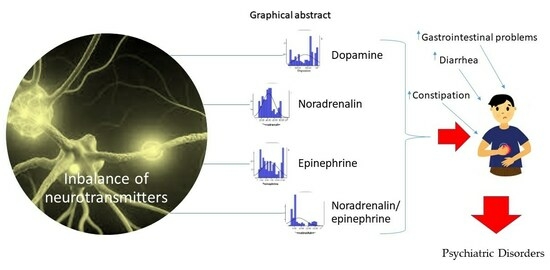
Catecholamine Variations in Pediatric Gastrointestinal Disorders and Their Neuropsychiatric Expression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Clinical Analysis
2.3. Paraclinical Analysis
2.4. Statistical Analysis
3. Results
3.1. Catecholamine Levels
3.1.1. Dopamine
3.1.2. Noradrenaline
3.1.3. Epinephrine
3.1.4. Noradrenaline/Epinephrine
3.2. Gastrointestinal Disorders in Patients with Neuropsychiatric Manifestations
3.3. Correlations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orr, W.C.; Crowell, M.D.; Lin, B.; Harnish, M.J.; Chen, J.D. Sleep and gastric function in irritable bowel syndrome: Derailing the brain-gut axis. Gut 1997, 41, 390–393. [Google Scholar] [CrossRef]
- Kaur, G.; Behl, T.; Bungau, S.; Kumar, A.; Uddin, S.M.; Mehta, V.; Zengin, G.; Mathew, B.; Shah, A.M.; Arora, S. Dysregulation of the Gut-Brain Axis, Dysbiosis and Influence of Numerous Factors on Gut Microbiota Associated Parkinson’s Disease. Curr. Neuropharmacol. 2021, 19, 233–247. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, I.; Sehgal, A.; Kumar, A.; Uddin, M.S.; Bungau, S. The Interplay of ABC Transporters in Aβ Translocation and Cholesterol Metabolism: Implicating Their Roles in Alzheimer’s Disease. Mol. Neurobiol. 2021, 58, 1564–1582. [Google Scholar] [CrossRef]
- Lagier, J.-C.; Dubourg, G.; Million, M.; Cadoret, F.; Bilen, M.; Fenollar, F.; Levasseur, A.; Rolain, J.-M.; Fournier, P.-E.; Raoult, D. Culturing the human microbiota and culturomics. Nat. Rev. Microbiol. 2018, 16, 540–550. [Google Scholar] [CrossRef]
- Șchiopu, C.G.; Ștefănescu, C.; Boloș, A.; Diaconescu, S.; Gilca-Blanariu, G.-E.; Ștefănescu, G. Functional Gastrointestinal Disorders with Psychiatric Symptoms: Involvement of the Microbiome–Gut–Brain Axis in the Pathophysiology and Case Management. Microorganisms 2022, 10, 2199. [Google Scholar]
- Anand, N.; Gorantla, V.R.; Chidambaram, S.B. The Role of Gut Dysbiosis in the Pathophysiology of Neuropsychiatric Disorders. Cells 2022, 12, 54. [Google Scholar] [CrossRef]
- Henjum, K.; Watne, L.O.; Godang, K.; Halaas, N.B.; Eldholm, R.S.; Blennow, K.; Zetterberg, H.; Saltvedt, I.; Bollerslev, J.; Knapskog, A.B. Cerebrospinal fluid catecholamines in Alzheimer’s disease patients with and without biological disease. Transl. Psychiatry 2022, 12, 151. [Google Scholar] [CrossRef]
- Neufeld, K.; Kang, N.; Bienenstock, J.; Foster, J.A. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 2011, 23, 255-e119. [Google Scholar] [CrossRef]
- Martin, C.R.; Osadchiy, V.; Kalani, A.; Mayer, E.A. The Brain-Gut-Microbiome Axis. Cell Mol. Gastroenterol. Hepatol. 2018, 6, 133–148. [Google Scholar] [CrossRef]
- Juárez Olguín, H.; Calderón Guzmán, D.; Hernández García, E.; Barragán Mejía, G. The Role of Dopamine and Its Dysfunction as a Consequence of Oxidative Stress. Oxid. Med. Cell Longev. 2016, 2016, 9730467. [Google Scholar] [CrossRef]
- Lewis, R.G.; Florio, E.; Punzo, D.; Borrelli, E. The Brain’s Reward System in Health and Disease. Adv. Exp. Med. Biol. 2021, 1344, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Leoni, J.; Owen, D. From Stress to Happiness. In Handbook of Happiness: Reflections and Praxis from around the World; Springer: Berlin/Heidelberg, Germany, 2023; pp. 339–361. [Google Scholar]
- Ahima, R.S.; Antwi, D.A. Brain regulation of appetite and satiety. Endocrinol. Metab. Clin. N. Am. 2008, 37, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Iversen, L.L. Dopamine Handbook; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Otte, C.; Neylan, T.C.; Pipkin, S.S.; Browner, W.S.; Whooley, M.A. Depressive symptoms and 24-hour urinary norepinephrine excretion levels in patients with coronary disease: Findings from the Heart and Soul Study. Am. J. Psychiatry 2005, 162, 2139–2145. [Google Scholar] [CrossRef]
- Thomas, D.H.; Taylor, J.D.; Barnaby, O.S.; Hage, D.S. Determination of free catecholamines in urine by tandem affinity/ion-pair chromatography and flow injection analysis. Clin. Chim. Acta 2008, 398, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Wang, F.; Zhang, K.; Wang, D.; Wang, X.; Li, X.; Zhang, J. l-Theanine Inhibits (−)-Epigallocatechin-3-gallate Oxidation via Chelating Copper. J. Agric. Food Chem. 2022, 70, 7751–7761. [Google Scholar] [CrossRef]
- Bloomfield, M.A.; McCutcheon, R.A.; Kempton, M.; Freeman, T.P.; Howes, O. The effects of psychosocial stress on dopaminergic function and the acute stress response. eLife 2019, 8, e46797. [Google Scholar] [CrossRef]
- Cordeiro, L.M.S.; Rabelo, P.C.R.; Moraes, M.M.; Teixeira-Coelho, F.; Coimbra, C.C.; Wanner, S.P.; Soares, D.D. Physical exercise-induced fatigue: The role of serotonergic and dopaminergic systems. Braz. J. Med. Biol. Res. 2017, 50, e6432. [Google Scholar] [CrossRef]
- National Collaborating Centre for Mental Health (Great Britain); National Institute for Health, Clinical Excellence (Great Britain); British Psychological Society; Royal College of Psychiatrists. Common Mental Health Disorders: Identification and Pathways to Care; 2011. [Google Scholar]
- Plotogea, O.-M.; Gheorghe, G.; Stan-Ilie, M.; Constantinescu, G.; Bacalbasa, N.; Bungau, S.; Diaconu, C.C. Assessment of Sleep among Patients with Chronic Liver Disease: Association with Quality of Life. J. Pers. Med. 2021, 11, 1387. [Google Scholar] [CrossRef]
- Plotogea, O.-M.; Ilie, M.; Bungau, S.; Chiotoroiu, A.L.; Stanescu, A.M.A.; Diaconu, C.C. Comprehensive Overview of Sleep Disorders in Patients with Chronic Liver Disease. Brain Sci. 2021, 11, 142. [Google Scholar] [CrossRef]
- Diana, M. The dopamine hypothesis of drug addiction and its potential therapeutic value. Front. Psychiatry 2011, 2, 64. [Google Scholar] [CrossRef]
- Delaville, C.; Deurwaerdère, P.D.; Benazzouz, A. Noradrenaline and Parkinson’s disease. Front. Syst. Neurosci. 2011, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Trifan, D.F.; Tirla, A.G.; Moldovan, A.F.; Moș, C.; Bodog, F.; Maghiar, T.T.; Manole, F.; Ghitea, T.C. Can Vitamin D Levels Alter the Effectiveness of Short-Term Facelift Interventions? Healthcare 2023, 11, 1490. [Google Scholar] [CrossRef] [PubMed]
- Trifan, D.F.; Tirla, A.G.; Mos, C.; Danciu, A.; Bodog, F.; Manole, F.; Ghitea, T.C. Involvement of Vitamin D3 in the Aging Process According to Sex. Cosmetics 2023, 10, 114. [Google Scholar] [CrossRef]
- Zielińska, M.; Łuszczki, E.; Dereń, K. Dietary Nutrient Deficiencies and Risk of Depression (Review Article 2018–2023). Nutrients 2023, 15, 2433. [Google Scholar] [CrossRef]
- Siegel, G.J.; Albers, R.W.; Katzman, R.; Agranoff, B. Basic Neurochemistry; Little, Brown Boston: Boston, MA, USA, 1981. [Google Scholar]
- Fitzgerald, P.J. Elevated Norepinephrine may be a Unifying Etiological Factor in the Abuse of a Broad Range of Substances: Alcohol, Nicotine, Marijuana, Heroin, Cocaine, and Caffeine. Subst. Abuse 2013, 7, 171–183. [Google Scholar] [CrossRef]
- Floriou-Servou, A.; von Ziegler, L.; Waag, R.; Schläppi, C.; Germain, P.-L.; Bohacek, J. The acute stress response in the multiomic era. Biol. Psychiatry 2021, 89, 1116–1126. [Google Scholar] [CrossRef]
- Hendrickson, R.C.; Raskind, M.A.; Millard, S.P.; Sikkema, C.; Terry, G.E.; Pagulayan, K.F.; Li, G.; Peskind, E.R. Evidence for altered brain reactivity to norepinephrine in Veterans with a history of traumatic stress. Neurobiol. Stress 2018, 8, 103–111. [Google Scholar] [CrossRef]
- Altevogt, B.M.; Colten, H.R. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem; 2006. [Google Scholar]
- Ekinci, G.N.; Sanlier, N. The relationship between nutrition and depression in the life process: A mini-review. Exp. Gerontol. 2023, 172, 112072. [Google Scholar] [CrossRef]
- Staner, L. Sleep and anxiety disorders. Dialogues Clin. Neurosci. 2003, 5, 249–258. [Google Scholar] [CrossRef]
- Oka, T. Psychogenic fever: How psychological stress affects body temperature in the clinical population. Temperature 2015, 2, 368–378. [Google Scholar] [CrossRef]
- Zhou, J. Norepinephrine transporter inhibitors and their therapeutic potential. Drugs Future 2004, 29, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Ambade, V.; Arora, M.M.; Singh, P.; Somani, B.L.; Basannar, D. Adrenaline, Noradrenaline and Dopamine Level Estimation in Depression : Does it Help? Med. J. Armed. Forces India 2009, 65, 216–220. [Google Scholar] [CrossRef]
- Seals, D.R.; Esler, M.D. Human ageing and the sympathoadrenal system. J. Physiol. 2000, 528, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Salleh, M.R. Life event, stress and illness. Malays J. Med. Sci. 2008, 15, 9–18. [Google Scholar] [PubMed]
- Herlenius, E.; Lagercrantz, H. Neurotransmitters and neuromodulators during early human development. Early Hum. Dev. 2001, 65, 21–37. [Google Scholar] [CrossRef]
- Murrin, L.C.; Sanders, J.D.; Bylund, D.B. Comparison of the maturation of the adrenergic and serotonergic neurotransmitter systems in the brain: Implications for differential drug effects on juveniles and adults. Biochem. Pharmacol. 2007, 73, 1225–1236. [Google Scholar] [CrossRef]
- Pearl, P.L.; Welch, W.P.; Johnston, M.; Michael; Adams, M., Jr.; Harold; Fatemi, M.; Ali, M.B.A. 417Pediatric Neurotransmitter Disorders. In Neurobiology of Disease; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Mittal, R.; Debs, L.H.; Patel, A.P.; Nguyen, D.; Patel, K.; O’Connor, G.; Grati, M.; Mittal, J.; Yan, D.; Eshraghi, A.A.; et al. Neurotransmitters: The Critical Modulators Regulating Gut-Brain Axis. J. Cell Physiol. 2017, 232, 2359–2372. [Google Scholar] [CrossRef]
- Spohn, S.N.; Mawe, G.M. Non-conventional features of peripheral serotonin signalling—The gut and beyond. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 412–420. [Google Scholar] [CrossRef]
- Gavra, D.I.; Endres, L.; Pető, Á.; Józsa, L.; Fehér, P.; Ujhelyi, Z.; Pallag, A.; Marian, E.; Vicas, L.G.; Ghitea, T.C.; et al. In Vitro and Human Pilot Studies of Different Topical Formulations Containing Rosa Species for the Treatment of Psoriasis. Molecules 2022, 27, 5499. [Google Scholar] [CrossRef]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Brain–Gut Axis in Psychiatric and Inflammatory Disorders. Front. Psychiatry 2018, 9, 44. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, G.; Fratila, O.; Buhas, C.; Judea-Pusta, C.T.; Negrut, N.; Bustea, C.; Bungau, S. Cross-talks among GBA mutations, glucocerebrosidase, and α-synuclein in GBA-associated Parkinson’s disease and their targeted therapeutic approaches: A comprehensive review. Transl. Neurodegener. 2021, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Reyes-Resina, I.; Navarro, G. Dopamine in Health and Disease: Much More Than a Neurotransmitter. Biomedicines 2021, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Kyriakoulis, P.; Kyrios, M. Biological and cognitive theories explaining panic disorder: A narrative review. Front. Psychiatry 2023, 14, 957515. [Google Scholar] [CrossRef] [PubMed]





| Parameters | Groups | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| I | II | III | |||||||
| Count % | |||||||||
| Constipation | No | 27 | 73.0 | 0 | 0.0 | 27 | 81.8 | 54 | 40.0 |
| Yes | 10 | 27.0 | 65 | 100.0 | 6 | 18.2 | 81 | 60.0 | |
| Diarrhea | No | 27 | 73.0 | 48 | 73.8 | 33 | 100.0 | 108 | 80.0 |
| Yes | 10 | 27.0 | 17 | 26.2 | 0 | 0.0 | 27 | 20.0 | |
| Gastrointestinal problems | No | 11 | 29.7 | 17 | 26.2 | 4 | 12.1 | 32 | 23.7 |
| Yes | 26 | 70.3 | 48 | 73.8 | 29 | 87.9 | 103 | 76.3 | |
| Parameters | Groups | p | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | ||||||||
| Count | % | Count | % | Count | % | Count | % | |||
| Initial | ||||||||||
| Constipation | No | 27 | 73.0 | 0 | 0.0 | 27 | 81.8 | 0.001 | 54 | 40.0 |
| Yes | 10 | 27.0 | 65 | 100.0 | 6 | 18.2 | 81 | 60.0 | ||
| Diarrhea | No | 27 | 73.0 | 48 | 73.8 | 33 | 100.0 | 0.004 | 108 | 80.0 |
| Yes | 10 | 27.0 | 17 | 26.2 | 0 | 0.0 | 27 | 20.0 | ||
| Gastrointestinal problems | No | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1.000 | 0 | 0.0 |
| Yes | 37 | 100.0 | 65 | 100.0 | 33 | 100.0 | 135 | 100.0 | ||
| Final | ||||||||||
| Constipation | No | 37 | 100.0 | 61 | 93.8 | 33 | 100.0 | 0.110 | 131 | 97.0 |
| Yes | 0 | 0.0 | 4 | 6.2 | 0 | 0.0 | 4 | 3.0 | ||
| Diarrhea | No | 37 | 100.0 | 61 | 93.8 | 33 | 100.0 | 0.110 | 131 | 97.0 |
| Yes | 0 | 0.0 | 4 | 6.2 | 0 | 0.0 | 4 | 3.0 | ||
| Gastrointestinal problems | No | 36 | 97.3 | 42 | 64.6 | 25 | 75.8 | 0.001 | 103 | 76.3 |
| Yes | 1 | 2.7 | 23 | 35.4 | 8 | 24.2 | 32 | 23.7 | ||
| Spearman’s Correlation | Dopamine | Noradrenalin | Adrenalin | Noradrenaline/Adrenaline | |
|---|---|---|---|---|---|
| Constipation | rho | −0.250 ** | −0.083 | −0.081 | −0.162 |
| p | 0.000 | 0.341 | 0.352 | 0.060 | |
| Diarrhea | rho | 0.307 ** | 0.632 ** | 0.070 | 0.623 ** |
| p | 0.000 | 0.000 | 0.417 | 0.000 | |
| Gastrointestinal problems | rho | 0.057 | 0.646 ** | 0.585 ** | −0.141 |
| p | 0.516 | 0.000 | 0.000 | 0.103 | |
| N | 135 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matiș, L.; Alexandru, B.A.; Ghitea, T.C. Catecholamine Variations in Pediatric Gastrointestinal Disorders and Their Neuropsychiatric Expression. Biomedicines 2023, 11, 2600. https://doi.org/10.3390/biomedicines11102600
Matiș L, Alexandru BA, Ghitea TC. Catecholamine Variations in Pediatric Gastrointestinal Disorders and Their Neuropsychiatric Expression. Biomedicines. 2023; 11(10):2600. https://doi.org/10.3390/biomedicines11102600
Chicago/Turabian StyleMatiș, Loredana, Bogdana Ariana Alexandru, and Timea Claudia Ghitea. 2023. "Catecholamine Variations in Pediatric Gastrointestinal Disorders and Their Neuropsychiatric Expression" Biomedicines 11, no. 10: 2600. https://doi.org/10.3390/biomedicines11102600
APA StyleMatiș, L., Alexandru, B. A., & Ghitea, T. C. (2023). Catecholamine Variations in Pediatric Gastrointestinal Disorders and Their Neuropsychiatric Expression. Biomedicines, 11(10), 2600. https://doi.org/10.3390/biomedicines11102600