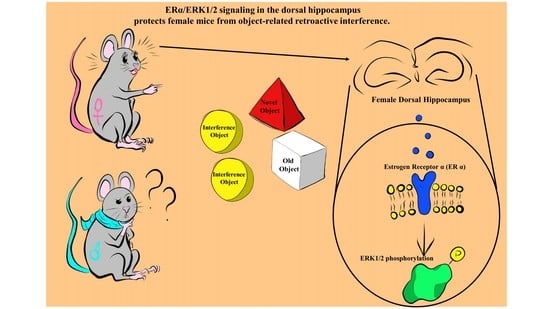
Hippocampal Estrogen Signaling Mediates Sex Differences in Retroactive Interference
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Ethics
2.3. Behavioral Analyses
2.4. Standard Novel Object Recognition Paradigm
2.5. Interference Paradigm
2.6. Surgery and Drug Administration
2.7. Western Blot
2.8. Statistical Analyses
3. Results
3.1. RI Induces Object-Memory Loss in Male but Not in Female C57bl/6 Mice
3.2. ERα Is Crucial for Resistance to RI in Female Mice
3.3. ERK1/2 Activation Plays a Critical Role in RI-Resistance of Female Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, M.C.; Neely, J.H. Interference and inhibition in memory retrieval. In Memory in Handbook of Perception and Cognition; Bjork, E.L., Bjork, R.A., Eds.; Academic Press: San Diego, CA, USA, 1996; pp. 237–313. [Google Scholar]
- Robertson, E.M. New insights in human memory interference and consolidation. Curr. Biol. 2012, 22, R66–R71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kliegl, O.; Bäuml, K.-H.T. The mechanisms underlying interference and inhibition: A review of current behavioral and neuroimaging research. Brain Sci. 2021, 11, 1246. [Google Scholar] [CrossRef] [PubMed]
- Wixted, J.T. The psychology and neuroscience of forgetting. Annu. Rev. Psychol. 2004, 55, 235–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medina, J.H. Neural, cellular and molecular mechanisms of active forgetting. Front. Syst. Neurosci. 2018, 12, 3. [Google Scholar] [CrossRef] [Green Version]
- Blondin, F.; Lepage, M. An fMRI study on memory discriminability for complex visual scenes. Hum. Brain Mapp. 2007, 29, 1159–1169. [Google Scholar] [CrossRef]
- Watson, H.C.; Lee, A.C.H. The perirhinal cortex and recognition memory interference. J. Neurosci. 2013, 33, 4192–4200. [Google Scholar] [CrossRef]
- Clarke, R.J.; Johnstone, T. Prefrontal inhibition of threat processing reduces working memory interference. Front. Hum. Neurosci. 2013, 7, 228. [Google Scholar] [CrossRef] [Green Version]
- Martínez, M.C.; Villar, M.E.; Ballarini, F.; Viola, H. Retroactive interference of object-in-context long-term memory: Role of dorsal hippocampus and medial prefrontal cortex. Hippocampus 2014, 24, 1482–1492. [Google Scholar] [CrossRef]
- Villar, M.E.; Martinez, M.C.; da Cunha, P.L.; Ballarini, F.; Viola, H. Memory consolidation and expression of object recognition are susceptible to retroactive interference. Neurobiol. Learn. Mem. 2017, 138, 198–205. [Google Scholar] [CrossRef]
- Teyler, T.J.; Rudy, J.W. The hippocampal indexing theory and episodic memory: Updating the index. Hippocampus 2007, 17, 1158–1169. [Google Scholar] [CrossRef]
- Kesner, R.P.; Rolls, E.T. A computational theory of hippocampal function, and tests of the theory: New developments. Neurosci. Biobehav. Rev. 2014, 48, 92–147. [Google Scholar] [CrossRef] [PubMed]
- Rajasethupathy, P.; Sankaran, S.; Marshel, J.; Kim, C.; Ferenczi, E.; Lee, S.Y.; Berndt, A.; Ramakrishnan, C.; Jaffe, A.; Lo, M.; et al. Projections from neocortex mediate top-down control of memory retrieval. Nature 2015, 526, 653–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loprinzi, P.D.; Frith, E. The role of sex in memory function: Considerations and recommendations in the context of exercise. J. Clin. Med. 2018, 7, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roof, R.L.; Stein, D.G. Gender differences in Morris water maze performance depend on task parameters. Physiol. Behav. 1999, 68, 81–86. [Google Scholar] [CrossRef]
- McCarthy, M.; Kusljic, S.; Gogos, A. The role of sex and sex steroids in the novel object recognition task. In Handbook of Object novelty Recognition; Ennaceur, A., de Souza Silva, M.A., Eds.; Elsevier Academic Press: Cambridge, MA, USA, 2018; pp. 499–529. [Google Scholar] [CrossRef]
- Florido, A.; Velasco, E.R.; Soto-Faguás, C.M.; Gomez-Gomez, A.; Perez-Caballero, L.; Molina, P.; Nadal, R.; Pozo, O.J.; Saura, C.A.; Andero, R. Sex differences in fear memory consolidation via Tac2 signaling in mice. Nat. Commun. 2021, 12, 2496. [Google Scholar] [CrossRef]
- Koss, W.A.; Haertel, J.M.; Philippi, S.M.; Frick, K.M. Sex differences in the rapid cell signaling mechanisms underlying the memory-enhancing effects of 17β-estradiol. eNeuro 2018, 5, 0267. [Google Scholar] [CrossRef] [Green Version]
- Yagi, S.; Galea, L.A.M. Sex differences in hippocampal cognition and neurogenesis. Neuropsychopharmacology 2019, 44, 200–213. [Google Scholar] [CrossRef] [Green Version]
- Gall, C.M.; Le, A.A.; Lynch, G. Sex differences in synaptic plasticity underlying learning. J. Neurosci. Res. 2021. [Google Scholar] [CrossRef]
- Taxier, L.R.; Gross, K.S.; Frick, K.M. Oestradiol as a neuromodulator of learning and memory. Nat. Rev. Neurosci. 2020, 21, 535–550. [Google Scholar] [CrossRef]
- Paciello, F.; Rinaudo, M.; Longo, V.; Cocco, S.; Conforto, G.; Pisani, A.; Podda, M.V.; Fetoni, A.R.; Paludetti, G.; Grassi, C. Auditory sensory deprivation induced by noise exposure exacerbates cognitive decline in a mouse model of Alzheimer’s disease. eLife 2021, 10, e70908. [Google Scholar] [CrossRef]
- Spinelli, M.; Natale, F.; Rinaudo, M.; Leone, L.; Mezzogori, D.; Fusco, S.; Grassi, C. Neural stem cell-derived exosomes revert HFD-dependent memory impairment via CREB-BDNF signalling. Int. J. Mol. Sci. 2020, 21, 8994. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Du, S.; Lv, L.; Lei, B.; Shi, W.; Tang, Y.; Wang, L.; Zhong, Y. Hippocampal activation of RAC1 regulates the forgetting of object recognition memory. Curr. Biol. 2016, 26, 2351–2357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-González, R.; Camacho-Arroyo, I.; Velasco, I. Progesterone and 17β-estradiol increase differentiation of mouse embryonic stem cells to motor neurons. IUBMB Life 2011, 63, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Al-Nakkash, L. Genistein stimulates jejunal chloride secretion via sex-dependent, estrogen receptor or adenylate cyclase mechanisms. Cell. Physiol. Biochem. 2012, 30, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Zheng, J.; Sun, X.; Deng, W.-K.; Li, B.M.; Liu, F. Prelimbic cortex extracellular signal-regulated kinase 1/2 activation is required for memory retrieval of long-term inhibitory avoidance. Brain Res. 2017, 1661, 88–99. [Google Scholar] [CrossRef]
- Natale, F.; Leone, L.; Rinaudo, M.; Sollazzo, R.; Barbati, S.A.; La Greca, F.; Spinelli, M.; Fusco, S.; Grassi, C. Neural stem cell-derived extracellular vesicles counteract insulin resistance-induced senescence of neurogenic niche. Stem Cells 2022, 40, 318–331. [Google Scholar] [CrossRef]
- Boenniger, M.M.; Staerk, C.; Coors, A.; Huijbers, W.; Ettinger, U.; Breteler, M.M.B. Ten German versions of Rey’s auditory verbal learning test: Age and sex effects in 4000 adults of the Rhineland Study. J. Clin. Exp. Neuropsychol. 2021, 43, 637–653. [Google Scholar] [CrossRef]
- Johnson, L.; Crawford, L.; Zou, L.; Loprinzi, P.D. Experimental effects of acute exercise in attenuating memory interference: Considerations by biological sex. Medicina 2019, 55, 331. [Google Scholar] [CrossRef] [Green Version]
- Dassanayake, T.L.; Hewawasam, C.; Baminiwatta, A.; Samarasekara, N.; Ariyasinghe, D.I. Sex-, age- and education-adjusted norms for the WHO/UCLA version of the Rey Auditory Verbal Learning Test for Sinhala-speaking Sri Lankan adults. Clin. Neuropsychol. 2020, 34, 127–142. [Google Scholar] [CrossRef]
- Sisson, E.D. Retroactive inhibition: The temporal position of interpolated activity. J. Exp. Psychol. 1939, 25, 228–233. [Google Scholar] [CrossRef]
- Postman, L.; Alper, T.G. Retroactive inhibition as a function of the time of interpolation of the inhibitor between learning and recall. Am. J. Psychol. 1946, 59, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Newton, J.M.; Wickens, D.D. Retroactive inhibition as a function of the temporal position of the interpolated learning. J. Exp. Psychol. 1956, 51, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Frick, K.M.; Gresack, J.E. Sex differences in the behavioral response to spatial and object novelty in adult C57BL/6 mice. Behav. Neurosci. 2003, 117, 1283–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceccarelli, I.; Scaramuzzino, A.; Aloisi, A.M. Effects of gonadal hormones and persistent pain on non-spatial working memory in male and female rats. Behav. Brain Res. 2001, 123, 65–76. [Google Scholar] [CrossRef]
- Benice, T.; Rizk, A.; Kohama, S.; Pfankuch, T.; Raber, J. Sex-differences in age-related cognitive decline in C57BL/6J mice associated with increased brain microtubule-associated protein 2 and synaptophysin immunoreactivity. Neuroscience 2006, 137, 413–423. [Google Scholar] [CrossRef]
- Walf, A.A.; Rhodes, M.E.; Frye, C.A. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol. Learn. Mem. 2006, 86, 35–46. [Google Scholar] [CrossRef] [Green Version]
- Sutcliffe, J.; Marshall, K.; Neill, J. Influence of gender on working and spatial memory in the novel object recognition task in the rat. Behav. Brain Res. 2007, 177, 117–125. [Google Scholar] [CrossRef]
- Paris, J.J.; Frye, C.A. Estrous cycle, pregnancy, and parity enhance performance of rats in object recognition or object placement tasks. Reproduction 2008, 136, 105–115. [Google Scholar] [CrossRef]
- van Goethem, N.P.; Rutten, K.; van der Staay, F.J.; Jans, L.A.; Akkerman, S.; Steinbusch, H.W.; Blokland, A.; van’t Klooster, J.V.; Prickaerts, J. Object recognition testing: Rodent species, strains, housing conditions, and estrous cycle. Behav. Brain Res. 2012, 232, 323–334. [Google Scholar] [CrossRef]
- Barbie-Shoshani, Y.; Shoham, S.; Bejar, C.; Weinstock, M. Sex-specific effects of prenatal stress on memory and markers of neuronal activity in juvenile rats. Dev. Neurosci. 2016, 38, 206–219. [Google Scholar] [CrossRef]
- Frick, K.M. Molecular mechanisms underlying the memory-enhancing effects of estradiol. Horm. Behav. 2015, 74, 4–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hojo, Y.; Hattori, T.-A.; Enami, T.; Furukawa, A.; Suzuki, K.; Ishii, H.-T.; Mukai, H.; Morrison, J.H.; Janssen, W.G.M.; Kominami, S.; et al. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017α and P450 aromatase localized in neurons. Proc. Natl. Acad. Sci. USA 2004, 101, 865–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vierk, R.; Glassmeier, G.; Zhou, L.; Brandt, N.; Fester, L.; Dudzinski, D.; Wilkars, W.; Bender, R.A.; Lewerenz, M.; Gloger, S.; et al. Aromatase inhibition abolishes LTP generation in female but not in male mice. J. Neurosci. 2012, 32, 8116–8126. [Google Scholar] [CrossRef] [PubMed]
- Alejandre-Gomez, M.; Garcia-Segura, L.M.; Gonzalez-Burgos, I. Administration of an inhibitor of estrogen biosynthesis facilitates working memory acquisition in male rats. Neurosci. Res. 2007, 58, 272–277. [Google Scholar] [CrossRef] [Green Version]
- Koss, W.A.; Frick, K.M. Activation of androgen receptors protects intact male mice from memory impairments caused by aromatase inhibition. Horm. Behav. 2019, 111, 96–104. [Google Scholar] [CrossRef]
- Pierman, S.; Sica, M.; Allieri, F.; Viglietti-Panzica, C.; Panzica, G.; Bakker, J. Activational effects of estradiol and dihydrotestosterone on social recognition and the arginine-vasopressin immunoreactive system in male mice lacking a functional aromatase gene. Horm. Behav. 2008, 54, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Fabian, C.; Tilzer, L.; Sternson, L. Comparative binding affinities of tamoxifen, 4-hydroxytamoxifen, and desmethyltamoxifen for estrogen receptors isolated from human breast carcinoma: Correlation with blood levels in patients with metastatic breast cancer. Biopharm. Drug Dispos. 1981, 2, 381–390. [Google Scholar] [CrossRef]
- Chen, D.; Wu, C.F.; Shi, B.; Xu, Y.M. Tamoxifen and toremifene cause impairment of learning and memory function in mice. Pharmacol. Biochem. Behav. 2002, 71, 269–276. [Google Scholar] [CrossRef]
- Chen, D.; Wu, C.-F.; Shi, B.; Xu, Y.-M. Tamoxifen and toremifene impair retrieval, but not acquisition, of spatial information processing in mice. Pharmacol. Biochem. Behav. 2002, 72, 417–421. [Google Scholar] [CrossRef]
- Esmaeili, B.; Basseda, Z.; Gholizadeh, S.; Paydar, M.J.; Dehpour, A.R. Tamoxifen disrupts consolidation and retrieval of morphine-associated contextual memory in male mice: Interaction with estradiol. Psychopharmacology 2009, 204, 191–201. [Google Scholar] [CrossRef]
- Wang, W.; Le, A.A.; Hou, B.; Lauterborn, J.C.; Cox, C.D.; Levin, E.R.; Lynch, G.; Gall, C.M. Memory-related synaptic plasticity is sexually dimorphic in rodent hippocampus. J. Neurosci. 2018, 38, 7935–7951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Frick, K.M. Distinct effects of estrogen receptor antagonism on object recognition and spatial memory consolidation in ovariectomized mice. Psychoneuroendocrinology 2017, 85, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Sinopoli, K.; Floresco, S.; Galea, L. Systemic and local administration of estradiol into the prefrontal cortex or hippocampus differentially alters working memory. Neurobiol. Learn. Mem. 2006, 86, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Oberlander, J.G.; Woolley, C.S. 17β-Estradiol acutely potentiates glutamatergic synaptic transmission in the hippocampus through distinct mechanisms in males and females. J. Neurosci. 2017, 37, 2677–2690. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.Z.; Woolley, C.S. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron 2012, 74, 801–808. [Google Scholar] [CrossRef] [Green Version]
- Fortress, A.M.; Fan, L.; Orr, P.T.; Zhao, Z.; Frick, K.M. Estradiol-induced object recognition memory consolidation is dependent on activation of mTOR signaling in the dorsal hippocampus. Learn. Mem. 2013, 20, 147–155. [Google Scholar] [CrossRef] [Green Version]
- Orr, P.T.; Rubin, A.J.; Fan, L.; Kent, B.A.; Frick, K.M. The progesterone-induced enhancement of object recognition memory consolidation involves activation of the extracellular signal-regulated kinase (ERK) and mammalian target of rapamycin (mTOR) pathways in the dorsal hippocampus. Horm. Behav. 2012, 61, 487–495. [Google Scholar] [CrossRef] [Green Version]
- Kahlert, S.; Nuedling, S.; van Eickels, M.; Vetter, H.; Meyer, R.; Grohé, C. Estrogen receptor α rapidly activates the IGF-1 receptor pathway. J. Biol. Chem. 2000, 275, 18447–18453. [Google Scholar] [CrossRef] [Green Version]
- Boulware, M.I.; Heisler, J.D.; Frick, K.M. The memory-enhancing effects of hippocampal estrogen receptor activation involve metabotropic glutamate receptor signaling. J. Neurosci. 2013, 33, 15184–15194. [Google Scholar] [CrossRef]
- Boulware, M.I.; Weick, J.P.; Becklund, B.R.; Kuo, S.P.; Groth, R.D.; Mermelstein, P.G. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J. Neurosci. 2005, 25, 5066–5078. [Google Scholar] [CrossRef]
- Spencer-Segal, J.; Tsuda, M.; Mattei, L.; Waters, E.; Romeo, R.; Milner, T.; McEwen, B.; Ogawa, S. Estradiol acts via estrogen receptors alpha and beta on pathways important for synaptic plasticity in the mouse hippocampal formation. Neuroscience 2012, 202, 131–146. [Google Scholar] [CrossRef] [Green Version]
- Wortzel, I.; Seger, R. The ERK Cascade: Distinct functions within various subcellular organelles. Genes Cancer 2011, 2, 195–209. [Google Scholar] [CrossRef]
- Medina, J.H.; Viola, H. ERK1/2: A key cellular component for the formation, retrieval, reconsolidation and persistence of memory. Front. Mol. Neurosci. 2018, 11, 361. [Google Scholar] [CrossRef] [PubMed]
- Zamorano, C.; Fernandez-Albert, J.; Storm, D.R.; Carné, X.; Sindreu, C. Memory retrieval re-activates Erk1/2 signaling in the same set of CA1 neurons recruited during conditioning. Neuroscience 2018, 370, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Mielke, M.M. Sex and gender differences in Alzheimer’s disease dementia. Psychiatr. Times 2018, 35, 14–17. [Google Scholar] [PubMed]
- Loewenstein, D.A.; Acevedo, A.; Luis, C.; Crum, T.; Barker, W.W.; Duara, R. Semantic interference deficits and the detection of mild Alzheimer’s disease and mild cognitive impairment without dementia. J. Int. Neuropsychol. Soc. 2004, 10, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Dewar, M.; Pesallaccia, M.; Cowan, N.; Provinciali, L.; Della Sala, S. Insights into spared memory capacity in amnestic MCI and Alzheimer’s Disease via minimal interference. Brain Cogn. 2012, 78, 189–199. [Google Scholar] [CrossRef]
- Gunes, S.; Aizawa, Y.; Sugashi, T.; Sugimoto, M.; Rodrigues, P.P. Biomarkers for Alzheimer’s disease in the current state: A narrative review. Int. J. Mol. Sci. 2022, 23, 4962. [Google Scholar] [CrossRef]
- Munetsuna, E.; Hojo, Y.; Hattori, M.; Ishii, H.; Kawato, S.; Ishida, A.; Kominami, S.A.J.; Yamazaki, T. Retinoic acid stimulates 17β-estradiol and testosterone synthesis in rat hippocampal slice cultures. Endocrinology 2009, 150, 4260–4269. [Google Scholar] [CrossRef] [Green Version]
- Biyong, E.F.; Tremblay, C.; Leclerc, M.; Caron, V.; Alfos, S.; Helbling, J.-C.; Rodriguez, L.; Pernet, V.; Bennett, D.A.; Pallet, V.; et al. Role of Retinoid X Receptors (RXRs) and dietary vitamin A in Alzheimer’s disease: Evidence from clinicopathological and preclinical studies. Neurobiol. Dis. 2021, 161, 105542. [Google Scholar] [CrossRef]



| Group | Training | Interference | Test |
|---|---|---|---|
| Males (Std-NOR; n = 9) | 17.0 ± 5.8 s | not applicable | 17.3 ± 6.1 s |
| Males (PI; n = 11) | 18.2 ± 4.9.2 s | 17.6 ± 4.6 s | 16.6 ± 4.7 s |
| Males (RI; n = 8) | 20.9 ± 5.9 s | 20.7 ± 6.5 s | 20.6 ± 6.6 s |
| Females (Std-NOR; n = 10) | 15.8 ± 4.3 s | not applicable | 16.2 ± 5.9 s |
| Females (PI; n = 10) | 19.0 ± 5.6 s | 17.6 ± 6.2 | 20.3 ± 3.1 s |
| Females (RI; n = 8) | 18.6 ± 4.4 s | 15.0 ± 6.5 s | 15.7 ± 6.1 s |
| Group | Training | Interference | Test |
|---|---|---|---|
| Veh (n = 8) | 21.5 ± 13.2 s | not applicable | 23.3 ± 10.0 s |
| 4-OHT (n = 9) | 23.5 ± 8.5 s | 19.6 ± 7.1 s | 21.6 ± 5.0 s |
| Veh (n = 8) | 20.2 ± 5.8 s | 24.7 ± 3.8 s | 20.1 ± 4.4 s |
| MPP (n = 8) | 22.3 ± 10.1 s | 23.3 ± 11.6 | 21.3 ± 7.9 s |
| PHTPP (n = 8) | 21.7 ± 2.4 s | 22.4 ± 4.2 | 23.9 ± 5.2 s |
| Group | Training | Interference | Test |
|---|---|---|---|
| Veh (n = 8) | 21.6 ± 5.0 s | not applicable | 20.3 ± 6.1 s |
| PD98059 (n = 8) | 20.3 ± 3.9 s | 22.3 ± 8.9 s | 23.4 ± 10.3 s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rinaudo, M.; Natale, F.; La Greca, F.; Spinelli, M.; Farsetti, A.; Paciello, F.; Fusco, S.; Grassi, C. Hippocampal Estrogen Signaling Mediates Sex Differences in Retroactive Interference. Biomedicines 2022, 10, 1387. https://doi.org/10.3390/biomedicines10061387
Rinaudo M, Natale F, La Greca F, Spinelli M, Farsetti A, Paciello F, Fusco S, Grassi C. Hippocampal Estrogen Signaling Mediates Sex Differences in Retroactive Interference. Biomedicines. 2022; 10(6):1387. https://doi.org/10.3390/biomedicines10061387
Chicago/Turabian StyleRinaudo, Marco, Francesca Natale, Francesco La Greca, Matteo Spinelli, Antonella Farsetti, Fabiola Paciello, Salvatore Fusco, and Claudio Grassi. 2022. "Hippocampal Estrogen Signaling Mediates Sex Differences in Retroactive Interference" Biomedicines 10, no. 6: 1387. https://doi.org/10.3390/biomedicines10061387
APA StyleRinaudo, M., Natale, F., La Greca, F., Spinelli, M., Farsetti, A., Paciello, F., Fusco, S., & Grassi, C. (2022). Hippocampal Estrogen Signaling Mediates Sex Differences in Retroactive Interference. Biomedicines, 10(6), 1387. https://doi.org/10.3390/biomedicines10061387