Isolation of Hepatocytes from Liver Tissue by a Novel, Semi-Automated Perfusion Technology
Abstract
:1. Introduction
2. Results
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Step-by-Step Protocol for the Isolation of Primary Hepatocytes with the gentleMACS Perfusion Technology
- Kit Components: The Liver Perfusion Kit, mouse and rat (Miltenyi Biotec; # 130-128-030) contains the following six components, which are suitable for 25 digestions:
- 1 vial of Enzyme D (lyophilized powder)
- 1 vial of Enzyme R (lyophilized powder)
- 1 vial of Enzyme A (lyophilized powder)
- 100 mL Buffer P (20×)
- 1 mL Reagent C
- 1 mL Reagent E
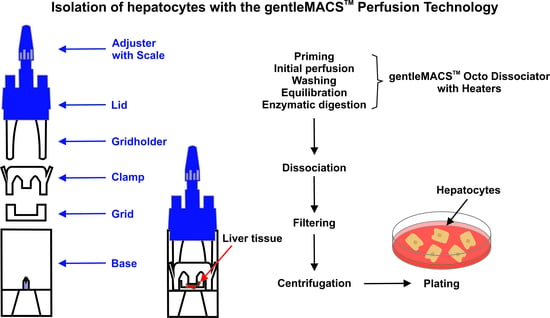
- Principle: The gentleMACS Perfusion Technology allows the disaggregation of mouse liver tissue into highly viable single-cell suspensions. The perfusion is performed on the gentleMACS Octo Dissociator with Heaters equipped with gentleMACS Perfusion Sleeves in combination with gentleMACS Perfusers. The liver tissue is enzymatically digested using the components of the Liver Perfusion Kit, thereby loosening the structural integrity of the extracellular matrix in the tissue during the perfusion process. Afterwards, single cells are liberated from the tissue by a short mechanical disruption of the perfused tissue using the gentleMACS Octo Dissociator with Heaters and a gentleMACS C Tube. The sample is then applied to a MACS SmartStrainer to remove any remaining larger particles from the single-cell suspension. Cells should be processed immediately for downstream applications, such as cell separation, cell culture, and cellular or molecular analyses.
- Background: The Liver Perfusion Kit in combination with the gentleMACS Perfusion Technology is suitable for the gentle, rapid, and efficient generation of single-cell suspensions from mouse liver. It is optimized for a high yield of hepatocytes, while preserving most cell surface epitopes.
- Additional Reagents
- Sterile, distilled water
- Phosphate-buffered saline (PBS), pH 7.2
- Culture medium (Dulbecco’s modified Eagle Medium containing 5% fetal bovine serum (FBS), 2 mM L-glutamine, 100 IU/mL penicillin, and 100 µg/mL streptomycin)
- Additional Disposables
- Disposable glass Pasteur pipettes with elongated tip (230 mm)
- 6 cm and 10 cm petri dishes
- Cell culture dishes (in desired format)
- 15 and 50 mL reagent tubes
- MACS SmartStrainers (100 μm) (Miltenyi, #130-098-463)
- gentleMACS C Tubes (Miltenyi, #130-093-237)
- gentleMACS Perfusers (Miltenyi, #130-128-151)
- gentleMACS Perfusion Sleeves (Miltenyi, #130-128-752)
- Additional Instruments
- Surgical instrument set
- Heatable water bath
- Liquid suction pump
- Cooling centrifuge with a swinging bucket rotor
- gentleMACS Octo Dissociator with Heaters (Miltenyi, #130-096-427)
- Preparation of Reagents from the Liver Perfusion Kit
- Pre-heat water bath at 37 °C.
- Warm sterile Buffer P (20×) at 37 °C for at least 45 min before use.
- Prepare Reconstitution buffer: The reconstitution buffer is prepared by diluting 400 μL Buffer P (20×) with 7.6 mL sterile water. Add 16 μL Reagent C and mix well.
- Reconstitute Enzyme D solution: Enzyme D solution is prepared by reconstitution of the lyophilized powder in the vial with 3 mL reconstitution buffer. After closing the lid, the vial is inverted to dissolve the enzyme for 5–10 min. The solution is sterile-filtered and aliquots can be stored at –20 °C for up to 6 months.
- Reconstitute Enzyme R solution: Enzyme R solution is prepared by reconstitution of the lyophilized powder in the vial with 3 mL reconstitution buffer. After closing the lid, the vial is inverted to dissolve the enzyme for 5–10 min. After the enzyme is dissolved, sterile-filter the solution. Aliquots can be stored at −20 °C for up to 6 months.
- Reconstitute Enzyme A solution: Enzyme A solution is prepared by sterile reconstitution of the lyophilized powder in the vial with 1 mL reconstitution buffer. Aliquots can be stored at −20 °C for up to 6 months.
- Prepare pre-digestion buffer: For each isolation, prepare 62 mL Buffer P (1×) by diluting 3.1 mL Buffer P (20×) with 58.9 mL sterile, distilled water. Store buffer at 37 °C.
- Prepare equilibration buffer: Add 36 µL Reagent C in 18 mL pre-digestion buffer. Transfer 9 mL of that buffer to a separate tube. This will be later used to prepare the enzyme digestion mix. Store both 9 mL buffer aliquots at 37 °C.
- Experimental Setup and Preparation of Instrument and gentleMACS Perfuser
- Exchange standard sleeves with gentleMACS Perfusion Sleeves on the gentleMACS Octo Dissociator with Heaters.
- Remove Grid and Clamp from the gentleMACS Perfuser (Figure 2A) and place the Base with the Lid of the Perfuser on the instrument. Leave out the Grid and Clamp. Note: Ensure the Adjuster is in the correct (delivered) position according to the tissue to be perfused (must not be changed in the case of mouse perfusion).
- Transfer 8 mL of warm pre-digestion buffer via one of the Luer lock openings using a 10 mL pipette (Figure 2G,H).
- Place a Heating Unit onto the gentleMACS Perfuser and start the program 37C_m_LIPK_1 (Priming: 5 s; Initial perfusion: 30 s; Washing: 3 × 30 s, 1 × 13 min; Equilibration: 30 s; Enzymatic perfusion: 10 min) (Figure 2F,I). Note: The program will automatically pause after the priming step.
- Put the Grid of the Perfuser into a sterile 10 cm petri dish.
- Perfusion Procedure
- Sacrifice the mouse.
- Carefully dissect an appropriate liver lobe (left lateral lobe is preferred) and rinse with PBS buffer. Note: Make sure that the liver lobe is not injured during dissection.
- Place the liver lobe in the center of the Grid using tweezers (see Figure 2B).
- Squeeze the Clamp and attach it into the Grid by pushing it all the way down to the petri dish (see Figure 2C).
- Remove the Lid from the Base and attach its Grid-holder channels through the Clamp into the Grid (Figure 2D). Note: A click is audible on each side of the two channels.
- Place the assembly of Lid, Grid, Clamp, and liver tissue onto the installed Base.
- Slowly turn the Lid counterclockwise without pressure until it reaches a lower position. Then, close Lid turning clockwise without tilting (Figure 2E).
- Click the respective position and resume the program to start the 30 s initial perfusion.
- After the initial perfusion step, select the flashing pause position.
- Remove used buffer from the Perfuser with a disposable elongated Pasteur pipette (230 mm) connected to a suction pump. This washing phase consists of 3 short cycles (30 s) and one long cycle (1 × 13 min) with each 8 mL pre-digestion buffer. For buffer exchange, the program will pause after each cycle. During the pause steps, remove the used pre-digestion buffer manually with the glass Pasteur pipette, add new pre-digestion buffer and resume the program. At the last washing step, remove the used pre-digestion buffer.
- Add 8 mL of warm equilibration buffer and resume the program.
- Prepare the enzyme digestion mix. For one perfusion add 110 μL Enzyme D, 110 μL Enzyme R, and 30 μL Enzyme A to the 9 mL pre-warmed equilibration buffer (see Preparation of Reagents from the Liver Perfusion Kit, step 8).
- Select paused position and remove the used buffer with the Pasteur pipette.
- Add 8 mL of warm enzyme digestion mix and resume program.
- After the enzymatic perfusion step (10 min), resume to finish the gentleMACS Program.
- Remove Heating Unit and Perfuser from instrument.
- Place Lid, Grid, and Clamp assembly in a petri dish.
- Transfer used enzyme digestion mix to a gentleMACS C Tube (Figure 2J–L).
- Lift Clamp with tweezers to remove perfused liver.
- Transfer the perfused tissue to the gentleMACS C Tube containing the used enzyme digestion mix and close the C Tube tightly (Figure 2M).
- Place gentleMACS C Tube upside-down in one position of the gentleMACS Dissociator equipped with a standard sleeve (Figure 2N).
- Run the gentleMACS program LIPK_HR_1 that releases the hepatocytes from the perfused tissue within 5 min (Figure 2O).
- Put a 15 mL tube on ice and place a MACS SmartStrainer (100 μm) on it.
- After termination of the program, remove the gentleMACS C Tube from the gentleMACS Dissociator.
- Open the gentleMACS C Tube and filter cell suspension through a MACS 100 µm SmartStrainer (Figure 2P). Note: Avoid producing air bubbles.
- Wash the C Tube with 6 mL culture medium and pour this solution on the MACS SmartStrainer.
- Centrifuge the cell suspension at 30× g for 5 min at 4 °C. A pellet containing hepatocytes will form (Figure 2Q).
- Remove the supernatant completely. Note: This solution is enriched in non-parenchymal cells.
- Carefully re-suspend the cell pellet consisting of hepatocytes by slowly pipetting up and down in an appropriate buffer for downstream application. Note: At this step you can use 5 mL cold culture medium.
- Count cells in a Neubauer chamber and plate required number of cells into an appropriate culture dish.
- Remarks
- A maximum of 1.2 g of liver tissue can be perfused in one gentleMACS Perfuser.
- The time from the dissection of the liver to the start of the perfusion should not exceed 10 min.
- It is recommended to use the biggest mouse liver lobe (left lateral lobe) instead of the whole mouse liver.
- The most critical step is screwing the Lid onto the Base. Do not use too much pressure during this step as the Clamp must not be lifted unless perfusion has been finished.
- A video showing the complete procedure for isolation of hepatocytes from mouse liver using the gentleMACS Perfusion Technology was launched at [26].
References
- Rose, S.; Ezan, F.; Cuvellier, M.; Bruyère, A.; Legagneux, V.; Langouët, S.; Baffet, G. Generation of proliferating human adult hepatocytes using optimized 3D culture conditions. Sci. Rep. 2021, 11, 515. [Google Scholar] [CrossRef] [PubMed]
- Ward, L.; Wilcock, J.; Butler, P. Drug metabolism assessment: Hepatocytes. In The ADME Encyclopedia; Talevi, A., Ed.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Sahi, J.; Grepper, S.; Smith, C. Hepatocytes as a tool in drug metabolism, transport and safety evaluations in drug discovery. Curr. Drug Discov. Technol. 2010, 7, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.C.; Kraaier, L.J.; Kluiver, T.A. Hepatocyte organoids and cell transplantation: What the future holds. Exp. Mol. Med. 2021, 53, 1512–1528. [Google Scholar] [CrossRef] [PubMed]
- Gamboa, C.M.; Wang, Y.; Xu, H.; Kalemba, K.; Wondisford, F.E.; Sabaawy, H.E. Optimized 3D culture of hepatic cells for liver organoid metabolic assays. Cells 2021, 10, 3280. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Sebastian, S.; Maharjan, S.; Lesha, A.; Carpenter, A.M.; Liu, X.; Xie, X.; Livermore, C.; Zhang, Y.S.; Zarrinpar, A. Liver-on-a-Chip Models of Fatty Liver Disease. Hepatology 2020, 71, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Seglen, P.O. Preparation of isolated rat liver cells. Methods Cell. Biol. 1976, 13, 29–83. [Google Scholar] [CrossRef] [PubMed]
- USDA. Animal and Plant Health Inspection Service. Research Facility Annual Summary & Archive Reports. Available online: https://www.aphis.usda.gov/aphis/ourfocus/animalwelfare/sa_obtain_research_facility_annual_report/ct_research_facility_annual_summary_reports (accessed on 25 August 2022).
- National Research Council (US) Committee on Recognition and Alleviation of Pain in Laboratory Animals. Recognition and Alleviation of Pain in Laboratory Animals; US Regulations and Guidelines Regarding Recognition and Alleviation of Pain in Laboratory Animals; National Academies Press (US): Washington, DC, USA, 2009. Available online: https://www.ncbi.nlm.nih.gov/books/NBK32657/ (accessed on 25 August 2022).
- EUR-Lex. Access to European Union Law. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes Text with EEA Relevance. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32010L0063&from=EN (accessed on 25 August 2022).
- Borkham-Kamphorst, E.; Huss, S.; Van de Leur, E.; Haas, U.; Weiskirchen, R. Adenoviral CCN3/NOV gene transfer fails to mitigate liver fibrosis in an experimental bile duct ligation model because of hepatocyte apoptosis. Liver Int. 2012, 32, 1342–1353. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Zhao, M.; Svensson, K.J. Isolation, culture, and functional analysis of hepatocytes from mice with fatty liver disease. STAR Protoc. 2020, 1, 100222. [Google Scholar] [CrossRef] [PubMed]
- Charni-Natan, M.; Goldstein, I. Protocol for primary mouse hepatocyte isolation. STAR Protoc. 2020, 1, 100086. [Google Scholar] [CrossRef] [PubMed]
- Mitry, R.R. Isolation of human hepatocytes. Methods Mol. Biol. 2009, 481, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Green, C.J.; Charlton, C.A.; Wang, L.M.; Silva, M.; Morten, K.J.; Hodson, L. The isolation of primary hepatocytes from human tissue: Optimising the use of small non-encapsulated liver resection surplus. Cell Tissue Bank 2017, 18, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Schelcher, C.; Demmel, M.; Hauner, M.; Thasler, W.E. Isolation of human hepatocytes by a two-step collagenase perfusion procedure. J. Vis. Exp. 2013, 79, 50615. [Google Scholar] [CrossRef] [PubMed]
- Kawai, M.; Saegusa, Y.; Kemmochi, S.; Harada, T.; Shimamoto, K.; Shibutani, M.; Mitsumori, K. Cytokeratin 8/18 is a useful immunohistochemical marker for hepatocellular proliferative lesions in mice. J. Vet. Med. Sci. 2010, 72, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Itoh, M.; Terada, M.; Sugimoto, H. The zonula occludens protein family regulates the hepatic barrier system in the murine liver. Biochim. Biophys Acta Mol. Basis Dis. 2021, 1867, 165994. [Google Scholar] [CrossRef] [PubMed]
- Walesky, C.; Apte, U. Role of hepatocyte nuclear factor 4α (HNF4α) in cell proliferation and cancer. Gene Expr. 2015, 16, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Borkham-Kamphorst, E.; Drews, F.; Weiskirchen, R. Induction of lipocalin-2 expression in acute and chronic experimental liver injury moderated by pro-inflammatory cytokines interleukin-1β through nuclear factor-κB activation. Liver Int. 2011, 31, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xue, J.; Yang, Y.; Zhou, X.; Qin, C.; Zheng, M.; Zhu, H.; Liu, Y.; Liu, W.; Lou, G.; et al. Lipocalin 2 upregulation protects hepatocytes from IL1-β-induced stress. Cell. Physiol. Biochem. 2015, 36, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Vazquez, J.T.; Boulger, E.; Liu, H.; Xue, P.; Hussain, M.A.; Wolfe, A. Hepatic estrogen receptor α is critical for regulation of gluconeogenesis and lipid metabolism in males. Sci. Rep. 2017, 7, 1661. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Reprinted 1992; Universities Federation for Animal Welfare: Wheathampstead, UK, 1959; Available online: https://caat.jhsph.edu/principles/the-principles-of-humane-experimental-technique (accessed on 25 August 2022).
- Baker, M. 1500 scientists lift the lid on reproducibility. Nature 2016, 533, 452–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirsch, C.; Schildknecht, S. In vitro research reproducibility: Keeping up high standards. Front. Pharmacol. 2019, 10, 1484. [Google Scholar] [CrossRef] [PubMed]
- Miltenyi Biotec’s Video Library. How to Perfuse Mouse Liver with the gentleMACS Perfusion Technology; Isolating Hepatocytes from Mouse Liver Has Never Been Easier! gentleMACSTM Perfusion Technology Makes It a Breeze to Isolate Hepatocytes from Rodent Liver. Available online: https://www.miltenyibiotec.com/tutorial-rodent-liver-perfusion (accessed on 25 August 2022).






| Number of Animals Used | Strain | Age (Weeks) | Total Hepatocyte Yield ** | Hepatocyte Yield/g ** | Viability (%) *** |
|---|---|---|---|---|---|
| n = 2 | CD1 | 5 | 7.0 × 106 | 1.5 × 107 | 81 |
| 1.9 × 107 | 5.8 × 107 | 91 | |||
| n = 2 | BALB/c | 9 | 1.5 × 107 | 4.5 × 107 | 85 |
| 5.5 × 106 | 1.7 × 107 | 87 | |||
| n = 2 | BALB/c | 8 | 1.1 × 107 | 3.9 × 107 | 90 |
| 6.7 × 106 | 1.9 × 107 | 88 | |||
| n = 2 | C57BL/6 | 12 | 1.4 × 107 | 2.9 × 107 | 88 |
| 9.6 × 106 | 2.0 × 107 | 88 | |||
| n = 2 | BALB/c | 10 | 1.2 × 107 | 3.0 × 107 | 93 |
| 1.2 × 107 | 3.5 × 107 | 90 | |||
| n = 4 | BALB/c | 10 | 9.5 × 106 | 3.2 × 107 | 93 |
| 3.2 × 106 | 1.1 × 107 | 86 | |||
| 1.1 × 107 | 3.8 × 107 | 93 | |||
| 1.5 × 107 | 4.8 × 107 | 92 | |||
| n = 4 | C57BL/6 | 9 | 1.1 × 107 | 3.5 × 107 | 91 |
| 6.2 × 106 | 1.7 × 107 | 85 | |||
| 1.4 × 107 | 4.1 × 107 | 91 | |||
| 9.8 × 106 | 2.6 × 107 | 95 | |||
| n = 8 | BALB/c | 8 | 1.2 × 107 | 3.4 × 107 | 91 |
| 1.0 × 107 | 2.5 × 107 | 91 | |||
| 1.6 × 107 | 5.4 × 107 | 90 | |||
| 1.1 × 107 | 3.3 × 107 | 93 | |||
| 7.8 × 106 | 2.4 × 107 | 90 | |||
| 1.3 × 107 | 4.1 × 107 | 85 | |||
| 1.5 × 107 | 4.3 × 107 | 90 | |||
| 7.2 × 106 | 1.9 × 107 | 84 | |||
| n = 3 | BALB/c | 6 | 9.3 × 106 | 2.3 × 107 | 84 |
| 1.1 × 107 | 3.0 × 107 | 83 | |||
| 7.4 × 106 | 2.2 × 107 | 86 | |||
| n = 4 | C57BL/6 | 9 | 1.4 × 107 | 4.6 × 107 | 90 |
| 1.7 × 107 | 5.2 × 107 | 94 | |||
| 1.0 × 107 | 5.9 × 107 | 90 | |||
| 1.7 × 107 | 5.6 × 107 | 88 |
| Feature | Conventional In Vivo Perfusion | gentleMACS Perfusion Technology |
|---|---|---|
| Approach | in situ | ex vivo |
| Perfusion environment | anaesthetized animal | resected tissue |
| Animals | best working with adult animals (cannulation of veins is necessary) | flexible in regard to age of animals (no cannulation of veins necessary) |
| Animal ethics approval | necessary | not needed |
| Sample material flexibility | no (whole organ) | yes (lobe rather than whole organ is recommended) |
| Perfusion enzymes | not standardized (lot-to-lot variations, activities must be determined by customer) | standardized (consistent enzyme activities contained in the corresponding Liver Perfusion Kit) |
| (Difficult) manual cannulation of liver vessels needed | yes | not needed |
| Sample processed in a closed system | no | yes |
| Perfusion steps and volumes (mL) | highly variable | 7 × 8 |
| Collection of used perfusion liquid | no | yes |
| Other lobes of the same liver available for other experiments | no (at least even more difficult to process) | yes |
| Robustness of needle insertion into the tissue | low-high (depending on user skills) | high (automatically done by the consumable) |
| Flow rate during perfusion | to be set by user | predefined by the device |
| Sterility of equipment | no (sterilization needs preparation time) | yes (usage of disposable items) |
| Sample size limitation | no (open system) | yes (closed system) |
| Needed instrumentation | peristaltic pump with tubing | gentleMACS Octo Dissociator with Heaters and gentleMACS Perfusion Sleeve |
| Needed one-way consumables | syringe needle, buffer reservoirs | gentleMACS Perfuser (for perfusion) and gentleMACS C Tube (for dissociation of perfused liver) |
| Ease of parallelization of perfusion process | low (would require additional peristaltic pumps and complex nesting of work steps) | high (Up to 8 cell preparations can be performed simultaneously on one instrument) |
| Disruption of perfused liver tissue | manually (mincing of tissue with forceps) | automated (within C Tube driven by instrument) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poggel, C.; Adams, T.; Janzen, R.; Hofmann, A.; Hardt, O.; Roeb, E.; Schröder, S.K.; Tag, C.G.; Roderfeld, M.; Weiskirchen, R. Isolation of Hepatocytes from Liver Tissue by a Novel, Semi-Automated Perfusion Technology. Biomedicines 2022, 10, 2198. https://doi.org/10.3390/biomedicines10092198
Poggel C, Adams T, Janzen R, Hofmann A, Hardt O, Roeb E, Schröder SK, Tag CG, Roderfeld M, Weiskirchen R. Isolation of Hepatocytes from Liver Tissue by a Novel, Semi-Automated Perfusion Technology. Biomedicines. 2022; 10(9):2198. https://doi.org/10.3390/biomedicines10092198
Chicago/Turabian StylePoggel, Carsten, Timo Adams, Ronald Janzen, Alexander Hofmann, Olaf Hardt, Elke Roeb, Sarah K. Schröder, Carmen G. Tag, Martin Roderfeld, and Ralf Weiskirchen. 2022. "Isolation of Hepatocytes from Liver Tissue by a Novel, Semi-Automated Perfusion Technology" Biomedicines 10, no. 9: 2198. https://doi.org/10.3390/biomedicines10092198
APA StylePoggel, C., Adams, T., Janzen, R., Hofmann, A., Hardt, O., Roeb, E., Schröder, S. K., Tag, C. G., Roderfeld, M., & Weiskirchen, R. (2022). Isolation of Hepatocytes from Liver Tissue by a Novel, Semi-Automated Perfusion Technology. Biomedicines, 10(9), 2198. https://doi.org/10.3390/biomedicines10092198