Clinical Value of Inflammatory and Neurotrophic Biomarkers in Bipolar Disorder: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Quantitative Analysis
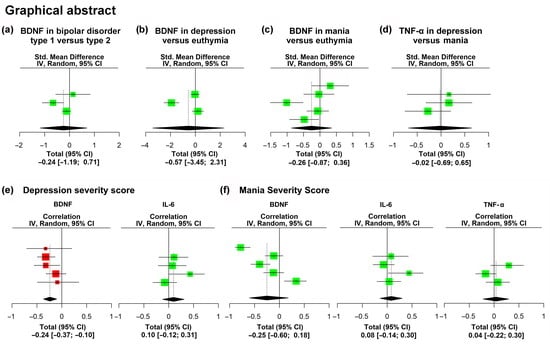
3. Results
3.1. Stratifying Value of Biomarkers: Analysis of Clinical Features of BD
3.1.1. Subtypes of the Disorder
3.1.2. Phases of the Disorder
3.1.3. Severity of Mood Symptoms
3.1.4. Factors Related to Clinical Staging of the Disorder
3.1.5. Number of Previous Episodes, Duration of Episodes and Polarity
3.1.6. Cognition and Functionality
3.1.7. Other Clinical Parameters and Comorbidity
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Merikangas, K.R.; Jin, R.; He, J.-P.; Kessler, R.C.; Lee, S.; Sampson, N.A.; Viana, M.C.; Andrade, L.H.; Hu, C.; Karam, E.G.; et al. Prevalence and Correlates of Bipolar Spectrum Disorder in the World Mental Health Survey Initiative. Arch. Gen. Psychiatry 2011, 68, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.; Petukhova, M.; Vilagut, G.; Chatterji, S.; Heeringa, S.; Üstün, T.B.; Alhamzawi, A.O.; Viana, M.C.; Angermeyer, M.; Bromet, E.; et al. Days out of Role Due to Common Physical and Mental Conditions: Results from the WHO World Mental Health Surveys. Mol. Psychiatry 2011, 16, 1234–1246. [Google Scholar] [CrossRef] [PubMed]
- Merikangas, K.R.; Akiskal, H.S.; Angst, J.; Greenberg, P.E.; Hirschfeld, R.M.A.; Petukhova, M.; Kessler, R.C. Lifetime and 12-Month Prevalence of Bipolar Spectrum Disorder in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 2007, 64, 543–552. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, R.S.; Konarski, J.Z.; Yatham, L.N. Comorbidity in Bipolar Disorder: A Framework for Rational Treatment Selection. Hum. Psychopharmacol. 2004, 19, 369–386. [Google Scholar] [CrossRef]
- Staudt Hansen, P.; Frahm Laursen, M.; Grøntved, S.; Puggard Vogt Straszek, S.; Licht, R.W.; Nielsen, R.E. Increasing Mortality Gap for Patients Diagnosed with Bipolar Disorder-A Nationwide Study with 20 Years of Follow-Up. Bipolar Disord. 2019, 21, 270–275. [Google Scholar] [CrossRef]
- Osby, U.; Brandt, L.; Correia, N.; Ekbom, A.; Sparén, P. Excess Mortality in Bipolar and Unipolar Disorder in Sweden. Arch. Gen. Psychiatry 2001, 58, 844–850. [Google Scholar] [CrossRef] [Green Version]
- Gonda, X.; Pompili, M.; Serafini, G.; Montebovi, F.; Campi, S.; Dome, P.; Duleba, T.; Girardi, P.; Rihmer, Z. Suicidal Behavior in Bipolar Disorder: Epidemiology, Characteristics and Major Risk Factors. J. Affect. Disord. 2012, 143, 16–26. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Berk, M.; Brietzke, E.; Goldstein, B.I.; López-Jaramillo, C.; Kessing, L.V.; Malhi, G.S.; Nierenberg, A.A.; Rosenblat, J.D.; Majeed, A.; et al. Bipolar Disorders. Lancet Lond. Engl. 2020, 396, 1841–1856. [Google Scholar] [CrossRef]
- Pompili, M.; Gonda, X.; Serafini, G.; Innamorati, M.; Sher, L.; Amore, M.; Rihmer, Z.; Girardi, P. Epidemiology of Suicide in Bipolar Disorders: A Systematic Review of the Literature. Bipolar Disord. 2013, 15, 457–490. [Google Scholar] [CrossRef]
- Perlis, R.H.; Dennehy, E.B.; Miklowitz, D.J.; Delbello, M.P.; Ostacher, M.; Calabrese, J.R.; Ametrano, R.M.; Wisniewski, S.R.; Bowden, C.L.; Thase, M.E.; et al. Retrospective Age at Onset of Bipolar Disorder and Outcome during Two-Year Follow-up: Results from the STEP-BD Study. Bipolar Disord. 2009, 11, 391–400. [Google Scholar] [CrossRef]
- Nivoli, A.M.A.; Pacchiarotti, I.; Rosa, A.R.; Popovic, D.; Murru, A.; Valenti, M.; Bonnin, C.M.; Grande, I.; Sanchez-Moreno, J.; Vieta, E.; et al. Gender Differences in a Cohort Study of 604 Bipolar Patients: The Role of Predominant Polarity. J. Affect. Disord. 2011, 133, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.L.; Kupfer, D.J. Bipolar Disorder Diagnosis: Challenges and Future Directions. Lancet Lond. Engl. 2013, 381, 1663–1671. [Google Scholar] [CrossRef] [Green Version]
- Bortolato, B.; Miskowiak, K.W.; Köhler, C.A.; Vieta, E.; Carvalho, A.F. Cognitive Dysfunction in Bipolar Disorder and Schizophrenia: A Systematic Review of Meta-Analyses. Neuropsychiatr. Dis. Treat. 2015, 11, 3111–3125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Aran, A.; Vieta, E.; Torrent, C.; Sanchez-Moreno, J.; Goikolea, J.M.; Salamero, M.; Malhi, G.S.; Gonzalez-Pinto, A.; Daban, C.; Alvarez-Grandi, S.; et al. Functional Outcome in Bipolar Disorder: The Role of Clinical and Cognitive Factors. Bipolar Disord. 2007, 9, 103–113. [Google Scholar] [CrossRef]
- Martinez-Aran, A.; Vieta, E. Cognition as a Target in Schizophrenia, Bipolar Disorder and Depression. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2015, 25, 151–157. [Google Scholar] [CrossRef]
- Torres, I.J.; Boudreau, V.G.; Yatham, L.N. Neuropsychological Functioning in Euthymic Bipolar Disorder: A Meta-Analysis. Acta Psychiatr. Scand. Suppl. 2007, 116, 17–26. [Google Scholar] [CrossRef]
- Torres, I.J.; DeFreitas, V.G.; DeFreitas, C.M.; Kauer-Sant’Anna, M.; Bond, D.J.; Honer, W.G.; Lam, R.W.; Yatham, L.N. Neurocognitive Functioning in Patients with Bipolar I Disorder Recently Recovered from a First Manic Episode. J. Clin. Psychiatry 2010, 71, 1234–1242. [Google Scholar] [CrossRef]
- Lee, R.S.C.; Hermens, D.F.; Scott, J.; Redoblado-Hodge, M.A.; Naismith, S.L.; Lagopoulos, J.; Griffiths, K.R.; Porter, M.A.; Hickie, I.B. A Meta-Analysis of Neuropsychological Functioning in First-Episode Bipolar Disorders. J. Psychiatr. Res. 2014, 57, 1–11. [Google Scholar] [CrossRef]
- Martino, D.J.; Samamé, C.; Ibañez, A.; Strejilevich, S.A. Neurocognitive Functioning in the Premorbid Stage and in the First Episode of Bipolar Disorder: A Systematic Review. Psychiatry Res. 2015, 226, 23–30. [Google Scholar] [CrossRef]
- Grande, I.; Goikolea, J.M.; de Dios, C.; González-Pinto, A.; Montes, J.M.; Saiz-Ruiz, J.; Prieto, E.; Vieta, E.; PREBIS group. Occupational Disability in Bipolar Disorder: Analysis of Predictors of Being on Severe Disablement Benefit (PREBIS Study Data). Acta Psychiatr. Scand. 2013, 127, 403–411. [Google Scholar] [CrossRef]
- Hirschfeld, R.M.A.; Lewis, L.; Vornik, L.A. Perceptions and Impact of Bipolar Disorder: How Far Have We Really Come? Results of the National Depressive and Manic-Depressive Association 2000 Survey of Individuals with Bipolar Disorder. J. Clin. Psychiatry 2003, 64, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Solé, B.; Jiménez, E.; Torrent, C.; Reinares, M.; Bonnin, C.D.M.; Torres, I.; Varo, C.; Grande, I.; Valls, E.; Salagre, E.; et al. Cognitive Impairment in Bipolar Disorder: Treatment and Prevention Strategies. Int. J. Neuropsychopharmacol. 2017, 20, 670–680. [Google Scholar] [CrossRef]
- Lima, I.M.M.; Peckham, A.D.; Johnson, S.L. Cognitive Deficits in Bipolar Disorders: Implications for Emotion. Clin. Psychol. Rev. 2018, 59, 126–136. [Google Scholar] [CrossRef]
- Battaglia, S.; Serio, G.; Scarpazza, C.; D’Ausilio, A.; Borgomaneri, S. Frozen in (e)Motion: How Reactive Motor Inhibition Is Influenced by the Emotional Content of Stimuli in Healthy and Psychiatric Populations. Behav. Res. Ther. 2021, 146, 103963. [Google Scholar] [CrossRef]
- Baune, B.T.; Malhi, G.S. A Review on the Impact of Cognitive Dysfunction on Social, Occupational, and General Functional Outcomes in Bipolar Disorder. Bipolar Disord. 2015, 17 (Suppl. S2), 41–55. [Google Scholar] [CrossRef]
- Depp, C.A.; Mausbach, B.T.; Harmell, A.L.; Savla, G.N.; Bowie, C.R.; Harvey, P.D.; Patterson, T.L. Meta-Analysis of the Association between Cognitive Abilities and Everyday Functioning in Bipolar Disorder. Bipolar Disord. 2012, 14, 217–226. [Google Scholar] [CrossRef] [Green Version]
- Mackala, S.A.; Torres, I.J.; Kozicky, J.; Michalak, E.E.; Yatham, L.N. Cognitive Performance and Quality of Life Early in the Course of Bipolar Disorder. J. Affect. Disord. 2014, 168, 119–124. [Google Scholar] [CrossRef]
- Mora, E.; Portella, M.J.; Forcada, I.; Vieta, E.; Mur, M. Persistence of Cognitive Impairment and Its Negative Impact on Psychosocial Functioning in Lithium-Treated, Euthymic Bipolar Patients: A 6-Year Follow-up Study. Psychol. Med. 2013, 43, 1187–1196. [Google Scholar] [CrossRef] [Green Version]
- Johnson, S.L.; Carver, C.S. Emotion-Relevant Impulsivity Predicts Sustained Anger and Aggression after Remission in Bipolar I Disorder. J. Affect. Disord. 2016, 189, 169–175. [Google Scholar] [CrossRef]
- Johnson, S.L.; Carver, C.S.; Tharp, J.A. Suicidality in Bipolar Disorder: The Role of Emotion-Triggered Impulsivity. Suicide Life. Threat. Behav. 2017, 47, 177–192. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.N.; Black, D.W. Bipolar Disorder and Suicide: A Review. Curr. Psychiatry Rep. 2020, 22, 6. [Google Scholar] [CrossRef]
- Ramírez-Martín, A.; Ramos-Martín, J.; Mayoral-Cleries, F.; Moreno-Küstner, B.; Guzman-Parra, J. Impulsivity, Decision-Making and Risk-Taking Behaviour in Bipolar Disorder: A Systematic Review and Meta-Analysis. Psychol. Med. 2020, 50, 2141–2153. [Google Scholar] [CrossRef]
- Zakowicz, P.; Skibińska, M.; Wasicka-Przewoźna, K.; Skulimowski, B.; Waśniewski, F.; Chorzepa, A.; Różański, M.; Twarowska-Hauser, J.; Pawlak, J. Impulsivity as a Risk Factor for Suicide in Bipolar Disorder. Front. Psychiatry 2021, 12, 706933. [Google Scholar] [CrossRef]
- Gvion, Y.; Levi-Belz, Y.; Hadlaczky, G.; Apter, A. On the Role of Impulsivity and Decision-Making in Suicidal Behavior. World J. Psychiatry 2015, 5, 255–259. [Google Scholar] [CrossRef]
- Battaglia, S.; Fabius, J.H.; Moravkova, K.; Fracasso, A.; Borgomaneri, S. The Neurobiological Correlates of Gaze Perception in Healthy Individuals and Neurologic Patients. Biomedicines 2022, 10, 627. [Google Scholar] [CrossRef]
- Tanaka, M.; Tóth, F.; Polyák, H.; Szabó, Á.; Mándi, Y.; Vécsei, L. Immune Influencers in Action: Metabolites and Enzymes of the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 734. [Google Scholar] [CrossRef]
- Rodrigues, J.E.; Martinho, A.; Santos, V.; Santa, C.; Madeira, N.; Martins, M.J.; Pato, C.N.; Macedo, A.; Manadas, B. Systematic Review and Meta-Analysis on MS-Based Proteomics Applied to Human Peripheral Fluids to Assess Potential Biomarkers of Bipolar Disorder. Int. J. Mol. Sci. 2022, 23, 5460. [Google Scholar] [CrossRef]
- Fabbri, C. The Role of Genetics in Bipolar Disorder. Curr. Top. Behav. Neurosci. 2021, 48, 41–60. [Google Scholar] [CrossRef]
- Johansson, V.; Kuja-Halkola, R.; Cannon, T.D.; Hultman, C.M.; Hedman, A.M. A Population-Based Heritability Estimate of Bipolar Disorder—In a Swedish Twin Sample. Psychiatry Res. 2019, 278, 180–187. [Google Scholar] [CrossRef]
- Craddock, N.; Jones, I. Genetics of Bipolar Disorder. J. Med. Genet. 1999, 36, 585–594. [Google Scholar] [CrossRef]
- Craddock, N.; Sklar, P. Genetics of Bipolar Disorder. Lancet Lond. Engl. 2013, 381, 1654–1662. [Google Scholar] [CrossRef]
- Grande, I.; Berk, M.; Birmaher, B.; Vieta, E. Bipolar Disorder. Lancet Lond. Engl. 2016, 387, 1561–1572. [Google Scholar] [CrossRef]
- Kendler, K.S. Reflections on the Relationship between Psychiatric Genetics and Psychiatric Nosology. Am. J. Psychiatry 2006, 163, 1138–1146. [Google Scholar] [CrossRef]
- Sanches, M.; Keshavan, M.S.; Brambilla, P.; Soares, J.C. Neurodevelopmental Basis of Bipolar Disorder: A Critical Appraisal. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 1617–1627. [Google Scholar] [CrossRef]
- Bora, E. Developmental Trajectory of Cognitive Impairment in Bipolar Disorder: Comparison with Schizophrenia. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2015, 25, 158–168. [Google Scholar] [CrossRef]
- Joseph, M.F.; Frazier, T.W.; Youngstrom, E.A.; Soares, J.C. A Quantitative and Qualitative Review of Neurocognitive Performance in Pediatric Bipolar Disorder. J. Child Adolesc. Psychopharmacol. 2008, 18, 595–605. [Google Scholar] [CrossRef]
- Post, R.M.; Fleming, J.; Kapczinski, F. Neurobiological Correlates of Illness Progression in the Recurrent Affective Disorders. J. Psychiatr. Res. 2012, 46, 561–573. [Google Scholar] [CrossRef]
- Arts, B.; Jabben, N.; Krabbendam, L.; van Os, J. Meta-Analyses of Cognitive Functioning in Euthymic Bipolar Patients and Their First-Degree Relatives. Psychol. Med. 2008, 38, 771–785. [Google Scholar] [CrossRef]
- Balanzá-Martínez, V.; Rubio, C.; Selva-Vera, G.; Martinez-Aran, A.; Sánchez-Moreno, J.; Salazar-Fraile, J.; Vieta, E.; Tabarés-Seisdedos, R. Neurocognitive Endophenotypes (Endophenocognitypes) from Studies of Relatives of Bipolar Disorder Subjects: A Systematic Review. Neurosci. Biobehav. Rev. 2008, 32, 1426–1438. [Google Scholar] [CrossRef]
- Bora, E.; Yucel, M.; Pantelis, C. Cognitive Endophenotypes of Bipolar Disorder: A Meta-Analysis of Neuropsychological Deficits in Euthymic Patients and Their First-Degree Relatives. J. Affect. Disord. 2009, 113, 1–20. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. Editorial of Special Issue “Crosstalk between Depression, Anxiety, and Dementia: Comorbidity in Behavioral Neurology and Neuropsychiatry”. Biomedicines 2021, 9, 517. [Google Scholar] [CrossRef]
- Goldstein, B.I.; Kemp, D.E.; Soczynska, J.K.; McIntyre, R.S. Inflammation and the Phenomenology, Pathophysiology, Comorbidity, and Treatment of Bipolar Disorder: A Systematic Review of the Literature. J. Clin. Psychiatry 2009, 70, 1078–1090. [Google Scholar] [CrossRef]
- Rosenblat, J.D.; Cha, D.S.; Mansur, R.B.; McIntyre, R.S. Inflamed Moods: A Review of the Interactions between Inflammation and Mood Disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 53, 23–34. [Google Scholar] [CrossRef]
- Rosenblat, J.D.; McIntyre, R.S. Bipolar Disorder and Inflammation. Psychiatr. Clin. N. Am. 2016, 39, 125–137. [Google Scholar] [CrossRef]
- Munkholm, K.; Braüner, J.V.; Kessing, L.V.; Vinberg, M. Cytokines in Bipolar Disorder vs. Healthy Control Subjects: A Systematic Review and Meta-Analysis. J. Psychiatr. Res. 2013, 47, 1119–1133. [Google Scholar] [CrossRef]
- Sayana, P.; Colpo, G.D.; Simões, L.R.; Giridharan, V.V.; Teixeira, A.L.; Quevedo, J.; Barichello, T. A Systematic Review of Evidence for the Role of Inflammatory Biomarkers in Bipolar Patients. J. Psychiatr. Res. 2017, 92, 160–182. [Google Scholar] [CrossRef]
- Grande, I.; Fries, G.R.; Kunz, M.; Kapczinski, F. The Role of BDNF as a Mediator of Neuroplasticity in Bipolar Disorder. Psychiatry Investig. 2010, 7, 243–250. [Google Scholar] [CrossRef]
- Rowland, T.; Perry, B.I.; Upthegrove, R.; Barnes, N.; Chatterjee, J.; Gallacher, D.; Marwaha, S. Neurotrophins, Cytokines, Oxidative Stress Mediators and Mood State in Bipolar Disorder: Systematic Review and Meta-Analyses. Br. J. Psychiatry 2018, 213, 514–525. [Google Scholar] [CrossRef] [Green Version]
- Gordovez, F.J.A.; McMahon, F.J. The Genetics of Bipolar Disorder. Mol. Psychiatry 2020, 25, 544–559. [Google Scholar] [CrossRef]
- Duffy, A.; Goodday, S.M.; Keown-Stoneman, C.; Scotti, M.; Maitra, M.; Nagy, C.; Horrocks, J.; Turecki, G. Epigenetic Markers in Inflammation-Related Genes Associated with Mood Disorder: A Cross-Sectional and Longitudinal Study in High-Risk Offspring of Bipolar Parents. Int. J. Bipolar Disord. 2019, 7, 17. [Google Scholar] [CrossRef] [Green Version]
- Alameda, L.; Trotta, G.; Quigley, H.; Rodriguez, V.; Gadelrab, R.; Dwir, D.; Dempster, E.; Wong, C.C.Y.; Forti, M.D. Can Epigenetics Shine a Light on the Biological Pathways Underlying Major Mental Disorders? Psychol. Med. 2022, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, C.; Hernandez, M.; Faedda, G.L. The Role of Environmental Exposures as Risk Factors for Bipolar Disorder: A Systematic Review of Longitudinal Studies. J. Affect. Disord. 2016, 193, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, C.; Faedda, G.L.; Baldessarini, R.J. Clinical and Environmental Risk Factors for Bipolar Disorder: Review of Prospective Studies. Harv. Rev. Psychiatry 2018, 26, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Aldinger, F.; Schulze, T.G. Environmental Factors, Life Events, and Trauma in the Course of Bipolar Disorder. Psychiatry Clin. Neurosci. 2017, 71, 6–17. [Google Scholar] [CrossRef]
- Bortolato, B.; Köhler, C.A.; Evangelou, E.; León-Caballero, J.; Solmi, M.; Stubbs, B.; Belbasis, L.; Pacchiarotti, I.; Kessing, L.V.; Berk, M.; et al. Systematic Assessment of Environmental Risk Factors for Bipolar Disorder: An Umbrella Review of Systematic Reviews and Meta-Analyses. Bipolar Disord. 2017, 19, 84–96. [Google Scholar] [CrossRef] [Green Version]
- Barichello, T.; Badawy, M.; Pitcher, M.R.; Saigal, P.; Generoso, J.S.; Goularte, J.A.; Simões, L.R.; Quevedo, J.; Carvalho, A.F. Exposure to Perinatal Infections and Bipolar Disorder: A Systematic Review. Curr. Mol. Med. 2016, 16, 106–118. [Google Scholar] [CrossRef]
- Pugliese, V.; Bruni, A.; Carbone, E.A.; Calabrò, G.; Cerminara, G.; Sampogna, G.; Luciano, M.; Steardo, L.; Fiorillo, A.; Garcia, C.S.; et al. Maternal Stress, Prenatal Medical Illnesses and Obstetric Complications: Risk Factors for Schizophrenia Spectrum Disorder, Bipolar Disorder and Major Depressive Disorder. Psychiatry Res. 2019, 271, 23–30. [Google Scholar] [CrossRef]
- Garno, J.L.; Goldberg, J.F.; Ramirez, P.M.; Ritzler, B.A. Impact of Childhood Abuse on the Clinical Course of Bipolar Disorder. Br. J. Psychiatry J. Ment. Sci. 2005, 186, 121–125. [Google Scholar] [CrossRef] [Green Version]
- Agnew-Blais, J.; Danese, A. Childhood Maltreatment and Unfavourable Clinical Outcomes in Bipolar Disorder: A Systematic Review and Meta-Analysis. Lancet Psychiatry 2016, 3, 342–349. [Google Scholar] [CrossRef]
- Palmier-Claus, J.E.; Berry, K.; Bucci, S.; Mansell, W.; Varese, F. Relationship between Childhood Adversity and Bipolar Affective Disorder: Systematic Review and Meta-Analysis. Br. J. Psychiatry J. Ment. Sci. 2016, 209, 454–459. [Google Scholar] [CrossRef]
- Gershon, A.; Johnson, S.L.; Miller, I. Chronic Stressors and Trauma: Prospective Influences on the Course of Bipolar Disorder. Psychol. Med. 2013, 43, 2583–2592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koenders, M.A.; Giltay, E.J.; Spijker, A.T.; Hoencamp, E.; Spinhoven, P.; Elzinga, B.M. Stressful Life Events in Bipolar I and II Disorder: Cause or Consequence of Mood Symptoms? J. Affect. Disord. 2014, 161, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Simhandl, C.; Radua, J.; König, B.; Amann, B.L. The Prevalence and Effect of Life Events in 222 Bipolar I and II Patients: A Prospective, Naturalistic 4 Year Follow-up Study. J. Affect. Disord. 2015, 170, 166–171. [Google Scholar] [CrossRef]
- Schepis, T.S.; Hakes, J.K. Non-Medical Prescription Use Increases the Risk for the Onset and Recurrence of Psychopathology: Results from the National Epidemiological Survey on Alcohol and Related Conditions. Addict. Abingdon Engl. 2011, 106, 2146–2155. [Google Scholar] [CrossRef] [PubMed]
- Feingold, D.; Weiser, M.; Rehm, J.; Lev-Ran, S. The Association between Cannabis Use and Mood Disorders: A Longitudinal Study. J. Affect. Disord. 2015, 172, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.; Hallmayer, J.; Wang, P.W.; Hill, S.J.; Johnson, S.L.; Ketter, T.A. Brain-Derived Neurotrophic Factor Val66met Genotype and Early Life Stress Effects upon Bipolar Course. J. Psychiatr. Res. 2013, 47, 252–258. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Leucht, S.; Fennema, H.; Engel, R.R.; Kaspers-Janssen, M.; Szegedi, A. Translating the HAM-D into the MADRS and Vice Versa with Equipercentile Linking. J. Affect. Disord. 2018, 226, 326–331. [Google Scholar] [CrossRef]
- IntHout, J.; Ioannidis, J.P.A.; Borm, G.F. The Hartung-Knapp-Sidik-Jonkman Method for Random Effects Meta-Analysis Is Straightforward and Considerably Outperforms the Standard DerSimonian-Laird Method. BMC Med. Res. Methodol. 2014, 14, 25. [Google Scholar] [CrossRef] [Green Version]
- Hartung, J.; Knapp, G. On Tests of the Overall Treatment Effect in Meta-Analysis with Normally Distributed Responses. Stat. Med. 2001, 20, 1771–1782. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Sutton, A.J.; Ioannidis, J.P.A.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for Examining and Interpreting Funnel Plot Asymmetry in Meta-Analyses of Randomised Controlled Trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arosio, B.; Guerini, F.R.; Voshaar, R.C.O.; Aprahamian, I. Blood Brain-Derived Neurotrophic Factor (BDNF) and Major Depression: Do We Have a Translational Perspective? Front. Behav. Neurosci. 2021, 15, 626906. [Google Scholar] [CrossRef]
- Fernandes, B.S.; Berk, M.; Turck, C.W.; Steiner, J.; Gonçalves, C.-A. Decreased Peripheral Brain-Derived Neurotrophic Factor Levels Are a Biomarker of Disease Activity in Major Psychiatric Disorders: A Comparative Meta-Analysis. Mol. Psychiatry 2014, 19, 750–751. [Google Scholar] [CrossRef]
- Chiou, Y.-J.; Huang, T.-L. Brain-Derived Neurotrophic Factor (BDNF) and Bipolar Disorder. Psychiatry Res. 2019, 274, 395–399. [Google Scholar] [CrossRef]
- Fernandes, B.S.; Molendijk, M.L.; Köhler, C.A.; Soares, J.C.; Leite, C.M.G.S.; Machado-Vieira, R.; Ribeiro, T.L.; Silva, J.C.; Sales, P.M.G.; Quevedo, J.; et al. Peripheral Brain-Derived Neurotrophic Factor (BDNF) as a Biomarker in Bipolar Disorder: A Meta-Analysis of 52 Studies. BMC Med. 2015, 13, 289. [Google Scholar] [CrossRef] [Green Version]
- Munkholm, K.; Vinberg, M.; Kessing, L.V. Peripheral Blood Brain-Derived Neurotrophic Factor in Bipolar Disorder: A Comprehensive Systematic Review and Meta-Analysis. Mol. Psychiatry 2016, 21, 216–228. [Google Scholar] [CrossRef]
- Fernandes, B.S.; Gama, C.S.; Ceresér, K.M.; Yatham, L.N.; Fries, G.R.; Colpo, G.; de Lucena, D.; Kunz, M.; Gomes, F.A.; Kapczinski, F. Brain-Derived Neurotrophic Factor as a State-Marker of Mood Episodes in Bipolar Disorders: A Systematic Review and Meta-Regression Analysis. J. Psychiatr. Res. 2011, 45, 995–1004. [Google Scholar] [CrossRef]
- Silva-Peña, D.; García-Marchena, N.; Alén, F.; Araos, P.; Rivera, P.; Vargas, A.; García-Fernández, M.I.; Martín-Velasco, A.I.; Villanúa, M.Á.; Castilla-Ortega, E.; et al. Alcohol-Induced Cognitive Deficits Are Associated with Decreased Circulating Levels of the Neurotrophin BDNF in Humans and Rats. Addict. Biol. 2019, 24, 1019–1033. [Google Scholar] [CrossRef]
- Simanek, A.M.; Parry, A.; Dowd, J.B. Differences in the Association between Persistent Pathogens and Mood Disorders among Young- to Middle-Aged Women and Men in the U.S. Brain Behav. Immun. 2018, 68, 56–65. [Google Scholar] [CrossRef] [Green Version]
- Mansur, R.B.; Subramaniapillai, M.; Lee, Y.; Pan, Z.; Carmona, N.E.; Shekotikhina, M.; Iacobucci, M.; Rodrigues, N.; Nasri, F.; Rosenblat, J.D.; et al. Effects of Infliximab on Brain Neurochemistry of Adults with Bipolar Depression. J. Affect. Disord. 2021, 281, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Solmi, M.; Suresh Sharma, M.; Osimo, E.F.; Fornaro, M.; Bortolato, B.; Croatto, G.; Miola, A.; Vieta, E.; Pariante, C.M.; Smith, L.; et al. Peripheral Levels of C-Reactive Protein, Tumor Necrosis Factor-α, Interleukin-6, and Interleukin-1β across the Mood Spectrum in Bipolar Disorder: A Meta-Analysis of Mean Differences and Variability. Brain Behav. Immun. 2021, 97, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Knorr, U.; Simonsen, A.H.; Jensen, C.S.; Zetterberg, H.; Blennow, K.; Akhøj, M.; Forman, J.; Hasselbalch, S.G.; Kessing, L.V. Alzheimer’s Disease Related Biomarkers in Bipolar Disorder—A Longitudinal One-Year Case-Control Study. J. Affect. Disord. 2022, 297, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Maloum, Z.; Taheri, M.; Ghafouri-Fard, S.; Shirvani-Farsani, Z. Significant Reduction of Long Non-Coding RNAs Expression in Bipolar Disorder. BMC Psychiatry 2022, 22, 256. [Google Scholar] [CrossRef] [PubMed]
- Steinacker, P.; Al Shweiki, M.R.; Oeckl, P.; Graf, H.; Ludolph, A.C.; Schönfeldt-Lecuona, C.; Otto, M. Glial Fibrillary Acidic Protein as Blood Biomarker for Differential Diagnosis and Severity of Major Depressive Disorder. J. Psychiatr. Res. 2021, 144, 54–58. [Google Scholar] [CrossRef]
- Isgren, A.; Sellgren, C.; Ekman, C.-J.; Holmén-Larsson, J.; Blennow, K.; Zetterberg, H.; Jakobsson, J.; Landén, M. Markers of Neuroinflammation and Neuronal Injury in Bipolar Disorder: Relation to Prospective Clinical Outcomes. Brain Behav. Immun. 2017, 65, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Niu, Z.; Wu, X.; Zhu, Y.; Yang, L.; Shi, Y.; Wang, Y.; Qiu, H.; Gu, W.; Wu, Y.; Long, X.; et al. Early Diagnosis of Bipolar Disorder Coming Soon: Application of an Oxidative Stress Injury Biomarker (BIOS) Model. Neurosci. Bull. 2022. [Google Scholar] [CrossRef]
- Stenzel, C.; Dalkner, N.; Unterrainer, H.-F.; Birner, A.; Bengesser, S.A.; Fellendorf, F.T.; Fink, A.; Fleischmann, E.; Lenger, M.; Maget, A.; et al. Effects of Metabolic Syndrome and Obesity on Suicidality in Individuals with Bipolar Disorder. J. Affect. Disord. 2022, 311, 1–7. [Google Scholar] [CrossRef]
- Wei, Y.; Womer, F.Y.; Sun, K.; Zhu, Y.; Sun, D.; Duan, J.; Zhang, R.; Wei, S.; Jiang, X.; Zhang, Y.; et al. Applying Dimensional Psychopathology: Transdiagnostic Prediction of Executive Cognition Using Brain Connectivity and Inflammatory Biomarkers. Psychol. Med. 2022, 1–11. [Google Scholar] [CrossRef]
- Cattarinussi, G.; Kubera, K.M.; Hirjak, D.; Wolf, R.C.; Sambataro, F. Neural Correlates of the Risk for Schizophrenia and Bipolar Disorder: A Meta-Analysis of Structural and Functional Neuroimaging Studies. Biol. Psychiatry, 2022; S0006-3223(22)01068-X, in press. [Google Scholar] [CrossRef]
- Sagar, R.; Pattanayak, R.D. Potential Biomarkers for Bipolar Disorder: Where Do We Stand? Indian J. Med. Res. 2017, 145, 7–16. [Google Scholar] [CrossRef]
- Scola, G.; Andreazza, A.C. Current State of Biomarkers in Bipolar Disorder. Curr. Psychiatry Rep. 2014, 16, 514. [Google Scholar] [CrossRef] [PubMed]
- Sigitova, E.; Fišar, Z.; Hroudová, J.; Cikánková, T.; Raboch, J. Biological Hypotheses and Biomarkers of Bipolar Disorder. Psychiatry Clin. Neurosci. 2017, 71, 77–103. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, C.; Antunes, B.M.; Giacon, T.R.; Vanderlei, L.C.M.; Campos, E.Z.; Peres, F.P.; Clark, N.W.; Panissa, V.L.G.; Lira, F.S. Influence of Acute and Chronic High-Intensity Intermittent Aerobic Plus Strength Exercise on BDNF, Lipid and Autonomic Parameters. J. Sports Sci. Med. 2019, 18, 359–368. [Google Scholar]
- Haack, M.; Hinze-Selch, D.; Fenzel, T.; Kraus, T.; Kühn, M.; Schuld, A.; Pollmächer, T. Plasma Levels of Cytokines and Soluble Cytokine Receptors in Psychiatric Patients upon Hospital Admission: Effects of Confounding Factors and Diagnosis. J. Psychiatr. Res. 1999, 33, 407–418. [Google Scholar] [CrossRef]
- Rosenberg-Hasson, Y.; Hansmann, L.; Liedtke, M.; Herschmann, I.; Maecker, H.T. Effects of Serum and Plasma Matrices on Multiplex Immunoassays. Immunol. Res. 2014, 58, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Vincent, F.B.; Nim, H.T.; Lee, J.P.W.; Morand, E.F.; Harris, J. Effect of Storage Duration on Cytokine Stability in Human Serum and Plasma. Cytokine 2019, 113, 453–457. [Google Scholar] [CrossRef]
- Gejl, A.K.; Enevold, C.; Bugge, A.; Andersen, M.S.; Nielsen, C.H.; Andersen, L.B. Associations between Serum and Plasma Brain-Derived Neurotrophic Factor and Influence of Storage Time and Centrifugation Strategy. Sci. Rep. 2019, 9, 9655. [Google Scholar] [CrossRef] [Green Version]




| ID | First Author (Year) | BD Sample | BD Age (Range) | BD Sex (M/F) | Exposure Factor | Measure Instrument | Biomarkers Evaluated | Main Findings |
|---|---|---|---|---|---|---|---|---|
| 1. | Bai et al. (2014) | 130 BD patients (77 BD-I, 53 BD-II) (75 euthymic, 14 (hypo)manic state, 41 depressive state) | 44.55 ± 11.08 (47.0 ± 11.7 BD-I, 41.0 ± 10.2 BD-II) (44.9 ± 12.4 euthymic, 46.1 ± 9.3 (hypo)manic, 43.4 ± 10.2 depressive) | 31.55%/68.44% (33.8%/66.2% BD-I, 28.3%/71.7% BD-II) (33.3%/66.7% euthymic, 21.4%/78.6% (hypo)manic, 33.3%/66.7% depressive) | Severity of manic symptoms Severity of depressive symptoms Subtype, phase of the disorder and other clinical features | YMRS MADRS MINI | sIL-2R sIL-6R sTNF-R1 | BB-I patients had higher levels of sIL-2R and sTNF-R1 than BD-II patients. Lower levels of sIL-2R and sTNF-R1 in depressive state than in a manic/hypomanic and euthymic state. Levels of sIL-6R and sTNF-R1 correlated positively with YMRS scores. Levels of sIL-6R, sIL-2R and sTNF-R1 correlated negatively with MADRS scores. Levels of sIL-6R, sIL-2R, sTNF-R1 correlated positively with length of illness. |
| 2. | Barbosa et al. (2010) | 53 BD-I patients (34 mania, 19 euthymia) | 47.77 ± 13.01 (49.6 ± 14.2 mania, 44.5 ± 10.9 euthymia) | 39.59%/60.40% (38.2%/61.8% mania, 42.1%/57.9% euthymia) | Severity of manic symptoms Severity of depressive symptoms Phase of the disorder, comorbidity and other clinical features | YMRS HDRS-17 MINI-plus | BDNF | No differences in BDNF levels between euthymic and manic states. No correlation between BDNF levels and YMRS nor HDRS scores. No correlation between BDNF levels and age nor in terms of length of illness. Higher levels of BDNF in patients suffering the disorder for more than 10 years. No differences in BDNF levels according to the presence of any psychiatric comorbidity. |
| 3. | Barbosa et al. (2011) | 49 BD-I patients (30 manic, 19 euthymic) | 46.85 ± 12.56 (48.03 ± 13.66 manic, 45.00 ± 10.84 euthymic) | 40.81%/59.18% (40.00%/60.00% manic, 42.1%/57.90% euthymic) | Severity of manic symptoms Severity of depressive symptoms Phase of the disorder, comorbidity and other clinical features | YMRS HAMD-17 MINI-plus | NGF | No differences in NGF levels between manic and euthymic patients. Levels of NGF negatively correlated with YMRS scores. No association between NGF levels and HAMD scores. No association between NGF levels and age nor number of hospitalisations in BD patients. In all BD patients, NGF levels were positively correlated with the length of illness but not for each sub-group (euthymia and mania). Levels of NGF did not differ in BD patients categorized according to the presence of psychiatric comorbidities, nor dependence of substances or nicotine. |
| 4. | Barbosa et al. (2012) | 25 BD-I euthymic patients | 50.88 ± 9.11 | 32%/68% | Cognitive function Executive function Comorbidity and other clinical features | MMSE FAB MINI | BDNF TNF-α sTNFR sTNFR2 | TNF-α levels were positively correlated with inhibitory control (FAB score). No other significant correlations between the biomarker levels and the cognitive parameters were evaluated (FAB and MMSE scores). No differences in BDNF, TNF-α, sTNFR1 or sTNFR2 levels according to the presence of psychiatric comorbidities, substance dependence and previous episodes of depression or psychosis. |
| 5. | Barbosa et al. (2014) | 46 BD-I patients (23 euthymic, 23 manic) | 49.74 ± 11.41 (50.39 ± 8.07 euthymic, 49.09 ± 14.76 manic) | 26.09%/73.91% (17.39%/82.61% euthymic, 34.79%/65.21% manic) | Severity of manic symptoms Severity of depressive symptoms Phase of the disorder and other clinical features | YMRS HDRS-17 MINI-Plus | IL-33 sSt2 | No significant differences in IL-33 or sST2 levels between BD patients in mania and in euthymia phase. No correlation between IL-33 or sST2 levels and clinical parameters such as HDRS scores, YMRS scores and length of illness in BD patients. |
| 6. | Benedetti et al. (2017) | 40 BD-I inpatients current major depressive episode | 46.3 ± 13.85 | 32.5%/67.5% | Child abuse and neglect | CTQ | BDNF | Early trauma was negatively associated with BDNF levels in BD patients. |
| 7. | Bonnin at al. (2019) | 102 BD patients (47 psychoeducation [25 BD-I], 39 FR [40 BD-I], 16 TAU [14 BD-I]) | 40.95 ± 8.20 (35.2 ± 9.2 psychoeducation, 48.5 ± 6.3 FR, 39.5 ± 9.9 TAU) | BD 63.20%36.79% (53.2%/46.8% psychoeducation, 73%/27% FR, 68.7%/31.3% TAU) | Severity of depressive symptoms Psychosocial functioning Clinical features | HAMD-17 FAST SCID | BDNF | Significant interaction between FAST scores at baseline and time on BDNF levels in BD patients. No significant interaction between age, HAMD scores and chronicity at baseline and time on BDNF levels. |
| 8. | Brietzke et al. (2009) | 61 BD-I patients (14 euthymic, 23 manic episode, 24 depressive episode) | 42.23 ± 13.00 (44.2 ± 13.75 euthymic, 40.8 ± 13.70 manic, 45.0 ± 11.91 depressive) | 39.33%/60.65% (28.6%/71.4% euthymic, 47.8%/52.2% manic, 37.5%/62.5% depressive) | Severity of manic symptoms Severity of depressive symptoms Phase of the disorder and other clinical features | YMRS HDRS SCID-I | IL-2 IL-4 IL-6 IL-10 IFN-γ TNF-α | No differences in levels of IL-2, IL-4, IL-6, IL-10, IFN-γ or TNF-α among euthymic, manic, depressive states. Positive correlation between IL-6 levels and YMRS and HDRS scores, and positive correlation between IL-2 levels and YMRS scores. No correlation between other biomarkers and the severity of depressive and manic symptoms. No correlation between any biomarker level and length of illness. |
| 9. | Cetin et al. (2012) | 45 BD-I patients in euthymic state (22 subsyndromal symptoms, 23 without subsyndromal symptoms) | 35.71 ± 7.15 (36.86 ± 7.03 subsyndromal, 34.61 ± 7.28 without subsyndromal) | 44.47%/55.52% (45.5%/54.5% subsyndromal, 43.5%/56.5% without subsyndromal) | Clinical characteristics and illness course Presence of subsyndromal symptoms | SKIP-TURK procedure | sIL-6R sTNF-R1 | No differences in sTNF-R1 or sIL-6R levels between BD subsyndromal and without subsyndromal symptoms. No significant association between sTNF-R1 nor sIL-6R levels and length of illness in bipolar patients. |
| 10. | Chang et al. (2017) | 91 BD patients | 43.0 ± 12.2 | 42.9%/57.1% | Dietary intake | BMI Daily caloric intake (kcal) Dietary LA (g) | IL-1β IL-1RA IL-6 IL-6RA IL-10 IL-18 IL-18BP sTNFR2 | Dietary Linoleic Acid (LA) intake was inversely associated with IL-18BP levels. Positive association between sTNFR1 and sTNFR2 levels and BMI. No association between IL-1β, IL-6, IL-6RA, IL-10 or IL-18 levels and BMI, daily caloric intake and dietary Linoleic Acid. Positive association between IL-18, IL-18BP, STNFR1 and sTNFR2 levels and age. |
| 11. | Chiou & Huang (2019) | 83 BD-I patients (61 mania, 22 depression) | 36.8 ± 11.4 | 45.78%/54.21% | Severity of manic symptoms Severity of depressive symptoms | YMRS HDRS-17 | BDNF | Significant positive correlations between BDNF levels and YMRS scores among bipolar manic patients. No significant correlation between BDNF levels and HDRS scores among bipolar depressed patients. |
| 12. | Dell’Osso et al. (2010) | 33 BD outpatients current major depressive episode (17 unipolar, 16 BD-I) | 46.4 ± 14.3 (22–65 years) (46.06 ± 10.78 unipolar, 46.75 ± 17.67 BD-I) | 36.37%/63.63% (23.5%/76.5% unipolar, 50%/50% BD-I) | Severity of depressive symptoms Global illness severity | HRSD-21 CGI-S | BDNF | Significant negative correlation between BDNF levels and HDRS scores and between BDNF and CGI score in all patients and in the bipolar depressed group. |
| 13. | Dolsen et al. (2018) | 22 BD-I euthymic patients (11 CBT for insomnia, 11 psychoeducation) | 36.4 ± 11.85 | 54,5%/45,5% | Severity of manic symptoms Severity of depressive symptoms Sleep disorders | YMRS, QIDS, DSISD, Sleep diary (TST and TWT) | IL-6 TNF-R2 | Levels of IL-6 were positively correlated with severity of depressive symptoms (YMRS and QIDS scores), and inversely correlated with total sleep time but not with total wake time. No association between TNF-R2 and manic or depressive symptoms, neither with sleep disorders. |
| 14. | Du et al. (2017) | 48 BD patients (typical BD group) 63 BD with anxiety disorders patients (atypical BD group) | 33.06 ± 14.53 BD 34.33 ± 15.55 atypical BD | 31.3%/68.8% BD 28.6%/71.4% atypical BD | Comorbid diagnosis of anxiety disorders (atypical BD group) | ICD-10 criteria for anxiety disorders | IL-6 IL-8 IL-10 TNF-α | Levels of IL-6 and TNF-α were significantly higher in the atypical group (with anxiety) than in the BD group. The IL-10 levels were significantly lower in the atypical group than in the BD group. No differences in IL-8 levels between the BD group and the atypical group. |
| 15. | Grande et al. (2014) | 115 BD patients (79 BD-I, 18 BD-II, 18 NOS) | 44.0 ± 20.0 (18–65 years) | 29.6%/70.4% | Classification patients into early-stage and late-stage | SCID-I | BDNF IL-6 | Differences in IL-6 levels between early-stage and late-stage. No differences in BDNF levels between early-stage and late-stage BD patients. |
| 16. | Hope et al. (2015) | 111 BD patients (65 BD-I, 40 BD-II, 6 NOS) | 33 ± 10 (18–63 years) | 54%/46% | General cognitive abilities | WASI, two subtests for verbal cognition and two tests for performance abilities | sTNF-R1 IL-1Ra sCD40L | Significant association between general cognitive abilities and levels of IL-1Ra and sCD40L, but not with sTNF-R1 levels. |
| 17. | Isgren et al. (2015) | 121 BD patients (65 BD-I, 46 BD-II, 10 NOS) | 36.0 ± 22.0 | 38.8%/61.2% | Severity of manic symptoms Severity of depressive symptoms Global illness severity Alcohol and substance abuse Subtype, comorbidity and other clinical features | YMRS MADRS CGI AUDIT DUDIT MINI | IL-8 | No association between changes of IL-8 levels and sex, bipolar subtype, CGI, MADRS and YMRS scores, length of illness, episode density, previous psychotic episodes, comorbid anxiety syndrome, attention deficit/hyperactivity disorder, smoking, alcohol abuse, substance abuse nor BMI. Levels of IL-8 were positively associated with age. |
| 18. | Jacoby et al. (2016) | 60 BD-I patients hospitalized with a manic or mixed episode | 42.7 ± 11.7 (20–60 years) | 61.7%/38.3% | Phase of the disorder | SCAN | BDNF TNF-α IL-6 IL-18 | No differences in levels of BDNF, IL-6 or TNF-α between any affective states (euthymic, manic, depressive, or mixed). Differences in levels of IL-18 between mixed states and remission and between mixed and hypomanic/manic states (no significant in the adjusted model). |
| 19. | Karabulut et al. (2019) | 107 BD patients (77 BD chronic [50 euthymic state, 20 manic state, 7 depressive state], 30 BD early-stage patients [12 euthymic state, 13 manic state, 2 depressive state, 2 hypomanic state, 1 mixed state]) | 34.29 ± 10.24 (37.81 ± 11.9 BD chronic, 25.27 ± 6 BD early-stage) | 52.34%/47.65% (57.2%/42.8% BD chronic, 23.4%/76.6% BD early-stage) | Severity of manic symptoms Severity of depressive symptoms Positive and negative symptoms Global illness severity Phase of the disorder, classification patients into early-stage and late-stage, comorbidity and other clinical features | YMRS MADRS PANSS CGI-S SCID-I | IL-1RA IL-6 TNF-α | Levels of IL-1RA, IL-6 and TNF-α were higher in chronic than early-stage patients. No differences in IL-1RA, IL-6 or TNF-α levels between chronic and early-stage patients in terms of mood state (euthymic, depressive and manic). Correlation between TNF-α levels and MADRS scores in chronic patients. Correlation between TNF-α levels and CGI scores in early-stage patients. No correlation between IL-1RA, IL-6 or TNF-α levels and YMRS nor PANNS scores among chronic and early-stage patients. No significant correlations between biomarkers levels and length of illness and the presence of psychotic symptoms. |
| 20. | Kauer-Sant’Anna et al. (2007) | 163 BD-I and BD-II patients (78 with life-time exposure to a traumatic event, 85 without-lifetime exposure to a traumatic event) | 42.58 ± 11.58 (42.13 ± 12.00 presence of trauma, 43.01 ± 11.21 absence of trauma) | 28.18%/71.82% (25.0%/75% presence of trauma, 31.1%/68.9% absence of trauma) | Trauma Comorbidity and other clinical features | DSM-IV A1 and A2 criteria from the PTSD module of the SCID SCID-I | BDNF | Patients with a history of trauma, especially sexual abuse, had lower levels of BDNF. No differences in BDNF levels in rapid cycling patients, or between patients with or without comorbid posttraumatic stress disorder (PTSD), alcohol abuse or dependence, or history of suicide attempts. |
| 21. | Kenna et al. (2014) | 47 BD euthymic patients (13 BD-I, 20 BD-II, 14 NOS) | 32.97 ± 6.5 (18–45 years) (32.9 ± 6.4 BD-I, 32.1 ± 6.3 BD-II, 34.3 ± 6.9 NOS) | 0%/100% | Severity of depressive symptoms Subtype | MADRS SCID | BDNF | No significant differences in BDNF levels between women with BD-I, BD-II and NOS. A significant negative correlation between BDNF levels and MADRS scores in BD women patients. |
| 22. | Kim et al. (2013) | 116 BD-I patients in manic episode, with pharmacological treatment during 6 weeks of follow-up | 35.90 ± 11.76 (35.99 ± 11.67) | 36.2%/63.8% (37.3%/62.7%) | Severity of manic symptoms | YMRS | IGF-1 β-NGF BDNF | No correlation between IGF-1, β-NGF or BDNF levels and YMRS scores (after 6 weeks of pharmacological treatment). |
| 23. | Lee et al. (2014) | 232 BD-II patients in depressed state, with VPA treatment during 12 weeks of follow-up (115 VPA + Memantine, 117 VPA + placebo) | 31.77 ± 11.56 | 50.86%/49.13% | Severity of manic symptoms Severity of depressive symptoms | YMRS HDRS | TNF-α IL-1β IL-6 IL-8 | The changes in IL-6 and IL-1β levels were significantly associated with the changes in HDRS scores (before and after pharmacological treatment). The changes in TNF-α levels were significantly associated with the changes in YMRS scores (before and after pharmacological treatment). The changes in IL-8 levels were not associated with the changes in HDRS scores, nor with the changes in YMRS scores (before and after pharmacological treatment). |
| 24. | Lee et al. (2016) | 541 BD patients (117 BD-I, 424 BD-II), with add-on memantine/placebo/DM treatment during 12 weeks of follow-up | 31.2 ± 11.2 | 48.79%/51.20% | Cognitive function Quality of life | WCST and CPT WHOQOL-BREF, Chinese version | BDNF | Only in BD-I patients was there a significant negative correlation between changes in BDNF levels and cognitive function (WCST scores) (after 12-weeks of pharmacological treatment) (not after a correction for multiple comparison). No correlation between changes of BDNF levels and CPT scores nor quality of life (WHOQOL scores). |
| 25. | Loch et al. (2015) | 23 BD patients in depressive episode (8 BD-I, 15 BD-II), with litium treatment during 6 weeks of follow-up | 28.0 (18–43 years) | 17.4%/82.6% | Severity of depressive symptoms Subtype, comorbidity and other clinical features | HAMD-21 SCID | NT-3 NT-4/5 | No correlation between NT-3 or NT-4/5 levels and changes in HAMD scores (after 6 weeks of treatment). No association between clinical features such as bipolar subtype, psychotic symptoms or tobacco use and baseline NT levels, except length of illness, which showed a positive correlation with NT-3 and NT-4/5 levels. |
| 26. | Machado-Vieira et al. (2007) | 30 BD-I patients in mania state (22 drug-naïve, 8 drug-free) | 26 ± 4 (20–40 years) | 23.3%/76.7% | Severity of manic symptoms | YMRS | BDNF | Significant negative correlation between BDNF levels and YMRS scores in unmedicated BD patients. |
| 27. | Maiti et al. (2017) | 25 BD patients current episode mania, 21 with oxcarbazepine treatment during 4 weeks of follow-up | 34.16 ± 9.89 (18–45 years) | 80%/20% | Severity of manic symptoms | YMRS | BDNF | Inverse relationship between BDNF levels and the YMRS score at baseline, and positive correlation between change of BDNF and change in the YMRS score (after four weeks of treatment). |
| 28. | Mansur et al. (2020) | 55 BD I-II patients current major depressive episode (27 Infliximab + 28 placebo) | 44.91 ± 10.90 (44.04 ± 11.55 Infliximab, 45.75 ± 10.28 placebo) | 20%/80% (25.9%/74.1% Infliximab, 14.3%/85.7% placebo) | Severity of depressive symptoms Child abuse and neglect Clinical features | MADRS CTQ and the subdomain of PA MINI 5.0.0 for DSM-IV-TR | TNFR1 TNFR2 | Levels of TNFR1 were significantly associated with MADRS scores, but there was no association between TNFR2 levels and MADRS scores. Levels of TNFR1 and TNFR2 were not associated with CTQ scores. No association between TNFR1 and TNFR2 and age, sex, BMI or tobacco use at baseline. |
| 29. | Mizuno et al. (2016) | 60 BD patients | 50.2 ± 13.8 (19–75 years) | 46.7%/53.3% | Resilience | Resilience Scale, 25-item | BDNF | No significant correlation between BDNF levels and resilience in the BD group. |
| 30a. | Mondin et al. (2016) | 48 BD patients (10 mania/mixed episode, 27 depressive episode, 11 euthymic) | 21.92 ± 2.32 (18–24 years) | 25%/75% | Chronotype | BRIAN | IL-6 IL-10 TNF-α | Association between subjects with day/night cycle reverse and IL-6 levels. BD patients active at night had IL-6 levels that were significantly decreased in comparison with BD patients who were not active at night. The reversed day/night cycle BD patients had IL-6 levels that were decreased in comparison with non-reversed day/night cycle BD patients. |
| 30b. | Wiener et al. (2017) | 48 BD patients | 21.92 ± 2.32 (18–24 years) | 25%/75% | Severity of depressive symptoms Functional impairment | HDRS FAST | IL-6 IL-10 | No correlation between IL-6 or IL-10 levels and HDRS scores in BD patients. Positive correlation between IL-6 and IL-10 levels and functional impairment (FAST scores). Higher IL-6 levels were associated with higher levels of impairment in the cognitive domain and in interpersonal relationships. Levels of IL-10 were positively correlated with impairment in the cognitive domain and in occupational functioning. |
| 31. | Monteleone et al. (2008) | 28 BD euthymic patients (17 BD-I, 11 BD-II) | 45.10 ± 11.08 (46.6 ± 9.4 BD-I, 42.8 ± 13.7 BD-II) | 39.28%/60.70% (29.41%/70.58% BD-I, 54.54%/45.45% BD-II) | Subtype and other clinical features | SCID-IP, Patient Edition | BDNF | No significant differences in BDNF levels among patient groups (BD-I, BD-II). No significant correlations between BDNF levels and age, age of onset, length of illness and number of depressive episodes in each diagnostic BD group. |
| 32. | Mora et al. (2019) | 84 BD patients (52 euthymic, 32 manic) | 45.13 ± 12.28 (18–65 years) (47.52 ± 11.9 euthymic BD, 41.25 ± 12.9 manic) | 52.38%/47.62% (50%/50% euthymic, 56.3%/43.7% manic) | Intelligence Attention Memory Executive function Subtype, phase of the disorder and other clinical features | WAIS III (vocabulary, block design and digits subtests), WCST, Stroop Color and Word Test, FAS verbal fluency task, TMT, CPT-II, CVLT, RCFT SCID-I | BDNF IL-6 IL-10 TNF-α | No differences in BDNF, IL-6, IL-10 or TNF-α levels between BD-I and BD-II euthymic patients. No differences between euthymic and manic patients in BDNF, IL-6, IL-10 or TNF-α levels. In a regression model, BDNF was the only biomarker associated with executive functioning and predicted worse performance in verbal memory in BD patient groups. Levels of IL-6 were not associated with cognitive domains in BD patients. Only IL-6 levels were associated with length of illness in BD manic and euthymic patients. |
| 33a. | Munkholm et al. (2014) | 37 BD rapid cycling patients (22 BD-I, 15 BD-II) | 40.9 ± 12.3 (18–70 years) | 32.43%/67.56% | Severity of manic and depressive symptoms Phase of the disorder and other clinical feature | YMRS HAMD-17 SCAN | BDNF | No difference in BDNF levels regardless of mood state (euthymic, depressive, manic/hypomanic, mixed). No association between BDNF levels and HAMD scores in a depressive state, neither between BDNF levels and YMRS scores in a manic/hypomanic state. Higher BDNF levels in patients with more than 10 years of illness in comparison with a shorter duration of disease. |
| 33b. | Munkholm et al. (2015) | 37 BD rapid cycling patients (22 BD-I, 15 BD-II) | 40.9 ± 12.3 (18–70 years) | 32.43%/67.56% | Severity of manic symptoms Severity of depressive symptoms Phase of the disorder and other clinical features | YMRS HAMD-17 SCAN | IL-6 IL-18 | Higher levels of IL-6 and IL-18 in manic/hypomanic rather than in depressive and euthymic patients. No differences in IL-6 or IL-18 levels between a depressed and euthymic state. No association between IL-6 and IL-18 levels and HAMD scores in depressive patients. No association between IL-6 and IL-18 levels and YMRS scores in manic/hypomanic patients. In mixed states, IL-6 levels were higher in episodes of 1–2 weeks compared with episodes below one week. In manic/hypomanic states, IL-6 levels were lower in episodes of more than one month compared with episodes below one week. In manic/hypomanic states, IL-18 levels were higher in episodes of 1–2 weeks compared with episodes below one week. In depressive states, IL-18 levels were lower in some episodes compared with episodes below one week. In euthymic states, IL-18 levels were higher in episodes of 3–4 weeks compared with episodes below one week. |
| 34. | Ortiz-Dominguez et al. (2007) | 20 BD-I patients (10 manic episode, 10 depressive episode) | 34.3 ± 9.94 (20–50 years) (28.9 ± 8.45 manic, 39.7 ± 11.43 depressive) | 25%/75% (30%/70% manic, 20%/80% depressive) | Phase of the disorder | MINI for DSM-IV | TNF-α IL-1β IL-2 IL-4 IL-6 | Bipolar patients in a manic state showed a significant increase in IL-4 levels and a significant decrease in IL-1β and IL-6 levels compared with depressed state patients. No significant differences between bipolar patients in a depressive state and in a manic state in IL-2 or TNF-α levels. |
| 35. | Pantović-Stefanović et al. (2016) | 83 BD-I patients, with pharmacological treatment during 10 weeks of follow-up | 45.61 ± 11.05 | 36.4%/63.60% | Severity of manic symptoms Severity of depressive symptoms Global illness severity Cardinal features of the disorder Clinical features | YMRS MADRS CGI-BP-S BPIX SCID-I | sICAM-1 sVCAM-1 IL-6 TNF-α | No differences in sICAM-1, sVCAM-1, IL-6, or TNF-α between patients in an acute phase and in remission phase. Levels of sVCAM-1 correlated negatively with the Bipolarity Index in the acute and remission phase. BD patients with manic polarity had significantly lower levels of sVCAM-1 compared with BD patients with depressive polarity. There were no differences regarding the polarity of the acute episode and levels of sICAM-1, IL-6 or TNF-α. Levels of sVCAM-1 in acute phase had a significant negative correlation with YMRS and a positive correlation with MADRS scores. Levels of sVCAM-1 correlated positively with the severity of the acute episode based on CGI-BP-S score. No correlation between sICAM-1, IL-6 or TNF-α levels and CGI, MADRS and YMRS scores in an acute or remission phase. Levels of sVCAM-1 in the acute and remission phase were positively correlated with the age of onset. Levels of sVCAM-1 in the acute phase were negatively correlated with the number of previous psychotic episodes. There were significantly lower levels of sVCAM-1 in the acute phase in patients with a family history of BD. Levels of TNF-α were inversely correlated with the duration of depressive episodes in BD patients in the acute phase. Significantly lower TNF- α levels in acute and remission phase in patients with a longer duration of untreated BD. No correlation between sICAM-1 nor IL-6 levels and clinical features. |
| 36. | Quidé et al. (2018) | 69 BD-I patients | 38.11 ± 12.31 (18–65 years) | 33.33%/66.66% | Childhood trauma | CTQ | IL-6 TNF-α | No association between IL-6 nor TNF-α levels and CTQ domains (emotional abuse, physical abuse, sexual abuse, emotional neglect and physical neglect) in the BD group. |
| 37. | Reininghaus et al. (2015) | 101 BD euthymic patients (64 BD-I, 37 BD-II) | 44.00 ± 12.99 | 48.51%/51.48% | Weigh cycling Clinical features | WCYC SCID-I | IL-6 | Significant differences in levels of IL-6 between weight cycling (WCY), BD patients and non-weight cycling (non-WCY) BD patients, WCY showed higher IL-6 levels than non-WCY. Significative correlation between IL-6 levels and the number of depressive episodes in BD patients. |
| 38. | Rosa et al. (2006) | 44 BD-I patients (15 manic, 14 depressed, 15 euthymic) | 40.83 ± 9.29 (30–51 years) (40.1 ± 9.3 manic, 42.1 ± 8.2 depressed, 40.4 ± 10.3 euthymic) | 41.07%/58.95% (56.3%/43.8% manic, 28.6%/71.4% depressed, 37.5%/62.5% euthymic) | Severity of manic symptoms Severity of depressive symptoms | YMRS HRSD-17 | GDNF | Higher GDNF levels in manic and depressed BD patients as compared with euthymic patients. |
| 39a. | Siwek et al. (2016) | 133 BD patients (65 BD-I and 64 BD-II) (23 manic phase, 61 depressive phase, 49 euthymic) (35 with melancholia, 26 without melancholia) | 44.3 ± 12.9 (21–70 years) | 65.41%/34.58% | Severity of manic symptoms Severity of depressive symptoms Subtype, phase of the disorder, comorbidity and other clinical features | YMRS MADRS HDRS SCID-I | sIL-1RA sIL-2R sIL-6R, sTNFR60 sTNFR80 | No differences in sIL-1RA levels regarding the mood phase (manic, depressive, or euthymic). Levels of sTNFR80 were significantly higher in depressive episodes in comparison with a (hypo)manic episode and remission. Levels of sTNFR80 were significantly higher in melancholia in comparison with all other patients (without melancholia). Positive correlation between sTNFR60 and sTNFR80 levels and HDRS and MADRS scores. No significant association between any biomarker and YMRS scores. No significant association between any biomarker and clinical variables such as subtype, number of lifetime episodes, age of onset, length of illness, rapid cycling, atypical depression, suicidal ideation, and psychotic symptoms. |
| 39b. | Sowa-Kućma et al. (2017) | 133 BD patients (69 BD-I, 64 BD-II all in depressed or euthymic phase) | 44.3 ± 12.9 BD (42.0 ± 14.6 BD-I, 46.8 ± 10.2 BD-II) | 65.41%/34.58% (42.02%/57.97% BD-I, 26.56%/73.43% BD-II) | Subtype and other clinical features | SCID-I | sIL-1RA IL-1α sIL-2R sIL-6R sTNFR60 sTNFR80 | No significant differences in any of the biomarkers between BD-I and BD-II. Levels of IL-1α and sTNFR80 were increased in BD with melancholia in comparison with BD without melancholia. |
| 40. | Soares et al. (2016) | 118 BD patients in euthymic state (91 BD-I, 27 BD-II) | 64.0 ± 9.7 (64.2 ± 9.8 BD-I, 63.3 ± 9.5 BD-II) | 31.29%/68.70% (30.7%/69.3% BD-I, 33.3%/66.7% BD-II) | Severity of manic and depressive symptoms Subtype and other clinical features | YMRS HAMD-17 SCID-I | BDNF | Patients with BD-I had significantly lower BDNF levels than patients with BD-II. No significant correlation between BDNF levels and age, length of illness, YMRS nor HAMD scores. |
| 41. | Tatay-Manteiga et al. (2017) | 48 BD euthymic patients (25 early-stage, 23 late-stage) | 44.21 ± 10.06 (18–60 years) (43.4 ± 10.3 early-stage, 45.1 ± 9.8 late-stage) | 47.91%/52.08% (48%/52% early-stage, 47.82%/52.17% late-stage) | Classification patients into early-stage and late-stage | Clinical interview | BDNF TNF-α IL-6 IL-10 NT-3 | Levels of IL-10 were significantly higher in early-stage bipolar patients compared with late-stage. There were no significant differences in BDNF, TNF-α, IL-6 or NT-3 levels between early-stage and late-stage bipolar patients. |
| 42. | Tunca et al. (2014) | 96 BD patients (92 BD-I, 4 BD-II) (37 euthymic, 33 manic, 26 depressed) | 38.12 ± 10.84 (36.24 ± 10.02 euthymia, 36.77 ± 12.02 mania, 42.54 ± 10.52 depression) | 44.74%/55.25% (37.8%/62.2% euthymia, 34.3%/65.7% mania, 67.9%/32.1% depression) | Severity of manic and depressive symptoms Global illness severity Social, occupational, and psychological functioning Phase of the disorder and other clinical features | YMRS HDRS CGI GAF SCID-I | BDNF GDNF | There were significantly lower BDNF levels in depressive and manic bipolar patients than in euthymic patients, but there were no differences in BDNF levels between manic and depressive episodes. Neither BDNF nor GDNF levels correlated with the duration of the mood state (euthymia, manic, depressive). There was a negative correlation between BDNF levels and YMRS, HDRS and CGI scores. There was a positive correlation between BDNF levels and GAF scores. Therew as a negative correlation between GDNF levels and HDRS scores and no correlation between GDNF levels and YMRS, CGI nor GAF scores. There were higher GDNF levels in early onset rather than late onset BD patients, while BDNF levels were similar in both groups. |
| 43. | Uyanik et al. (2015) | 30 BD-I patients in manic episode, with pharmacological treatment during 6 weeks of follow-up | 33.4 ± 8.6 (18–65 years) | 53.3%/46.7% | Severity of manic symptoms | YMRS | IL-4 IL-6 IL-10 TNF-α IFN-γ | No correlation between IL-4, IL-6, IL-10, TNF-α nor IFN-γ levels and YMRS scores (before and after pharmacological treatment). |
| 44a. | van den Ameele et al. (2017) | 67 BD patients (35 depressive episode [16 BD-I, 19 BD-II], 32 (hypo)manic episode [26 BD-I, 4 BD-II, 2 schizoaffective]) | 43.3 ± 11.1 (23–62 years) (43.7 ± 9.7 (28–61 years) depressive, 42.9 ± 12.7 (23–62 years) (hypo)manic) | 41.8%/58.2% (31.4%/68.6% depressive 53.1%/46.9% (hypo)manic) | Severity of manic symptoms Severity of depressive symptoms Positive symptoms Subtype, phase of the disorder, comorbidity and other clinical features | YMRS HDRS-17 PANSS, positive subscale MINI-plus, version 5.0.0 | TNF-α BDNF VEGF sFlt-1 | In a subgroup analysis, patients with mood symptoms had a significant increase in TNF-α levels in comparison with euthymic patients, but there were no significant differences between euthymic patients and patients with mood symptoms in BDNF, VEGF and sFlt-1 levels. No strong correlation between TNF-α, BDNF, VEGF and sFlt-1 levels and HDRS, YMRS nor PANSS positive subscale scores in BD patients. There was no significant association between TNF-α, BDNF, VEGF and sFlt-1 levels and clinical characteristics such as BD type I or II, mixed features, current psychotic features, lifetime psychosis features, age of onset, length of illness nor number of hospitalizations. There were higher concentrations of all the biomarkers levels, and this effect was significant for TNF-α, VEGF and sFlt-1, when the duration of the current mood episode was shorter. |
| 44b. | van den Ameele et al. (2018) | 67 BD patients (42 BD-I, 23 BD-II, 2 schizoaffective disorder) (29 depressive episode, 29 (hypo)manic episode, 9 mixed episode) | 43.3 ± 11.1 (23–62 years) | 41.8%/58.2% | Severity of manic symptoms Severity of depressive symptoms Positive symptoms Subtype, phase of the disorder, comorbidity and other clinical features | YMRS HDRS-17 PANSS, positive subscale MINI-plus | TNF-α IFN-y IL-6 | No significant differences were found between levels of TNF-α, IFN-y or IL-6 and YMRS, HDRS, nor between PANSS scores in BD patients. No significant relations were found between any of the biomarkers or illness characteristics such as BD type I or II, mood state, duration of current mood state, number of mood episodes and number of hospitalizations, nor lifetime psychotic features. A longer duration of illness was associated with higher TNF-α. The older patient group (above 45 years old) had higher levels of IL-6 and TNF-α. |
| 45. | Wang et al. (2016) | 48 BD-II patients (placebo) 41 SBD (more than 2-days but less than 4-days duration of hypomania) patients (placebo) | 31.5 ± 11.3 BD-II 28.5 ± 10.2 SBD | 54.16%/45.83% BD-II 51.21%/48.78% SBD | History of subthreshold hypomania (SBD group) | SADS-L, Chinese version | TNF-α TGF-β 1 IL-6 IL-8 IL-1β BDNF | No significant differences were found in TNF-α, TGF-ß1 or IL-8 levels between the BD group and the SBD group at baseline. Significantly higher levels of IL-1ß but significantly lower levels of IL-6 and BDNF in the SBD group were found at baseline. Levels of BDNF were significantly lower in the SBD group (after 12 weeks of treatment). No other significant differences in biomarker levels between the BD and SBD group (after 12 weeks of treatment) were found. |
| 46. | Wang et al. (2016) | 737 BD patients (234 BD-I, 260 BD-II, 243 SBD) | 32.69 ± 12.20 (33.6 ± 11.7 BD-I, 31.6 ± 12.3 BD-II, 33.0 ± 12.6 SBD) | 47.48%/52.50% (49.14%/50.85% BD-I, 51.92%/48.07% BD-II, 41.15%/58.84% SBD) | Severity of manic and depressive symptoms Subtype, subthreshold hypomania (SBD group), comorbidity and other clinical features | YMRS HDRS SADS-L, Chinese version | BDNF TNF-α TGF-ß1 IL-8 | BDNF levels were not associated with bipolar patient groups (BD-I, BD-II, SBD). BD-II and SBD patients had lower levels of IL-8 than BD-I patients.There were no significant differences in TNF-α, TGF-ß1, or IL-8 levels between BD-II and SBD patients. There was a significant association between TNF-α levels and HDRS scores and between TGF-ß1 levels and HDRS and YMRS scores, length of illness and comorbidities. |
| 47. | Yoshimura et al. (2006) | 18 BD-I patients (12 manic episode, 6 depressive episode), with risperidone treatment during 4 weeks of follow-up | 34 ± 15 (23–51 years) | 44.44%/55.55% | Phase of the disorder | Clinical interview | BDNF | Levels of BDNF were significantly decreased in bipolar depressive patients compared with manic patients (before and after pharmacological treatment). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vega-Núñez, A.; Gómez-Sánchez-Lafuente, C.; Mayoral-Cleries, F.; Bordallo, A.; Rodríguez de Fonseca, F.; Suárez, J.; Guzmán-Parra, J. Clinical Value of Inflammatory and Neurotrophic Biomarkers in Bipolar Disorder: A Systematic Review and Meta-Analysis. Biomedicines 2022, 10, 1368. https://doi.org/10.3390/biomedicines10061368
Vega-Núñez A, Gómez-Sánchez-Lafuente C, Mayoral-Cleries F, Bordallo A, Rodríguez de Fonseca F, Suárez J, Guzmán-Parra J. Clinical Value of Inflammatory and Neurotrophic Biomarkers in Bipolar Disorder: A Systematic Review and Meta-Analysis. Biomedicines. 2022; 10(6):1368. https://doi.org/10.3390/biomedicines10061368
Chicago/Turabian StyleVega-Núñez, Amanda, Carlos Gómez-Sánchez-Lafuente, Fermín Mayoral-Cleries, Antonio Bordallo, Fernando Rodríguez de Fonseca, Juan Suárez, and José Guzmán-Parra. 2022. "Clinical Value of Inflammatory and Neurotrophic Biomarkers in Bipolar Disorder: A Systematic Review and Meta-Analysis" Biomedicines 10, no. 6: 1368. https://doi.org/10.3390/biomedicines10061368
APA StyleVega-Núñez, A., Gómez-Sánchez-Lafuente, C., Mayoral-Cleries, F., Bordallo, A., Rodríguez de Fonseca, F., Suárez, J., & Guzmán-Parra, J. (2022). Clinical Value of Inflammatory and Neurotrophic Biomarkers in Bipolar Disorder: A Systematic Review and Meta-Analysis. Biomedicines, 10(6), 1368. https://doi.org/10.3390/biomedicines10061368