High Rate of Discontinuation during Long-Acting Injectable Antipsychotic Treatment in Patients with Psychotic Disorders
Abstract
:1. Introduction
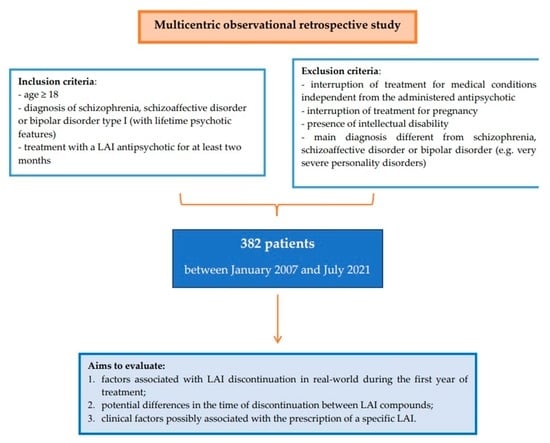
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Christensen, K.; Doblhammer, G.; Rau, R.; Vaupel, J.W. Ageing populations: The challenges ahead. Lancet 2009, 374, 1196–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodenheimer, T.; Lorig, K.; Holman, H.; Grumbach, K. Patient self-management of chronic disease in primary care. JAMA 2002, 288, 2469–2475. [Google Scholar] [CrossRef] [PubMed]
- Sabaté, E. Adherence to Long-Term Therapies: Evidence for Action; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Keramatian, K.; Chakrabarty, T.; Yatham, L.N. Long-Acting Injectable Second-Generation/Atypical Antipsychotics for the Management of Bipolar Disorder: A Systematic Review. CNS Drugs 2019, 33, 431–456. [Google Scholar] [CrossRef] [PubMed]
- Sheffield, J.M.; Karcher, N.R.; Barch, D.M. Cognitive Deficits in Psychotic Disorders: A Lifespan Perspective. Neuropsychol. Rev. 2018, 28, 509–533. [Google Scholar] [CrossRef]
- Kane, J.M.; Garcia-Ribera, C. Clinical guideline recommendations for antipsychotic long-acting injections. Br. J. Psychiatry Suppl. 2009, 52, S63–S67. [Google Scholar] [CrossRef] [Green Version]
- Buoli, M.; Kahn, R.S.; Serati, M.; Altamura, A.C.; Cahn, W. Haloperidol versus second-generation antipsychotics in the long-term treatment of schizophrenia. Hum. Psychopharmacol. 2016, 31, 325–331. [Google Scholar] [CrossRef]
- Carbon, M.; Correll, C.U. Clinical predictors of therapeutic response to antipsychotics in schizophrenia. Dialogues Clin. Neurosci. 2014, 16, 505–524. [Google Scholar] [CrossRef]
- Shimomura, Y.; Kikuchi, Y.; Suzuki, T.; Uchida, H.; Mimura, M.; Takeuchi, H. Antipsychotic treatment in the maintenance phase of schizophrenia: An updated systematic review of the guidelines and algorithms. Schizophr. Res. 2020, 215, 8–16. [Google Scholar] [CrossRef]
- Kane, J.M.; Kishimoto, T.; Correll, C.U. Assessing the comparative effectiveness of long-acting injectable vs. oral antipsychotic medications in the prevention of relapse provides a case study in comparative effectiveness research in psychiatry. J. Clin. Epidemiol. 2013, 66, S37–S41. [Google Scholar] [CrossRef] [Green Version]
- Correll, C.U.; Citrome, L.; Haddad, P.M.; Lauriello, J.; Olfson, M.; Calloway, S.M.; Kane, J.M. The Use of Long-Acting Injectable Antipsychotics in Schizophrenia: Evaluating the Evidence. J. Clin. Psychiatry. 2016, 77, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Tiihonen, J.; Mittendorfer-Rutz, E.; Majak, M.; Mehtälä, J.; Hoti, F.; Jedenius, E.; Enkusson, D.; Leval, A.; Sermon, J.; Tanskanen, A.; et al. Real-World Effectiveness of Antipsychotic Treatments in a Nationwide Cohort of 29 823 Patients With Schizophrenia. JAMA Psychiatry. 2017, 74, 686–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabrazzo, M.; Cipolla, S.; Camerlengo, A.; Perris, F.; Catapano, F. Second-Generation Antipsychotics’ Effectiveness and Tolerability: A Review of Real-World Studies in Patients with Schizophrenia and Related Disorders. J. Clin. Med. 2022, 11, 4530. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.; Chen, N.; Glick, I.D. A meta-analysis of the efficacy of second-generation antipsychotics. Arch. Gen. Psychiatry. 2003, 60, 553–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCreath, J.; Larson, E.; Bharatiya, P.; Labanieh, H.A.; Weiss, Z.; Lozovatsky, M. Long-Acting Injectable Antipsychotics for Schizophrenia: Sociodemographic Characteristics and Treatment Adherence. Prim. Care Companion CNS Disord. 2017, 19. [Google Scholar] [CrossRef]
- Gentile, S. Discontinuation rates during long-term, second-generation antipsychotic long-acting injection treatment: A systematic review. Psychiatry Clin. Neurosci. 2019, 73, 216–230. [Google Scholar] [CrossRef]
- Wu, C.S.; Hsieh, M.H.; Tang, C.H.; Chang, C.J. Comparative effectiveness of long-acting injectable risperidone vs. long-acting injectable first-generation antipsychotics in bipolar disorder. J. Affect. Disord. 2016, 197, 189–195. [Google Scholar] [CrossRef]
- Tatini, L.; D’Anna, G.; Pietrini, F.; Calligaris, E.; Ballerini, A.; Ricca, V. Predictors of long-acting injectable antipsychotic treatment discontinuation in outpatients with schizophrenia: Relevance of the Drug Attitude Inventory-10. Int. Clin. Psychopharmacol. 2021, 36, 181–187. [Google Scholar] [CrossRef]
- Aguglia, A.; Fusar-Poli, L.; Natale, A.; Amerio, A.; Espa, I.; Villa, V.; Martinotti, G.; Carrà, G.; Bartoli, F.; D’Agostino, A.; et al. Factors Associated with Medication Adherence to Long-Acting Injectable Antipsychotics: Results from the STAR Network Depot Study. Pharmacopsychiatry 2022, 55, 281–289. [Google Scholar] [CrossRef]
- Barbui, C.; Bertolini, F.; Bartoli, F.; Calandra, C.; Callegari, C.; Carrà, G.; D’Agostino, A.; Lucii, C.; Martinotti, G.; Mastromo, D.; et al. STAR Network Investigators. Reasons for initiating long-acting antipsychotics in psychiatric practice: Findings from the STAR Network Depot Study. Ther. Adv. Psychopharmacol. 2020, 10, 8102. [Google Scholar] [CrossRef]
- Perkins, D.O.; Gu, H.; Weiden, P.J.; McEvoy, J.P.; Hamer, R.M.; Lieberman, J.A. Comparison of Atypicals in First Episode study group. Predictors of treatment discontinuation and medication nonadherence in patients recovering from a first episode of schizophrenia, schizophreniform disorder, or schizoaffective disorder: A randomized, double-blind, flexible-dose, multicenter study. J. Clin. Psychiatry 2008, 69, 106–113. [Google Scholar] [CrossRef]
- Leucht, S.; Cipriani, A.; Spineli, L.; Mavridis, D.; Orey, D.; Richter, F.; Samara, M.; Barbui, C.; Engel, R.R.; Geddes, J.R.; et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: A multiple-treatments meta-analysis. Lancet 2013, 382, 951–962. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5; American Psychiatric Press: Washington, DC, USA, 2013. [Google Scholar] [CrossRef]
- Lora, A.; Monzani, E.; Ibrahim, B.; Soranna, D.; Corrao, G. Routine quality care assessment of schizophrenic disorders using information systems. Int. J. Qual. Health Care. 2018, 30, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buoli, M.; Cesana, B.M.; Fagiolini, A.; Albert, U.; Maina, G.; de Bartolomeis, A.; Pompili, M.; Bondi, E.; Steardo, L., Jr.; Amore, M.; et al. ISBD Italian Chapter Epidemiologic Group. Which factors delay treatment in bipolar disorder? A nationwide study focussed on duration of untreated illness. Early Interv. Psychiatry. 2021, 15, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Mahlich, J.; Olbrich, K.; Wilk, A.; Wimmer, A.; Wolff-Menzler, C. Time to Treatment Discontinuation in German Patients with Schizophrenia: Long-Acting Injectables versus Oral Antipsychotics. Clin. Drug Investig. 2021, 41, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Rittmannsberger, H.; Rosenleitner, J.; Malsiner-Walli, G.; Werl, R.; Rittmannsberger, B.; Yazdi, K. Treatment Duration With Long-Acting Injectable Antipsychotics After In-hospital Initiation: A Retrospective Cohort Study. J. Clin. Psychopharmacol. 2017, 37, 250–254. [Google Scholar] [CrossRef]
- Goel, M.K.; Khanna, P.; Kishore, J. Understanding survival analysis: Kaplan-Meier estimate. Int. J. Ayurveda Res. 2010, 1, 274–278. [Google Scholar] [CrossRef] [Green Version]
- Buoli, M.; Dell’osso, B.; Zaytseva, Y.; Gurovich, I.Y.; Movina, L.; Dorodnova, A.; Shmuckler, A.; Altamura, A.C. Duration of untreated illness (DUI) and schizophrenia sub-types: A collaborative study between the universities of Milan and Moscow. Int. J. Soc. Psychiatry. 2013, 59, 765–770. [Google Scholar] [CrossRef]
- Stahl, S.M. Long-acting injectable antipsychotics: Shall the last be first? CNS Spectr. 2014, 19, 3–5. [Google Scholar] [CrossRef] [Green Version]
- Ostuzzi, G.; Mazzi, M.A.; Terlizzi, S.; Bertolini, F.; Aguglia, A.; Bartoli, F.; Bortolaso, P.; Callegari, C.; Caroleo, M.; Carrà, G.; et al. STAR Network Investigators. Factors associated with first- versus second-generation long-acting antipsychotics prescribed under ordinary clinical practice in Italy. PloS ONE 2018, 13, e0201371. [Google Scholar] [CrossRef] [Green Version]
- Şahin, O.Ş.; Mursalova, Z.; Gadimov, S.; Üçok, A. Predictors of long-acting injectable antipsychotic prescription at discharge in patients with schizophrenia and other psychotic disorders. Int. Clin. Psychopharmacol. 2021, 36, 251–256. [Google Scholar] [CrossRef]
- Waddell, L.; Taylor, M. Attitudes of patients and mental health staff to antipsychotic long-acting injections: Systematic review. Br. J. Psychiatry Suppl. 2009, 52, S43–S50. [Google Scholar] [CrossRef] [Green Version]
- Grover, S.; Sahoo, S.; Mehra, A. Perceptions of Psychiatrists Toward the Use of Long-Acting Injectable Antipsychotics: An Online Survey Study From India. J. Clin. Psychopharmacol. 2019, 39, 611–619. [Google Scholar] [CrossRef]
- Carr, C.N.; Hall, C.P.; Roche-Desilets, J.E.; Burant, C.J.; Fuller, M.A. Evaluation of adherence in patients prescribed long-acting injectable antipsychotics: A comparison of biweekly versus monthly administered neuroleptics. Ment Health Clin. 2016, 6, 248–253. [Google Scholar] [CrossRef]
- Llorca, P.M.; Bobes, J.; Fleischhacker, W.W.; Heres, S.; Moore, N.; Bent-Ennakhil, N.; Sapin, C.; Loze, J.Y.; Nylander, A.G.; Patel, M.X. Baseline results from the European non-interventional Antipsychotic Long acTing injection in schizOphrenia (ALTO) study. Eur. Psychiatry 2018, 52, 85–94. [Google Scholar] [CrossRef]
- Üçok, A.; Yağcioğlu, E.A.; Aydin, M.; Kara, İ.A.; Erbasan, V.; Türkoğlu, Ö.; Ergün, S.; Chousein, M.G.; Oktar, N.; Uçar, N.; et al. Predictors of discontinuation and hospitalization during long-acting injectable antipsychotic treatment in patients with schizophrenia spectrum disorder. Int. Clin. Psychopharmacol. 2021, 36, 89–96. [Google Scholar] [CrossRef]
- Greene, M.; Yan, T.; Chang, E.; Hartry, A.; Touya, M.; Broder, M.S. Medication adherence and discontinuation of long-acting injectable versus oral antipsychotics in patients with schizophrenia or bipolar disorder. J. Med. Econ. 2018, 21, 127–134. [Google Scholar] [CrossRef]
- Vita, A.; Perin, A.P.; Cavanna, M.; Cobelli, F.; Rosa, J.; Valsecchi, P.; Zanigni, M.; Reggiardo, G.; Sacchetti, E. Negative symptom severity at discharge from an index hospitalization and subsequent use of psychiatric care resources: A retrospective 1-year follow-up study on 450 patients with schizophrenia spectrum disorders. Schizophr. Res. 2020, 216, 243–248. [Google Scholar] [CrossRef]
- Barlati, S.; Nibbio, G.; Calzavara-Pinton, I.; Invernizzi, E.; Cadei, L.; Lisoni, J.; Valsecchi, P.; Deste, G.; Vita, A. Primary and secondary negative symptoms severity and the use of psychiatric care resources in schizophrenia spectrum disorders: A 3-year follow-up longitudinal retrospective study. Schizophr. Res. 2022, 250, 31–38. [Google Scholar] [CrossRef]
- Misawa, F.; Kishimoto, T.; Hagi, K.; Kane, J.M.; Correll, C.U. Safety and tolerability of long-acting injectable versus oral antipsychotics: A meta-analysis of randomized controlled studies comparing the same antipsychotics. Schizophr. Res. 2016, 176, 220–230. [Google Scholar] [CrossRef]
- Kishimoto, T.; Robenzadeh, A.; Leucht, C.; Leucht, S.; Watanabe, K.; Mimura, M.; Borenstein, M.; Kane, J.M.; Correll, C.U. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: A meta-analysis of randomized trials. Schizophr. Bull. 2014, 40, 192–213. [Google Scholar] [CrossRef] [Green Version]
- Brnabic, A.J.; Kelin, K.; Ascher-Svanum, H.; Montgomery, W.; Kadziola, Z.; Karagianis, J. Medication discontinuation with depot and oral antipsychotics in outpatients with schizophrenia: Comparison of matched cohorts from a 12-month observational study. Int. J. Clin. Pract. 2011, 65, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, F.; Ostuzzi, G.; Pievani, M.; Aguglia, A.; Bartoli, F.; Bortolaso, P.; Callegari, C.; Caroleo, M.; Carrà, G.; Corbo, M.; et al. STAR Network Investigators. Comparing Long-Acting Antipsychotic Discontinuation Rates Under Ordinary Clinical Circumstances: A Survival Analysis from an Observational, Pragmatic Study. CNS Drugs 2021, 35, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Greene, M.; Chang, E.; Hartry, A.; Touya, M.; Broder, M.S. Medication Adherence and Discontinuation of Aripiprazole Once-Monthly 400 mg (AOM 400) Versus Oral Antipsychotics in Patients with Schizophrenia or Bipolar I Disorder: A Real-World Study Using US Claims Data. Adv. Ther. 2018, 35, 1612–1625. [Google Scholar] [CrossRef] [PubMed]
- Saucedo Uribe, E.; Carranza Navarro, F.; Guerrero Medrano, A.F.; García Cervantes, K.I.; Álvarez Villalobos, N.A.; Acuña Rocha, V.D.; Méndez Hernández, M.; Millán Alanís, J.M.; Hinojosa Cavada, C.M.; Zúñiga Hernández, J.A.; et al. Preliminary efficacy and tolerability profiles of first versus second-generation Long-Acting Injectable Antipsychotics in schizophrenia: A systematic review and meta-analysis. J. Psychiatr. Res. 2020, 129, 222–233. [Google Scholar] [CrossRef]
- Stone, J.M.; Roux, S.; Taylor, D.; Morrison, P.D. First-generation versus second-generation long-acting injectable antipsychotic drugs and time to relapse. Ther. Adv. Psychopharmacol. 2018, 8, 333–336. [Google Scholar] [CrossRef]
- Taipale, H.; Mittendorfer-Rutz, E.; Alexanderson, K.; Majak, M.; Mehtälä, J.; Hoti, F.; Jedenius, E.; Enkusson, D.; Leval, A.; Sermon, J.; et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr. Res. 2018, 197, 274–280. [Google Scholar] [CrossRef]
- Ostuzzi, G.; Bertolini, F.; Tedeschi, F.; Vita, G.; Brambilla, P.; Del Fabro, L.; Gastaldon, C.; Papola, D.; Purgato, M.; Nosari, G.; et al. Oral and long-acting antipsychotics for relapse prevention in schizophrenia-spectrum disorders: A network meta-analysis of 92 randomized trials including 22,645 participants. World Psychiatry 2022, 21, 295–307. [Google Scholar] [CrossRef]
- Bellavia, A.; Centorrino, F.; Jackson, J.W.; Fitzmaurice, G.; Valeri, L. The role of weight gain in explaining the effects of antipsychotic drugs on positive and negative symptoms: An analysis of the CATIE schizophrenia trial. Schizophr. Res. 2019, 206, 96–102. [Google Scholar] [CrossRef]
- Ostuzzi, G.; Bertolini, F.; Del Giovane, C.; Tedeschi, F.; Bovo, C.; Gastaldon, C.; Nosé, M.; Ogheri, F.; Papola, D.; Purgato, M.; et al. Maintenance Treatment With Long-Acting Injectable Antipsychotics for People With Nonaffective Psychoses: A Network Meta-Analysis. Am. J. Psychiatry 2021, 178, 424–436. [Google Scholar] [CrossRef]
- Joo, S.W.; Shon, S.H.; Choi, G.; Koh, M.; Cho, S.W.; Lee, J. Continuation of schizophrenia treatment with three long-acting injectable antipsychotics in South Korea: A nationwide population-based study. Eur. Neuropsychopharmacol. 2019, 29, 1051–1060. [Google Scholar] [CrossRef]
- Ringen, P.A.; Reponen, E.J.; Vedal, T.S.J.; Andreassen, O.A.; Steen, N.E.; Melle, I. Predictors for Antipsychotic Dosage Change in the First Year of Treatment in Schizophrenia Spectrum and Bipolar Disorders. Front. Psychiatry 2019, 10, 649. [Google Scholar] [CrossRef]
- Ascher-Svanum, H.; Zhao, F.; Detke, H.C.; Nyhuis, A.W.; Lawson, A.H.; Stauffer, V.L.; Montgomery, W.; Witte, M.M.; McDonnell, D.P. Early response predicts subsequent response to olanzapine long-acting injection in a randomized, double-blind clinical trial of treatment for schizophrenia. BMC psychiatry 2011, 11, 152. [Google Scholar] [CrossRef] [Green Version]
- Velligan, D.I.; Sajatovic, M.; Hatch, A.; Kramata, P.; Docherty, J.P. Why do psychiatric patients stop antipsychotic medication? A systematic review of reasons for nonadherence to medication in patients with serious mental illness. Patient Prefer Adherence 2017, 11, 449–468. [Google Scholar] [CrossRef] [Green Version]
- Surace, T.; Capuzzi, E.; Caldiroli, A.; Ceresa, A.; Esposito, C.M.; Auxilia, A.M.; Tagliabue, I.; Capellazzi, M.; Legnani, F.; Di Paolo, M.; et al. Which Clinical and Biochemical Parameters Are Associated with Lifetime Suicide Attempts in Bipolar Disorder? Diagnostics 2022, 12, 2215. [Google Scholar] [CrossRef]
- Koola, M.M.; Wehring, H.J.; Kelly, D.L. The Potential Role of Long-acting Injectable Antipsychotics in People with Schizophrenia and Comorbid Substance Use. J. Dual Diagn. 2012, 8, 50–61. [Google Scholar] [CrossRef] [Green Version]
- Capuzzi, E.; Caldiroli, A.; Besana, F.; Cova, F.; Buoli, M.; Clerici, M. Factors associated with psychotic symptoms among a sample of male prisoners with substance use disorder: A cross-sectional study. J. Subst Abuse Treat. 2020, 118, 108104. [Google Scholar] [CrossRef]
- McCleery, A.; Nuechterlein, K.H. Cognitive impairment in psychotic illness: Prevalence, profile of impairment, developmental course, and treatment considerations. Dialogues Clin. Neurosci. 2019, 21, 239–248. [Google Scholar] [CrossRef]
- Buoli, M.; Caldiroli, A.; Panza, G.; Altamura, A.C. Prominent clinical dimension, duration of illness and treatment response in schizophrenia: A naturalistic study. Psychiatry Investig. 2012, 9, 354–360. [Google Scholar] [CrossRef] [Green Version]
- Gicas, K.M.; Parmar, P.K.; Fabiano, G.F.; Mashhadi, F. Substance-induced psychosis and cognitive functioning: A systematic review. Psychiatry Res. 2022, 308, 114361. [Google Scholar] [CrossRef]
- Lui, S.S.Y.; Lam, J.P.Y.; Lam, J.W.S.; Chui, W.W.H.; Mui, J.H.C.; Siu, B.W.M.; Cheng, K.M.; Cheung, E.F.C.; Chan, R.C.K. Cognitive insight is correlated with cognitive impairments and contributes to medication adherence in schizophrenia patients. Asian J. Psychiatr. 2021, 60, 102644. [Google Scholar] [CrossRef]
- Schoeler, T.; Petros, N.; Di Forti, M.; Klamerus, E.; Foglia, E.; Murray, R.; Bhattacharyya, S. Poor medication adherence and risk of relapse associated with continued cannabis use in patients with first-episode psychosis: A prospective analysis. Lancet Psychiatry 2017, 4, 627–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capuzzi, E.; Ceresa, A.; Caldiroli, A.; Esposito, C.M.; Ossola, P.; Buoli, M. The Relation between the Plasma Concentrations of Long-Acting Atypical Antipsychotics and Clinical Effectiveness in Patients Affected by Schizophrenia or Schizoaffective Disorder: A Comprehensive Overview. Curr. Pharm Des. 2021, 27, 4070–4077. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Marwaha, R. Haloperidol. 2022 Jul 4. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Tang, C.T.; Chua, E.C.; Chew, Q.H.; He, Y.L.; Si, T.M.; Chiu, H.F.; Xiang, Y.T.; Kato, T.A.; Kanba, S.; Shinfuku, N.; et al. Patterns of long acting injectable antipsychotic use and associated clinical factors in schizophrenia among 15 Asian countries and region. Asia Pac. Psychiatry 2020, 12, e12393. [Google Scholar] [CrossRef] [PubMed]
- Lejoyeux, M.; Nivoli, F.; Basquin, A.; Petit, A.; Chalvin, F.; Embouazza, H. An Investigation of Factors Increasing the Risk of Aggressive Behavior among Schizophrenic Inpatients. Front. Psychiatry 2013, 4, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becarevic, N.; Softic, R.; Osmanovic, E. Does the Duration of the Illness Affect the Severity of Negative Symptoms of Schizophrenia? Mater. Sociomed. 2022, 34, 25–27. [Google Scholar] [CrossRef]
- Magliocco, F.; de Filippis, R.; Aloi, M.; Staltari, F.A.; Gaetano, R.; Segura-Garcia, C.; De Fazio, P. Second-generation long-acting injections anti-psychotics improve executive functions in patients with schizophrenia: A 12-month real-world study. Int. J. Psychiatry Clin. Pract. 2020, 24, 201–207. [Google Scholar] [CrossRef]
- Lin, C.H.; Chan, H.Y.; Hsu, C.C.; Chen, F.C. Time to rehospitalization in patients with bipolar mania discharged on long-acting injectable or oral antipsychotics. J. Affect. Disord. 2021, 279, 292–298. [Google Scholar] [CrossRef]
- Biagi, E.; Capuzzi, E.; Colmegna, F.; Mascarini, A.; Brambilla, G.; Ornaghi, A.; Santambrogio, J.; Clerici, M. Long-Acting Injectable Antipsychotics in Schizophrenia: Literature Review and Practical Perspective, with a Focus on Aripiprazole Once-Monthly. Adv. Ther. 2017, 34, 1036–1048. [Google Scholar] [CrossRef] [Green Version]
- Brown, E.S.; Jeffress, J.; Liggin, J.D.; Garza, M.; Beard, L. Switching outpatients with bipolar or schizoaffective disorders and substance abuse from their current antipsychotic to aripiprazole. J. Clin. Psychiatry 2005, 66, 756–760. [Google Scholar] [CrossRef]
- Kishi, T.; Matsuda, Y.; Iwata, N.; Correll, C.U. Antipsychotics for cocaine or psychostimulant dependence: Systematic review and meta-analysis of randomized, placebo-controlled trials. J. Clin. Psychiatry 2013, 74, e1169–e1180. [Google Scholar] [CrossRef]
- Cuomo, I.; Kotzalidis, G.D.; de Persis, S.; Piacentino, D.; Perrini, F.; Amici, E.; De Filippis, S. Head-to-head comparison of 1-year aripiprazole long-acting injectable (LAI) versus paliperidone LAI in comorbid psychosis and substance use disorder: Impact on clinical status, substance craving, and quality of life. Neuropsychiatr. Dis. Treat. 2018, 14, 1645–1656. [Google Scholar] [CrossRef] [Green Version]
- Szerman, N.; Basurte-Villamor, I.; Vega, P.; Martinez-Raga, J.; Parro-Torres, C.; Cambra Almerge, J.; Grau-López, L.; De Matteis, M.; Arias, F. Once-Monthly Long-Acting Injectable Aripiprazole for the Treatment of Patients with Schizophrenia and Co-occurring Substance Use Disorders: A Multicentre, Observational Study. Drugs Real. World Outcomes 2020, 7, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Higashi, K.; Medic, G.; Littlewood, K.J.; Diez, T.; Granström, O.; De Hert, M. Medication adherence in schizophrenia: Factors influencing adherence and consequences of nonadherence, a systematic literature review. Ther. Adv. Psychopharmacol. 2013, 3, 200–218. [Google Scholar] [CrossRef] [Green Version]
- Vita, A.; De Peri, L.; Deste, G.; Barlati, S.; Sacchetti, E. The Effect of Antipsychotic Treatment on Cortical Gray Matter Changes in Schizophrenia: Does the Class Matter? A Meta-analysis and Meta-regression of Longitudinal Magnetic Resonance Imaging Studies. Biol. Psychiatry 2015, 78, 403–412. [Google Scholar] [CrossRef]
- Capuzzi, E.; Caldiroli, A.; Ciscato, V.; Russo, S.; Buoli, M. Experimental Serotonergic Agents for the Treatment of Schizophrenia. J. Exp. Pharmacol. 2021, 13, 49–67. [Google Scholar] [CrossRef]
- Chen, A.T.; Nasrallah, H.A. Neuroprotective effects of the second generation antipsychotics. Schizophr. Res. 2019, 208, 1–7. [Google Scholar] [CrossRef]
- Nasrallah, H.A.; Chen, A.T. Multiple neurotoxic effects of haloperidol resulting in neuronal death. Ann. Clin. Psychiatry 2017, 29, 195–202. [Google Scholar]
- Caldiroli, A.; Capuzzi, E.; Barkin, J.L.; Grassi, S.; Esposito, C.M.; Auxilia, A.M.; Russo, S.; Tagliabue, I.; Carnevali, G.S.; Mucci, F.; et al. Is there an association between inflammatory/anti-oxidant markers and the presence of psychotic symptoms or severity of illness in mood and psychotic disorders? A multi-centric study on a drug-free sample. Brain Behav. Immun. Health 2022, 22, 100453. [Google Scholar] [CrossRef]
- Capuzzi, E.; Bartoli, F.; Crocamo, C.; Clerici, M.; Carrà, G. Acute variations of cytokine levels after antipsychotic treatment in drug-naïve subjects with a first-episode psychosis: A meta-analysis. Neurosci. Biobehav. Rev. 2017, 77, 122–128. [Google Scholar] [CrossRef]
- Taipale, H.; Tanskanen, A.; Correll, C.U.; Tiihonen, J. Real-world effectiveness of antipsychotic doses for relapse prevention in patients with first-episode schizophrenia in Finland: A nationwide, register-based cohort study. Lancet Psychiatry 2022, 9, 271–279. [Google Scholar] [CrossRef]
- Kane, J.M.; Kishimoto, T.; Correll, C.U. Non-adherence to medication in patients with psychotic disorders: Epidemiology, contributing factors and management strategies. World Psychiatry 2013, 12, 216–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Variables | Total Sample N = 382 | |
|---|---|---|
| Gender | Male | 221 (57.9) |
| Female | 161 (42.1) | |
| Age (years) | 45.43 (12.79) | |
| Work status | Employed * | 304 (79.6) |
| Unemployed | 78 (20.4) | |
| Marital status | Single | 221 (57.9) |
| Married/cohabitant | 161 (42.1) | |
| Age at onset (years) | 27.04 (8.29) | |
| Diagnosis | Bipolar Disorder | 101 (26.4) |
| Schizoaffective disorder | 59 (15.4) | |
| Schizophrenia | 222 (58.2) | |
| Duration of illness (years) | 18.52 (12.70) | |
| Duration of Untreated Illness (DUI) (years) | 2.60 (5.32) | |
| Presence of personality disorders | Yes | 52 (13.6) |
| No | 330 (86.4) | |
| Family history of psychiatric disorders Missing: 10 | Yes | 144 (38.7) |
| No | 228 (61.3) | |
| Multiple family history of psychiatric disorders Missing: 11 | Yes | 42 (11.3) |
| No | 329 (88.7) | |
| Pre-onset psychiatric comorbidity | Yes | 65 (17.0) |
| No | 317 (83.0) | |
| Pre-onset psychiatric poly-comorbidity | Yes | 3 (0.8) |
| No | 379 (99.2) | |
| Post-onset psychiatric comorbidity | Yes | 14 (3.7) |
| No | 368 (96.3) | |
| Post-onset psychiatric poly-comorbidity | Yes | 2 (0.5) |
| No | 380 (99.5) | |
| Pre-onset medical comorbidity | Yes | 63 (16.5) |
| No | 319 (83.5) | |
| Pre-onset medical poly-comorbidity | Yes | 13 (3.4) |
| No | 369 (96.6) | |
| Post-onset medical comorbidity | Yes | 158 (41.4) |
| No | 224 (58.6) | |
| Post-onset medical poly-comorbidity | Yes | 82 (21.5) |
| No | 300 (78.5) | |
| Pre-onset substance misuse | Yes | 80 (20.9) |
| No | 302 (79.1) | |
| Pre-onset poly-substance misuse | Yes | 41 (10.7) |
| No | 341 (89.3) | |
| Post-onset substance misuse | Yes | 86 (22.5) |
| No | 296 (77.5) | |
| Post-onset poly-substance misuse | Yes | 49 (12.8) |
| No | 333 (87.2) | |
| Presence of previous suicide attempts | Yes | 54 (14.1) |
| No | 328 (85.9) | |
| Number of previous suicide attempts | 0.22 (0.67) | |
| Presence of previous hospitalizations | Yes | 362 (94.8) |
| No | 20 (5.2) | |
| Number of previous hospitalizations | 4.60 (4.64) | |
| History of criminal acts | Yes | 44 (11.5) |
| No | 338 (88.5) | |
| LAI antipsychotic treatment | Haloperidol decanoate | 150 (39.3) |
| Zuclopenthixol decanoate | 44 (11.5) | |
| Paliperidone palmitate | 77 (20.2) | |
| Olanzapine pamoate | 22 (5.7) | |
| Aripiprazole | 56 (14.7) | |
| Risperidone | 33 (8.6) | |
| First/Second generation LAI antipsychotic treatment | First generation | 194 (50.8) |
| Second generation | 188 (49.2) | |
| Survival at 12 months | Yes | 272 (71.2) |
| No | 110 (28.8) | |
| Months of survival | Haloperidol decanoate | 10.24 (3.14) |
| Zuclopenthixol decanoate | 9.34 (3.66) | |
| Paliperidone palmitate | 10.06 (3.43) | |
| Olanzapine pamoate | 11.36 (1.76) | |
| Aripiprazole | 10.55 (3.16) | |
| Risperidone | 9.30 (3.72) | |
| Total | 10.13 (3.28) | |
| Reason for discontinuation of LAI antipsychotic | No discontinuation | 244 (63.9) |
| Recurrence (including hospitalization) | 20 (5.2) | |
| Side effects | 40 (10.5) | |
| No compliance | 78 (20.4) | |
| Current poly-pharmacotherapy | Yes | 210 (55.0) |
| No | 172 (45.0) | |
| Treatment side effects | Yes | 150 (39.3) |
| No | 232 (60.7) | |
| Presence of multiple side effects | Yes | 35 (9.2) |
| No | 347 (90.8) | |
| Lifetime psychotherapy | Yes | 63 (16.5) |
| No | 319 (83.5) | |
| Type of lifetime psychotherapy | None | 319 (83.5) |
| Psychoeducation/supportive | 40 (10.5) | |
| Cognitive-Behavioral Therapy | 16 (4.2) | |
| Psychodynamic | 7 (1.8) | |
| Current psychotherapy | Yes | 9 (2.4) |
| No | 373 (97.6) | |
| Type of current psychotherapy | None | 373 (97.6) |
| Psychoeducation/supportive | 6 (1.6) | |
| Cognitive-Behavioral Therapy | 2 (0.5) | |
| Psychodynamic | 1 (0.3) | |
| Predictors | B | p | Exp(B) | CI |
|---|---|---|---|---|
| Age | 0.204 | 0.520 | 1.226 | 0.659–2.279 |
| Age at onset | −0.207 | 0.514 | 0.813 | 0.437–1.512 |
| Duration of illness | −0.215 | 0.497 | 0.807 | 0.434–1.499 |
| Duration of untreated illness | 0.026 | 0.200 | 1.027 | 0.986–1.069 |
| Presence of personality disorders (yes/no) | −0.057 | 0.871 | 0.944 | 0.473–1.884 |
| Family history for psychiatric disorders (yes/no) | −0.051 | 0.835 | 0.950 | 0.588–1.536 |
| Work status (employed versus the others) | 0.023 | 0.929 | 1.023 | 0.619–1.691 |
| Marital status (married/in partnership versus the others) | 0.050 | 0.840 | 1.051 | 0.648–1.706 |
| Pre-onset psychiatric comorbidity (yes/no) | 0.191 | 0.549 | 1.210 | 0.648–2.259 |
| Post-onset psychiatric comorbidity (yes/no) | 0.184 | 0.764 | 1.202 | 0.360–4.013 |
| Pre-onset substance use disorders (yes/no) | −0.073 | 0.862 | 0.930 | 0.411–2.105 |
| Post-onset substance use disorders (yes/no) | 0.193 | 0.640 | 1.213 | 0.540–2.724 |
| Pre-onset medical comorbidity (yes/no) | −0.145 | 0.634 | 0.865 | 0.475–1.573 |
| Post-onset medical comorbidity (yes/no) | −0.492 | 0.098 | 0.612 | 0.342–1.095 |
| Diagnosis | NA | 0.532 | NA | NA |
| History of criminal acts (yes/no) | −0.332 | 0.290 | 0.718 | 0.388–1.328 |
| Gender | 0.191 | 0.463 | 0.463 | 0.727–2.014 |
| Multiple family history of psychiatric disorders (yes/no) | −0.037 | 0.921 | 0.964 | 0.462–2.010 |
| Pre-onset multiple substance use disorders (yes/no) | −1.049 | 0.058 | 0.350 | 0.118–1.035 |
| Post-onset multiple substance use disorders (yes/no) | 0.433 | 0.444 | 1.542 | 0.509–4.677 |
| Pre-onset multiple medical comorbidity (yes/no) | −0.061 | 0.929 | 0.941 | 0.244–3.622 |
| Post-onset multiple medical comorbidity (yes/no) | 0.424 | 0.217 | 1.528 | 0.779–2.999 |
| Type of LAI antipsychotic | NA | 0.033 | NA | NA |
| Poly-therapy (yes/no) | 0.128 | 0.556 | 1.137 | 0.743–1.739 |
| Lifetime psychotherapy (yes/no) | −0.404 | 0.158 | 0.668 | 0.381–1.169 |
| Lifetime attempted suicide (yes/no) | −0.370 | 0.288 | 0.691 | 0.349–1.368 |
| Lifetime hospitalizations (yes/no) | −0.108 | 0.812 | 0.897 | 0.369–2.185 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Auxilia, A.M.; Buoli, M.; Caldiroli, A.; Carnevali, G.S.; Tringali, A.; Nava, R.; Clerici, M.; Capuzzi, E. High Rate of Discontinuation during Long-Acting Injectable Antipsychotic Treatment in Patients with Psychotic Disorders. Biomedicines 2023, 11, 314. https://doi.org/10.3390/biomedicines11020314
Auxilia AM, Buoli M, Caldiroli A, Carnevali GS, Tringali A, Nava R, Clerici M, Capuzzi E. High Rate of Discontinuation during Long-Acting Injectable Antipsychotic Treatment in Patients with Psychotic Disorders. Biomedicines. 2023; 11(2):314. https://doi.org/10.3390/biomedicines11020314
Chicago/Turabian StyleAuxilia, Anna Maria, Massimiliano Buoli, Alice Caldiroli, Greta Silvia Carnevali, Agnese Tringali, Roberto Nava, Massimo Clerici, and Enrico Capuzzi. 2023. "High Rate of Discontinuation during Long-Acting Injectable Antipsychotic Treatment in Patients with Psychotic Disorders" Biomedicines 11, no. 2: 314. https://doi.org/10.3390/biomedicines11020314
APA StyleAuxilia, A. M., Buoli, M., Caldiroli, A., Carnevali, G. S., Tringali, A., Nava, R., Clerici, M., & Capuzzi, E. (2023). High Rate of Discontinuation during Long-Acting Injectable Antipsychotic Treatment in Patients with Psychotic Disorders. Biomedicines, 11(2), 314. https://doi.org/10.3390/biomedicines11020314