Development and Characterization of a Cancer Cachexia Rat Model Transplanted with Cells of the Rat Lung Adenocarcinoma Cell Line Sato Lung Cancer (SLC)
Abstract
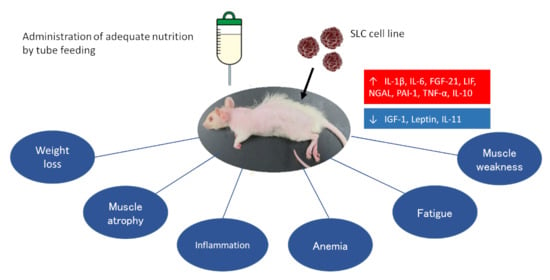
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cell Culture and Tumor Inoculation
2.3. Grip Strength Test
2.4. Open Field Test
2.5. Blood Measurements
2.6. Cytokine Array
2.7. RNA Extraction, Reverse Transcriptase Polymerase Chain Reaction (PCR), and mRNA Quantification
2.8. Tube Feeding following Gastrostomy
2.9. Histological Analysis of the Skeletal Muscles
2.10. Statistical Analysis
3. Results
3.1. SLC-Transplanted Rats Exhibited Severe Weight Loss and Reduced Food Intake
3.2. SLC Transplantation Induced Muscle Fiber Atrophy and Upregulated the Expression of Muscle-Specific E3 Ubiquitin Ligases in Rats
3.3. Muscle Atrophy Associated with SLC Transplantation Reduced Muscle Function and Activity in Rats
3.4. SLC-Transplanted Rats Developed Severe Anemia, Malnutrition, and Hypercalcemia
3.5. The Cytokine Production Patterns in SLC-Transplanted Rats Were Significantly Different from Those in the Control Rats
3.6. SLC Transplantation Induced Muscle Atrophy and Loss of Muscle Function in Rats Subjected to Tube Feeding
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evans, W.J.; Morley, J.E.; Argilés, J.; Bales, C.; Baracos, V.; Guttridge, D.; Jatoi, A.; Kalantar-Zadeh, K.; Lochs, H.; Mantovani, G.; et al. Cachexia: A New Definition. Clin. Nutr. 2008, 27, 793–799. [Google Scholar] [CrossRef]
- Amitani, M.; Asakawa, A.; Amitani, H.; Inui, A. Control of Food Intake and Muscle Wasting in Cachexia. Int. J. Biochem. Cell Biol. 2013, 45, 2179–2185. [Google Scholar] [CrossRef] [PubMed]
- Baracos, V.E.; Martin, L.; Korc, M.; Guttridge, D.C.; Fearon, K.C.H. Cancer-associated Cachexia. Nat. Rev. Dis. Primers 2018, 4, 17105. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and Classification of Cancer Cachexia: An International Consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Argilés, J.M.; Busquets, S.; Stemmler, B.; Lopéz-Soriano, F.J. Cancer Cachexia: Understanding the Molecular Basis. Nat. Rev. Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, T.; Terracina, K.P.; Raza, A.; Matsubara, H.; Takabe, K. Cancer Cachexia, Mechanism and Treatment. World J. Gastrointest. Oncol. 2015, 7, 17–29. [Google Scholar] [CrossRef]
- Parmar, M.P.; Swanson, T.; Jagoe, R.T. Weight Changes Correlate with Alterations in Subjective Physical Function in Advanced Cancer Patients Referred to a Specialized Nutrition and Rehabilitation Team. Support. Care Cancer 2013, 21, 2049–2057. [Google Scholar] [CrossRef]
- Evans, W.K.; Nixon, D.W.; Daly, J.M.; Ellenberg, S.S.; Gardner, L.; Wolfe, E.; Shepherd, F.A.; Feld, R.; Gralla, R.; Fine, S.; et al. A Randomized Study of Oral Nutritional Support Versus ad lib Nutritional Intake During Chemotherapy for Advanced Colorectal and Non-small-cell lung Cancer. J. Clin. Oncol. 1987, 5, 113–124. [Google Scholar] [CrossRef]
- Ovesen, L.; Allingstrup, L.; Hannibal, J.; Mortensen, E.L.; Hansen, O.P. Effect of Dietary Counseling on Food Intake, Body Weight, Response Rate, Survival, and Quality of Life in Cancer Patients Undergoing Chemotherapy: A Prospective, Randomized Study. J. Clin. Oncol. 1993, 11, 2043–2049. [Google Scholar] [CrossRef]
- Hall, K.D.; Baracos, V.E. Computational Modeling of Cancer Cachexia. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 214–221. [Google Scholar] [CrossRef]
- Peixoto da Silva, S.; Santos, J.M.O.; Costa e Silva, M.P.; Gil da Costa, R.M.; Medeiros, R. Cancer Cachexia and its Pathophysiology: Links with Sarcopenia, Anorexia and Asthenia. J. Cachexia Sarcopenia Muscle 2020, 11, 619–635. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Feng, X.; Wu, X.; Lu, Y.; Chen, K.; Ye, Y. Fat Wasting Is Damaging: Role of Adipose Tissue in Cancer-Associated Cachexia. Front. Cell Dev. Biol. 2020, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Arends, J.; Baracos, V. Understanding the Mechanisms and Treatment Options in Cancer Cachexia. Nat. Rev. Clin. Oncol. 2013, 10, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Kadakia, K.C.; Hamilton-Reeves, J.M.; Baracos, V.E. Current Therapeutic Targets in Cancer Cachexia: A Pathophysiologic Approach. Am. Soc. Clin. Oncol. Educ. Book 2023, 43, e389942. [Google Scholar] [CrossRef] [PubMed]
- da Fonseca, G.W.P.; Sato, R.; de Nazaré Nunes Alves, M.J.; von Haehling, S. Current Advancements in Pharmacotherapy for Cancer Cachexia. Expert Opin. Pharmacother. 2023, 24, 629–639. [Google Scholar] [CrossRef]
- Suzuki, H.; Asakawa, A.; Amitani, H.; Fujitsuka, N.; Nakamura, N.; Inui, A. Cancer Cachexia Pathophysiology and Translational Aspect of Herbal Medicine. Jpn. J. Clin. Oncol. 2013, 43, 695–705. [Google Scholar] [CrossRef]
- Terawaki, K.; Kashiwase, Y.; Sawada, Y.; Hashimoto, H.; Yoshimura, M.; Ohbuchi, K.; Sudo, Y.; Suzuki, M.; Miyano, K.; Shiraishi, S.; et al. Development of Ghrelin Resistance in a Cancer Cachexia Rat Model Using Human Gastric Cancer-derived 85As2 Cells and the Palliative Effects of the Kampo Medicine Rikkunshito on the Model. PLoS ONE 2017, 12, e0173113. [Google Scholar] [CrossRef]
- Hamauchi, S.; Furuse, J.; Takano, T.; Munemoto, Y.; Furuya, K.; Baba, H.; Takeuchi, M.; Choda, Y.; Higashiguchi, T.; Naito, T.; et al. A Multicenter, Open-label, Single-arm Study of Anamorelin (ONO-7643) in Advanced Gastrointestinal Cancer Patients with Cancer Cachexia. Cancer 2019, 125, 4294–4302. [Google Scholar] [CrossRef]
- Nishie, K.; Sato, S.; Hanaoka, M. Anamorelin for Cancer Cachexia. Drugs Today 2022, 58, 97–104. [Google Scholar] [CrossRef]
- Morita-Tanaka, S.; Yamada, T.; Takayama, K. The Landscape of Cancer Cachexia in Advanced Non-small Cell Lung Cancer: A Narrative Review. Transl. Lung Cancer Res. 2023, 12, 168–180. [Google Scholar] [CrossRef]
- Kasumi, E.; Kuzumaki, Y.; Sadakata, M.; Kuzuoka, H.; Sato, N. A Novel Highly Concentrated Enteral Nutrition Formula, EN-P05, Shows Nutritional Effectiveness Comparable to the Approved OSN-001 in Gastrostomized Rats. Drug Discov. Ther. 2019, 13, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Advani, S.M.; Advani, P.G.; VonVille, H.M.; Jafri, S.H. Pharmacological Management of Cachexia in Adult Cancer Patients: A Systematic Review of Clinical Trials. BMC Cancer 2018, 18, 1174. [Google Scholar] [CrossRef]
- Vigano, A.A.L.; Morais, J.A.; Ciutto, L.; Rosenthall, L.; di Tomasso, J.; Khan, S.; Olders, H.; Borod, M.; Kilgour, R.D. Use of Routinely Available Clinical, Nutritional, and Functional Criteria to Classify Cachexia in Advanced Cancer Patients. Clin. Nutr. 2017, 36, 1378–1390. [Google Scholar] [CrossRef] [PubMed]
- Vignjević Petrinović, S.; Jauković, A.; Milošević, M.; Bugarski, D.; Budeč, M. Targeting Stress Erythropoiesis Pathways in Cancer. Front. Physiol. 2022, 13, 844042. [Google Scholar] [CrossRef] [PubMed]
- Madeddu, C.; Gramignano, G.; Astara, G.; Demontis, R.; Sanna, E.; Atzeni, V.; Macciò, A. Pathogenesis and Treatment Options of Cancer Related Anemia: Perspective for a Targeted Mechanism-Based Approach. Front. Physiol. 2018, 9, 1294. [Google Scholar] [CrossRef]
- Kir, S.; White, J.P.; Kleiner, S.; Kazak, L.; Cohen, P.; Baracos, V.E.; Spiegelman, B.M. Tumour-derived PTH-related Protein Triggers Adipose Tissue Browning and Cancer Cachexia. Nature 2014, 513, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Wolff, B.S.; Raheem, S.A.; Saligan, L.N. Comparing Passive Measures of Fatigue-like Behavior in Mice. Sci. Rep. 2018, 8, 14238. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.C.; Voss, A.C.; Hustead, D.S.; Cancer Cachexia Study Group. Definition of Cancer Cachexia: Effect of Weight Loss, Reduced Food Intake, and Systemic Inflammation on Functional Status and Prognosis. Am. J. Clin. Nutr. 2006, 83, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Bozzetti, F. Is Enteral Nutrition a Primary Therapy in Cancer Patients? Gut 1994, 35, S65–S68. [Google Scholar] [CrossRef]
- Gullett, N.P.; Mazurak, V.C.; Hebbar, G.; Ziegler, T.R. Nutritional Interventions for Cancer-Induced Cachexia. Curr. Probl. Cancer 2011, 35, 58–90. [Google Scholar] [CrossRef]
- Kotler, D.P. Cachexia. Ann. Intern. Med. 2000, 133, 622–634. [Google Scholar] [CrossRef]
- Setiawan, T.; Sari, I.N.; Wijaya, Y.T.; Julianto, N.M.; Muhammad, J.A.; Lee, H.; Chae, J.H.; Kwon, H.Y. Cancer Cachexia: Molecular Mechanisms and Treatment Strategies. J. Hematol. Oncol. 2023, 16, 54. [Google Scholar] [CrossRef]
- Santoso, P.; Nakata, M.; Shiizaki, K.; Boyang, Z.; Parmila, K.; Otgon-Uul, Z.; Hashimoto, K.; Satoh, T.; Mori, M.; Kuro, O.M.; et al. Fibroblast Growth Factor 21, Assisted by Elevated Glucose, Activates Paraventricular Nucleus NUCB2/Nesfatin-1 Neurons to Produce Satiety Under Fed States. Sci. Rep. 2017, 7, 45819. [Google Scholar] [CrossRef]
- Søberg, S.; Sandholt, C.H.; Jespersen, N.Z.; Toft, U.; Madsen, A.L.; von Holstein-Rathlou, S.; Grevengoed, T.J.; Christensen, K.B.; Bredie, W.L.P.; Potthoff, M.J.; et al. FGF21 Is a Sugar-Induced Hormone Associated with Sweet Intake and Preference in Humans. Cell Metab. 2017, 25, 1045–1053.e6. [Google Scholar] [CrossRef]
- von Holstein-Rathlou, S.; Gillum, M.P. Fibroblast Growth Factor 21: An Endocrine Inhibitor of Sugar and Alcohol Appetite. J. Physiol. 2019, 597, 3539–3548. [Google Scholar] [CrossRef]
- Zhang, Y.; Chua, S., Jr. Leptin Function and Regulation. Compr. Physiol. 2017, 8, 351–369. [Google Scholar] [CrossRef]
- Inui, A. Cancer Anorexia-cachexia Syndrome: Are Neuropeptides the Key? Cancer Res. 1999, 59, 4493–4501. [Google Scholar] [PubMed]
- Zeng, R.; Tong, C.; Xiong, X. The Molecular Basis and Therapeutic Potential of Leukemia Inhibitory Factor in Cancer Cachexia. Cancers 2022, 14, 2955. [Google Scholar] [CrossRef] [PubMed]
- Kandarian, S.C.; Nosacka, R.L.; Delitto, A.E.; Judge, A.R.; Judge, S.M.; Ganey, J.D.; Moreira, J.D.; Jackman, R.W. Tumour-derived Leukaemia Inhibitory Factor is a Major Driver of Cancer Cachexia and Morbidity in C26 Tumour-bearing Mice. J. Cachexia Sarcopenia Muscle 2018, 9, 1109–1120. [Google Scholar] [CrossRef]
- Hong, N.; Yoon, H.J.; Lee, Y.H.; Kim, H.R.; Lee, B.W.; Rhee, Y.; Kang, E.S.; Cha, B.S.; Lee, H.C. Serum PTHrP Predicts Weight Loss in Cancer Patients Independent of Hypercalcemia, Inflammation, and Tumor Burden. J. Clin. Endocrinol. Metab. 2016, 101, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Hong, N.; Kim, H.R.; Lee, B.W.; Kang, E.S.; Cha, B.S.; Lee, Y.H. Effects of Serum Albumin, Calcium Levels, Cancer Stage and Performance Status on Weight Loss in Parathyroid Hormone-Related Peptide Positive or Negative Patients with Cancer. Endocrinol. Metab. 2018, 33, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Costelli, P.; Muscaritoli, M.; Bossola, M.; Penna, F.; Reffo, P.; Bonetto, A.; Busquets, S.; Bonelli, G.; Lopez-Soriano, F.J.; Doglietto, G.B.; et al. IGF-1 is Downregulated in Experimental Cancer Cachexia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R674–R683. [Google Scholar] [CrossRef] [PubMed]
- Timmer, L.T.; Hoogaars, W.M.H.; Jaspers, R.T. The Role of IGF-1 Signaling in Skeletal Muscle Atrophy. Adv. Exp. Med. Biol. 2018, 1088, 109–137. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Semprun-Prieto, L.; Sukhanov, S.; Delafontaine, P. IGF-1 Prevents ANG II-induced Skeletal Muscle Atrophy via Akt- and Foxo-dependent Inhibition of the Ubiquitin Ligase Atrogin-1 Expression. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H1565–H1570. [Google Scholar] [CrossRef]
- Romejko, K.; Markowska, M.; Niemczyk, S. The Review of Current Knowledge on Neutrophil Gelatinase-Associated Lipocalin (NGAL). Int. J. Mol. Sci. 2023, 24, 10470. [Google Scholar] [CrossRef]
- Roli, L.; Pecoraro, V.; Trenti, T. Can NGAL be Employed as Prognostic and Diagnostic Biomarker in Human Cancers? A Systematic Review of Current Evidence. Int. J. Biol. Markers 2017, 32, e53–e61. [Google Scholar] [CrossRef]
- Song, E.; Jahng, J.W.; Chong, L.P.; Sung, H.K.; Han, M.; Luo, C.; Wu, D.; Boo, S.; Hinz, B.; Cooper, M.A.; et al. Lipocalin-2 Induces NLRP3 Inflammasome Activation via HMGB1 induced TLR4 Signaling in Heart Tissue of Mice Under Pressure Overload Challenge. Am. J. Transl. Res. 2017, 9, 2723–2735. [Google Scholar]
- Riuzzi, F.; Sorci, G.; Sagheddu, R.; Chiappalupi, S.; Salvadori, L.; Donato, R. RAGE in the Pathophysiology of Skeletal Muscle. J. Cachexia Sarcopenia Muscle 2018, 9, 1213–1234. [Google Scholar] [CrossRef]
- Mosialou, I.; Shikhel, S.; Liu, J.M.; Maurizi, A.; Luo, N.; He, Z.; Huang, Y.; Zong, H.; Friedman, R.A.; Barasch, J.; et al. MC4R-dependent Suppression of Appetite by Bone-derived Lipocalin 2. Nature 2017, 543, 385–390. [Google Scholar] [CrossRef]
- Bolignano, D.; Coppolino, G.; Donato, V.; Lacquaniti, A.; Bono, C.; Buemi, M. Neutrophil Gelatinase-associated Lipocalin (NGAL): A New Piece of the Anemia Puzzle? Med. Sci. Monit. 2010, 16, RA131–RA135. [Google Scholar]
- Tamura, Y.; Kawao, N.; Shimoide, T.; Okada, K.; Matsuo, O.; Kaji, H. Role of Plasminogen Activator Inhibitor-1 in Glucocorticoid-induced Muscle Change in Mice. J. Bone Miner. Metab. 2018, 36, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Naderi, J.; Bernreuther, C.; Grabinski, N.; Putman, C.T.; Henkel, B.; Bell, G.; Glatzel, M.; Sultan, K.R. Plasminogen Activator Inhibitor Type 1 Up-regulation is Associated with Skeletal Muscle Atrophy and Associated Fibrosis. Am. J. Pathol. 2009, 175, 763–771. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasumi, E.; Chiba, M.; Kuzumaki, Y.; Kuzuoka, H.; Sato, N.; Takahashi, B. Development and Characterization of a Cancer Cachexia Rat Model Transplanted with Cells of the Rat Lung Adenocarcinoma Cell Line Sato Lung Cancer (SLC). Biomedicines 2023, 11, 2824. https://doi.org/10.3390/biomedicines11102824
Kasumi E, Chiba M, Kuzumaki Y, Kuzuoka H, Sato N, Takahashi B. Development and Characterization of a Cancer Cachexia Rat Model Transplanted with Cells of the Rat Lung Adenocarcinoma Cell Line Sato Lung Cancer (SLC). Biomedicines. 2023; 11(10):2824. https://doi.org/10.3390/biomedicines11102824
Chicago/Turabian StyleKasumi, Eiji, Miku Chiba, Yoshie Kuzumaki, Hiroyuki Kuzuoka, Norifumi Sato, and Banyu Takahashi. 2023. "Development and Characterization of a Cancer Cachexia Rat Model Transplanted with Cells of the Rat Lung Adenocarcinoma Cell Line Sato Lung Cancer (SLC)" Biomedicines 11, no. 10: 2824. https://doi.org/10.3390/biomedicines11102824
APA StyleKasumi, E., Chiba, M., Kuzumaki, Y., Kuzuoka, H., Sato, N., & Takahashi, B. (2023). Development and Characterization of a Cancer Cachexia Rat Model Transplanted with Cells of the Rat Lung Adenocarcinoma Cell Line Sato Lung Cancer (SLC). Biomedicines, 11(10), 2824. https://doi.org/10.3390/biomedicines11102824