory focal onset epilepsy. Seizure 2015, 31, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Shang, K.; Wang, J.; Fan, X.; Cui, B.; Ma, J.; Yang, H.; Zhou, Y.; Zhao, G.; Lu, J. Clinical Value of Hybrid TOF-PET/MR Imaging–Based Multiparametric Imaging in Localizing Seizure Focus in Patients with MRI-Negative Temporal Lobe Epilepsy. AJNR Am. J. Neuroradiol. 2018, 39, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Werner, P.; Barthel, H.; Drzezga, A.; Sabri, O. Current status and future role of brain PET/MRI in clinical and research settings. Eur. J. Pediatr. 2015, 42, 512–526. [Google Scholar] [CrossRef]
- Jadvar, H.; Colletti, P.M. Competitive advantage of PET/MRI. Eur. J. Radiol. 2014, 83, 84–94. [Google Scholar] [CrossRef] [Green Version]
- Traub-Weidinger, T.; Muzik, O.; Sundar, L.K.S.; Aull-Watschinger, S.; Beyer, T.; Hacker, M.; Hahn, A.; Kasprian, G.; Klebermass, E.-M.; Lanzenberger, R.; et al. Utility of Absolute Quantification in Non-lesional Extratemporal Lobe Epilepsy Using FDG PET/MR Imaging. Front. Neurol. 2020, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, W.; Huang, P.; Lin, X.; Huang, X.; Meng, H.; Wang, J.; Hu, K.; Li, J.; Lin, M.; et al. Utility of hybrid PET/MRI multiparametric imaging in navigating SEEG placement in refractory epilepsy. Seizure 2020, 81, 295–303. [Google Scholar] [CrossRef]
- Guo, K.; Wang, J.; Cui, B.; Wang, Y.; Hou, Y.; Zhao, G.; Lu, J. [18F]FDG PET/MRI and magnetoencephalography may improve presurgical localization of temporal lobe epilepsy. Eur. Radiol. 2021, 1–11. [Google Scholar] [CrossRef]
- Varrone, A.; Asenbaum, S.; Borght, T.V.; Booij, J.; Nobili, F.; Någren, K.; Darcourt, J.; Kapucu, Ö.L.; Tatsch, K.; Bartenstein, P.; et al. EANM procedure guidelines for PET brain imaging using [18F]FDG, version 2. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 2103–2110. [Google Scholar] [CrossRef]
- FreeSurfer Software Suite. Available online: http://surfer.nmr.mgh.harvard.edu (accessed on 21 March 2020).
- Dale, A.M.; Fischl, B.; Sereno, M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage 1999, 9, 179–194. [Google Scholar] [CrossRef]
- Fischl, B.; Sereno, M.I.; Dale, A.M. Cortical Surface-Based Analysis. II: Inflation, Flattening, and a Surface-Based Coordinate System. NeuroImage 1999, 9, 195–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkinson, M.; Beckmann, C.F.; Behrens, T.E.; Woolrich, M.W.; Smith, S.M. FSL. NeuroImage 2012, 62, 782–790. [Google Scholar] [CrossRef] [Green Version]
- Avants, B.B.; Tustison, N.J.; Song, G.; Cook, P.A.; Klein, A.; Gee, J.C. A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage 2011, 54, 2033–2044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Della Rosa, P.A.; Cerami, C.; Gallivanone, F.; Prestia, A.; Caroli, A.; Castiglioni, I.; Gilardi, M.C.; Frisoni, G.; Friston, K.; Ashburner, J.; et al. A Standardized [18F]-FDG-PET Template for Spatial Normalization in Statistical Parametric Mapping of Dementia. Neuroinformatics 2014, 12, 575–593. [Google Scholar] [CrossRef] [PubMed]
- Desikan, R.S.; Ségonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.T.; et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 2006, 31, 968–980. [Google Scholar] [CrossRef]
- Ashburner, J. SPM: A history. NeuroImage 2012, 62, 791–800. [Google Scholar] [CrossRef] [Green Version]
- House, P.M.; Lanz, M.; Holst, B.; Martens, T.; Stodieck, S.; Huppertz, H.-J. Comparison of morphometric analysis based on T1- and T2-weighted MRI data for visualization of focal cortical dysplasia. Epilepsy Res. 2013, 106, 403–409. [Google Scholar] [CrossRef]
- Benjamini, Y.; Drai, D.; Elmer, G.; Kafkafi, N.; Golani, I. Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 2001, 125, 279–284. [Google Scholar] [CrossRef] [Green Version]
- Gwet, K.L. Computing inter-rater reliability and its variance in the presence of high agreement. Br. J. Math. Stat. Psychol. 2008, 61, 29–48. [Google Scholar] [CrossRef] [Green Version]
- Wongpakaran, N.; Wongpakaran, T.; Wedding, D.; Gwet, K.L. A comparison of Cohen’s Kappa and Gwet’s AC1 when calculating inter-rater reliability coefficients: A study conducted with personality disorder samples. BMC Med. Res. Methodol. 2013, 13, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salamon, N.; Kung, J.; Shaw, S.J.; Koo, J.; Koh, S.; Wu, J.Y.; Lerner, J.T.; Sankar, R.; Shields, W.D.; Engel, J., Jr.; et al. FDG-PET/MRI coregistration improves detection of cortical dysplasia in patients with epilepsy. Neurology 2008, 71, 1594–1601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, K.; Ren, Z.; Yang, D.; Wang, X.; Yu, T.; Ni, D.; Qiao, L.; Xu, C.; Gao, R.; Lin, Y.; et al. Voxel-based morphometric MRI post-processing and PET/MRI co-registration reveal subtle abnormalities in cingulate epilepsy. Epilepsy Res. 2021, 171, 106568. [Google Scholar] [CrossRef]
- Kikuchi, K.; Togao, O.; Yamashita, K.; Momosaka, D.; Nakayama, T.; Kitamura, Y.; Kikuchi, Y.; Baba, S.; Sagiyama, K.; Ishimatsu, K.; et al. Diagnostic accuracy for the epileptogenic zone detection in focal epilepsy could be higher in FDG-PET/MRI than in FDG-PET/CT. Eur. Radiol. 2021, 31, 2915–2922. [Google Scholar] [CrossRef] [PubMed]
- Coan, A.C.; Chaudhary, U.J.; Grouiller, F.; Campos, B.M.; Perani, S.; De Ciantis, A.; Vulliemoz, S.; Diehl, B.; Beltramini, G.C.; Carmichael, D.W.; et al. EEG-fMRI in the presurgical evaluation of temporal lobe epilepsy. J. Neurol. Neurosurg. Psychiatry 2016, 87, 642–649. [Google Scholar] [CrossRef] [Green Version]
- Meletti, S.; Vignoli, A.; Benuzzi, F.; Avanzini, P.; Ruggieri, A.; Pugnaghi, M.; Nichelli, P.; Canevini, M.P. Ictal involvement of the nigrostriatal system in subtle seizures of ring chromosome 20 epilepsy. Epilepsia 2012, 53, e156–e160. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, U.J.; Carmichael, D.W.; Rodionov, R.; Thornton, R.C.; Bartlett, P.; Vulliemoz, S.; Micallef, C.; McEvoy, A.W.; Diehl, B.; Walker, M.C.; et al. Mapping preictal and ictal haemodynamic networks using video-electroencephalography and functional imaging. Brain J. Neurol. 2012, 135, 3645–3663. [Google Scholar] [CrossRef] [Green Version]
- Vaudano, A.E.; Carmichael, D.W.; Salek-Haddadi, A.; Rampp, S.; Stefan, H.; Lemieux, L.; Koepp, M.J. Networks involved in seizure initiation: A reading epilepsy case studied with EEG-fMRI and MEG. Neurology 2012, 79, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Maesawa, S.; Bagarinao, E.; Okai, Y.; Nakatsubo, D.; Yamamoto, H.; Kidokoro, H.; Usui, N.; Natsume, J.; Hoshiyama, M.; et al. Subsecond EEG-fMRI analysis for presurgical evaluation in focal epilepsy. J. Neurosurg. 2020, 134, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Chatzistefanidis, D.; Huang, D.; Dümpelmann, M.; Jacobs, J.; Schulze-Bonhage, A.; LeVan, P. Topography-Related EEG-fMRI in Surgically Confirmed Epileptic Foci: A Comparison to Spike-Related EEG-fMRI in Clinical Practice. Brain Topogr. 2021, 34, 373–383. [Google Scholar] [CrossRef] [PubMed]



| MR Sequence | TR (ms) | TE (ms) | FA | Slice Thickness | Imaging Matrix | Voxel Size | TA |
|---|---|---|---|---|---|---|---|
| Axial T2 UTE (MRAC) | 11.94 | TE1:0.07, TE:2:2.46 | 10 | 1.6 × 1.6 × 1.6 mm | 1:38 | ||
| Sagittal MPRAGE | 2300 | 2.98 | 9 | 1.2 mm | 240 × 256 | 1.0 × 1.0 × 1.2 | 9:14 |
| Axial T2 TSE | 6000 | 106 | 150 | 4 mm | 358 × 448 | 0.5 × 0.5 × 4 mm | 4:08 |
| Coronal T2 TSE HR | 6770 | 89 | 150 | 3 mm | 307 × 384 | 0.5 × 0.5 × 3 mm | 3:04 |
| Coronal FLAIR HR | 9000 | 128 | 120 | 3 mm | 192 × 256 | 0.9 × 0.9 × 3 mm | 5:44 |
| Axial DTI | 3600 | 95 | - | 4 mm | 128 × 128 | 1.7 × 1.7 × 4 mm | 3:59 |
| Axial T2 HEMO | 620 | 19.9 | 20 | 4 mm | 205 × 256 | 0.4 × 0.4 × 4 mm | 2:09 |
| SagittalT2 SPC 3D | 3200 | 409 | 120 | 1.0 mm | 261 × 256 | 0.5 × 0.5 × 1 mm | 4:43 |
| Sagittal T2 FLAIR 3D | 5000 | 395 | 120 | 1.0 mm | 261 × 256 | 0.5 × 0.5 × 1 mm | 5:52 |
| Resting state fMRI | 2580 | 30 | 90 | 3 mm | 74 × 74 | 3 × 3 × 3 mm | 10:54 |
| GRE Field Mapping | 400 | 4.92/7.38 | 60 | 3 mm | 64 × 64 | 3.4 × 3.4 × 3 | 0:54 |
| Axial ASL | 3060.4 | 17 | 90 | 5 mm | 64 × 64 | 3.6 × 3.6 × 5 mm | 5:14 |
| Image Processing Data | Description of PET Data | Source |
|---|---|---|
| voi.min | minimal [18F]-FDG uptake value | the globally normalized and spatially standardized [18F]-FDG PET image |
| voi.max | maximal [18F]-FDG uptake value | |
| voi.mean | average of mean values according to Harvard-Oxford Cortical and Subcortical atlases (HOVOI) | |
| voi.median | median of HOVOI medians values | |
| voi.sd | maximal HOVOI based standard deviation | |
| ai.min | minimum of the asymmetry of minimal HOVOI’s [18F]-FDG values | |
| ai.max | maximum of the asymmetry of maximal HOVOI’s [18F]-FDG values | |
| ai.mean | the maximum value of the asymmetry of HOVOI’s [18F]-FDG value means | |
| ai.median | the maximum value of the asymmetry of HOVOI’s [18F]-FDG value medians | |
| ai.sd | the maximum value of the asymmetry of standard deviations of HOVOI’s [18F]-FDG values | |
| spm.max | highest Student-t value in the HOVOI region | SPM generated Student-t map |
| spm.vol | the relative volume of hypometabolic area (thresholded by uncorrected p < 0.001) in the HOVOI region | |
| map.max | maximum z-value in the HOVOI region | Combined z-score image produced by MAP07 |
| map.mean | maximum value of the HOVOI’s mean z-values in the HOVOI’s region |
| Diagnostic Parameters | Description | Value |
|---|---|---|
| Semiology | Possible localization considered by semiology in the given EPILOBE region. | 0.0: certainly not 0.3: slightly possible 0.6: possible 1.0: the most likely |
| iiEEG.mfl | Occurrence of interictal EEG activity in the given EPILOBE region (most frequent localization). | 0: no 1: yes |
| iiEEG | Occurrence of interictal EEG activity in the given EPILOBE region. | 0: no 1: yes |
| iEEG.mfl | Possible ictal EEG activity in the given EPILOBE region (most frequent localization). | 0.0: certainly not 0.3: slightly possible 0.6: possible 1.0: the most likely |
| iEEG | Possible ictal EEG activity in the given EPILOBE region. | 0.0: certainly not 0.3: slightly possible 0.6: possible 1.0: the most likely |
| MRI1 | Specific epileptogenic MRI lesions found by radiologist experts (before this study). | 0: no 1: yes |
| MRI2 | Possible specific epileptogenic MRI lesions found by radiologist experts (during this study). | 0.0: certainly not 0.5: possible 1.0: exist |
| PETvis | Visual PET findings detected by nuclear medicine experts (during this study). | 0: no abnormal pattern 0.5: possible 1.0: the most likely |
| Source | Image Processing Data | EPILOBE Region | p-Value | FDR Adjusted p-Value | Meaning in the Detected Lesion |
|---|---|---|---|---|---|
| iiEEG | ai.max | lTemp | 0.0039 | 0.0467 | lower asymmetry index |
| map.max | rTemp | 0.0014 | 0.0172 | higher z-score | |
| voi.mean | rFroLat | 0.0020 | 0.0245 | lower [18F]-FDG | |
| voi.median | rFroLat | <0.0001 | 0.0086 | ||
| voi.sd | rFroLat | <0.0001 | 0.0025 | ||
| iiEEG.mfl | spm.vol | rTemp | 0.0040 | 0.0396 | larger SPM hypometabolism area |
| MRI2 | ai.median | rTemp | 0.0013 | 0.0179 | lower asymmetry index |
| ai.mean | rTemp | 0.0016 | 0.0225 | ||
| PET.vis | ai.max | lFroMed | 0.0065 | 0.0276 | |
| lOcc | 0.0166 | 0.0465 | |||
| lTemp | 0.0012 | 0.0081 | |||
| rIns | 0.0076 | 0.0267 | |||
| rTemp | 0.0004 | 0.0057 | |||
| ai.median | lTemp | <0.0001 | 0.0004 | ||
| rFroLat | 0.0041 | 0.0145 | |||
| rIns | 0.0012 | 0.0083 | |||
| rTemp | 0.0037 | 0.0145 | |||
| ai.mean | lFroLat | 0.0091 | 0.0254 | ||
| lTemp | 0.0002 | 0.0031 | |||
| rFroLat | 0.0067 | 0.0234 | |||
| rIns | 0.0013 | 0.0060 | |||
| rTemp | 0.0006 | 0.0044 | |||
| ai.sd | lTemp | 0.0005 | 0.0068 | ||
| rFroLat | 0.0055 | 0.0382 | |||
| spm.max | lTemp | <0.0001 | 0.0012 | higher Student-t value | |
| spm.vol | lTemp | <0.0001 | 0.0016 | larger SPM hypometabolism area | |
| rTemp | <0.0001 | 0.0019 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borbély, K.; Emri, M.; Kenessey, I.; Tóth, M.; Singer, J.; Barsi, P.; Vajda, Z.; Pál, E.; Tóth, Z.; Beyer, T.; et al. PET/MRI in the Presurgical Evaluation of Patients with Epilepsy: A Concordance Analysis. Biomedicines 2022, 10, 949. https://doi.org/10.3390/biomedicines10050949
Borbély K, Emri M, Kenessey I, Tóth M, Singer J, Barsi P, Vajda Z, Pál E, Tóth Z, Beyer T, et al. PET/MRI in the Presurgical Evaluation of Patients with Epilepsy: A Concordance Analysis. Biomedicines. 2022; 10(5):949. https://doi.org/10.3390/biomedicines10050949
Chicago/Turabian StyleBorbély, Katalin, Miklós Emri, István Kenessey, Márton Tóth, Júlia Singer, Péter Barsi, Zsolt Vajda, Endre Pál, Zoltán Tóth, Thomas Beyer, and et al. 2022. "PET/MRI in the Presurgical Evaluation of Patients with Epilepsy: A Concordance Analysis" Biomedicines 10, no. 5: 949. https://doi.org/10.3390/biomedicines10050949
APA StyleBorbély, K., Emri, M., Kenessey, I., Tóth, M., Singer, J., Barsi, P., Vajda, Z., Pál, E., Tóth, Z., Beyer, T., Dóczi, T., Bajzik, G., Fabó, D., Janszky, J., Jordán, Z., Fajtai, D., Kelemen, A., Juhos, V., Wintermark, M., ... Repa, I. (2022). PET/MRI in the Presurgical Evaluation of Patients with Epilepsy: A Concordance Analysis. Biomedicines, 10(5), 949. https://doi.org/10.3390/biomedicines10050949