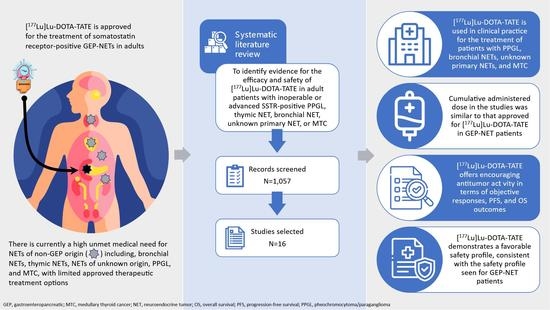
Efficacy and Safety of [177Lu]Lu-DOTA-TATE in Adults with Inoperable or Metastatic Somatostatin Receptor-Positive Pheochromocytomas/Paragangliomas, Bronchial and Unknown Origin Neuroendocrine Tumors, and Medullary Thyroid Carcinoma: A Systematic Literature Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection Process
2.3. Inclusion and Exclusion Criteria
2.4. Types of Intervention
2.5. Types of Outcome
2.6. Types of Study
2.7. Data Extraction and Synthesis
3. Results
3.1. PPGL
3.2. Bronchial NETs
3.3. NETs of Unknown Primary Origin
3.4. MTC
3.5. Safety of [177Lu]Lu-DOTA-TATE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hofland, J.; Kaltsas, G.; de Herder, W.W. Advances in the diagnosis and management of well-differentiated neuroendocrine neoplasms. Endocr. Rev. 2020, 41, 371–403. [Google Scholar] [CrossRef] [Green Version]
- Rindi, G.; Klimstra, D.S.; Abedi-Ardekani, B.; Asa, S.L.; Bosman, F.T.; Brambilla, E.; Busam, K.J.; de Krijger, R.R.; Dietel, M.; El-Naggar, A.K.; et al. A common classification framework for neuroendocrine neoplasms: An International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod. Pathol. 2018, 31, 1770–1786. [Google Scholar] [CrossRef] [Green Version]
- Stueven, A.K.; Kayser, A.; Wetz, C.; Amthauer, H.; Wree, A.; Tacke, F.; Wiedenmann, B.; Roderburg, C.; Jann, H. Somatostatin analogues in the treatment of neuroendocrine tumors: Past, present and future. Int. J. Mol. Sci. 2019, 20, 3049. [Google Scholar] [CrossRef] [Green Version]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- Advanced Accelerator Applications USA, Inc. LUTATHERA® US Prescribing Information. Available online: https://www.novartis.us/sites/www.novartis.us/files/lutathera.pdf (accessed on 5 January 2022).
- European Medicines Agency. Lutathera: EPAR-Product Information. Available online: https://www.ema.europa.eu/documents/product-information/lutathera-epar-product-information_en.pdf (accessed on 3 February 2022).
- Strosberg, J.R.; Caplin, M.E.; Kunz, P.L.; Ruszniewski, P.B.; Bodei, L.; Hendifar, A.; Mittra, E.; Wolin, E.M.; Yao, J.C.; Pavel, M.E.; et al. 177Lu-Dotatate plus long-acting octreotide versus high-dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): Final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 1752–1763. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Hope, T.A.; Bodei, L.; Chan, J.A.; El-Haddad, G.; Fidelman, N.; Kunz, P.L.; Mailman, J.; Menda, Y.; Metz, D.C.; Mittra, E.S.; et al. NANETS/SNMMI consensus statement on patient selection and appropriate use of 177Lu-DOTATATE peptide receptor radionuclide therapy. J. Nucl. Med. 2020, 61, 222–227. [Google Scholar] [CrossRef]
- Righi, L.; Volante, M.; Tavaglione, V.; Billè, A.; Daniele, L.; Angusti, T.; Inzani, F.; Pelosi, G.; Rindi, G.; Papotti, M. Somatostatin receptor tissue distribution in lung neuroendocrine tumours: A clinicopathologic and immunohistochemical study of 218 ‘clinically aggressive’ cases. Ann. Oncol. 2010, 21, 548–555. [Google Scholar] [CrossRef]
- Hauso, O.; Gustafsson, B.I.; Kidd, M.; Waldum, H.L.; Drozdov, I.; Chan, A.K.; Modlin, I.M. Neuroendocrine tumor epidemiology: Contrasting Norway and North America. Cancer 2008, 113, 2655–2664. [Google Scholar] [CrossRef]
- Riihimäki, M.; Hemminki, A.; Sundquist, K.; Sundquist, J.; Hemminki, K. The epidemiology of metastases in neuroendocrine tumors. Int. J. Cancer 2016, 139, 2679–2686. [Google Scholar] [CrossRef]
- Brabander, T.; van der Zwan, W.A.; Teunissen, J.J.M.; Kam, B.L.R.; Feelders, R.A.; de Herder, W.W.; van Eijck, C.H.J.; Franssen, G.J.H.; Krenning, E.P.; Kwekkeboom, D.J. Long-term efficacy, survival, and safety of [177Lu-DOTA0,Tyr3]octreotate in patients with gastroenteropancreatic and bronchial neuroendocrine tumors. Clin. Cancer Res. 2017, 23, 4617–4624. [Google Scholar] [CrossRef] [Green Version]
- Satapathy, S.; Mittal, B.R.; Bhansali, A. Peptide receptor radionuclide therapy in the management of advanced pheochromocytoma and paraganglioma: A systematic review and meta-analysis. Clin. Endocrinol. 2019, 91, 718–727. [Google Scholar] [CrossRef]
- Rindi, G.; Wiedenmann, B. Neuroendocrine neoplasia of the gastrointestinal tract revisited: Towards precision medicine. Nat. Rev. Endocrinol. 2020, 16, 590–607. [Google Scholar] [CrossRef]
- Baudin, E.; Caplin, M.; Garcia-Carbonero, R.; Fazio, N.; Ferolla, P.; Filosso, P.L.; Frilling, A.; de Herder, W.W.; Hörsch, D.; Knigge, U.; et al. Lung and thymic carcinoids: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 439–451. [Google Scholar] [CrossRef]
- Fassnacht, M.; Assie, G.; Baudin, E.; Eisenhofer, G.; de la Fouchardiere, C.; Haak, H.R.; de Krijger, R.; Porpiglia, F.; Terzolo, M.; Berruti, A. Adrenocortical carcinomas and malignant phaeochromocytomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1476–1490. [Google Scholar] [CrossRef]
- Filetti, S.; Durante, C.; Hartl, D.; Leboulleux, S.; Locati, L.D.; Newbold, K.; Papotti, M.G.; Berruti, A. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1856–1883. [Google Scholar] [CrossRef] [Green Version]
- National Comprehensive Cancer Network. Neuroendocrine and Adrenal Tumors. Version 4.2021. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1448 (accessed on 12 January 2022).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Pinato, D.J.; Black, J.R.; Ramaswami, R.; Tan, T.M.; Adjogatse, D.; Sharma, R. Peptide receptor radionuclide therapy for metastatic paragangliomas. Med. Oncol. 2016, 33, 47. [Google Scholar] [CrossRef]
- van Essen, M.; Krenning, E.P.; Kooij, P.P.; Bakker, W.H.; Feelders, R.A.; de Herder, W.W.; Wolbers, J.G.; Kwekkeboom, D.J. Effects of therapy with [177Lu-DOTA0, Tyr3]octreotate in patients with paraganglioma, meningioma, small cell lung carcinoma, and melanoma. J. Nucl. Med. 2006, 47, 1599–1606. [Google Scholar]
- Vyakaranam, A.R.; Crona, J.; Norlén, O.; Granberg, D.; Garske-Román, U.; Sandström, M.; Fröss-Baron, K.; Thiis-Evensen, E.; Hellman, P.; Sundin, A. Favorable outcome in patients with pheochromocytoma and paraganglioma treated with 177Lu-DOTATATE. Cancers 2019, 11, 909. [Google Scholar] [CrossRef] [Green Version]
- Zandee, W.T.; Feelders, R.A.; Smit Duijzentkunst, D.A.; Hofland, J.; Metselaar, R.M.; Oldenburg, R.A.; van Linge, A.; Kam, B.L.R.; Teunissen, J.J.M.; Korpershoek, E.; et al. Treatment of inoperable or metastatic paragangliomas and pheochromocytomas with peptide receptor radionuclide therapy using 177Lu-DOTATATE. Eur. J. Endocrinol. 2019, 181, 45–53. [Google Scholar] [CrossRef]
- Jaiswal, S.K.; Sarathi, V.; Memon, S.S.; Garg, R.; Malhotra, G.; Verma, P.; Shah, R.; Sehemby, M.K.; Patil, V.A.; Jadhav, S.; et al. 177Lu-DOTATATE therapy in metastatic/inoperable pheochromocytoma-paraganglioma. Endocr. Connect. 2020, 9, 864–873. [Google Scholar] [CrossRef]
- Parghane, R.V.; Talole, S.; Basu, S. 131I-MIBG negative progressive symptomatic metastatic paraganglioma: Response and outcome with 177Lu-DOTATATE peptide receptor radionuclide therapy. Ann. Nucl. Med. 2021, 35, 92–101. [Google Scholar] [CrossRef]
- Roll, W.; Müther, M.; Sporns, P.B.; Zinnhardt, B.; Suero Molina, E.; Seifert, R.; Schäfers, M.; Weckesser, M.; Stegger, L.; Beule, A.G.; et al. Somatostatin receptor-targeted radioligand therapy in head and neck paraganglioma. World Neurosurg. 2020, 143, e391–e399. [Google Scholar] [CrossRef]
- Ianniello, A.; Sansovini, M.; Severi, S.; Nicolini, S.; Grana, C.M.; Massri, K.; Bongiovanni, A.; Antonuzzo, L.; Di Iorio, V.; Sarnelli, A.; et al. Peptide receptor radionuclide therapy with 177Lu-DOTATATE in advanced bronchial carcinoids: Prognostic role of thyroid transcription factor 1 and 18F-FDG PET. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1040–1046. [Google Scholar] [CrossRef]
- van Essen, M.; Krenning, E.P.; Bakker, W.H.; de Herder, W.W.; van Aken, M.O.; Kwekkeboom, D.J. Peptide receptor radionuclide therapy with 177Lu-octreotate in patients with foregut carcinoid tumours of bronchial, gastric and thymic origin. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1219–1227. [Google Scholar] [CrossRef] [Green Version]
- Garske-Román, U.; Sandström, M.; Fröss Baron, K.; Lundin, L.; Hellman, P.; Welin, S.; Johansson, S.; Khan, T.; Lundqvist, H.; Eriksson, B.; et al. Prospective observational study of 177Lu-DOTA-octreotate therapy in 200 patients with advanced metastasized neuroendocrine tumours (NETs): Feasibility and impact of a dosimetry-guided study protocol on outcome and toxicity. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 970–988. [Google Scholar] [CrossRef] [Green Version]
- Mariniello, A.; Bodei, L.; Tinelli, C.; Baio, S.M.; Gilardi, L.; Colandrea, M.; Papi, S.; Valmadre, G.; Fazio, N.; Galetta, D.; et al. Long-term results of PRRT in advanced bronchopulmonary carcinoid. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 441–452. [Google Scholar] [CrossRef]
- Mirvis, E.; Toumpanakis, C.; Mandair, D.; Gnanasegaran, G.; Caplin, M.; Navalkissoor, S. Efficacy and tolerability of peptide receptor radionuclide therapy (PRRT) in advanced metastatic bronchial neuroendocrine tumours (NETs). Lung Cancer 2020, 150, 70–75. [Google Scholar] [CrossRef]
- Sabet, A.; Haug, A.R.; Eiden, C.; Auernhammer, C.J.; Simon, B.; Bartenstein, P.; Biersack, H.J.; Ezziddin, S. Efficacy of peptide receptor radionuclide therapy with 177Lu-octreotate in metastatic pulmonary neuroendocrine tumors: A dual-centre analysis. Am. J. Nucl. Med. Mol. Imaging 2017, 7, 74–83. [Google Scholar]
- Vaisman, F.; Rosado de Castro, P.H.; Lopes, F.P.; Kendler, D.B.; Pessoa, C.H.; Bulzico, D.A.; de Carvalho Leal, D.; Vilhena, B.; Vaisman, M.; Carneiro, M.; et al. Is there a role for peptide receptor radionuclide therapy in medullary thyroid cancer? Clin. Nucl. Med. 2015, 40, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Parghane, R.V.; Naik, C.; Talole, S.; Desmukh, A.; Chaukar, D.; Banerjee, S.; Basu, S. Clinical utility of 177Lu-DOTATATE PRRT in somatostatin receptor-positive metastatic medullary carcinoma of thyroid patients with assessment of efficacy, survival analysis, prognostic variables, and toxicity. Head Neck 2020, 42, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Beukhof, C.M.; Brabander, T.; van Nederveen, F.H.; van Velthuysen, M.F.; de Rijke, Y.B.; Hofland, L.J.; Franssen, G.J.H.; Fröberg, L.A.C.; Kam, B.L.R.; Visser, W.E.; et al. Peptide receptor radionuclide therapy in patients with medullary thyroid carcinoma: Predictors and pitfalls. BMC Cancer 2019, 19, 325. [Google Scholar] [CrossRef] [Green Version]
- Brabander, T.; Teunissen, J.J.; Van Eijck, C.H.; Franssen, G.J.; Feelders, R.A.; de Herder, W.W.; Kwekkeboom, D.J. Peptide receptor radionuclide therapy of neuroendocrine tumours. Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 103–114. [Google Scholar] [CrossRef]
- Saravana-Bawan, B.; Bajwa, A.; Paterson, J.; McEwan, A.J.B.; McMullen, T.P.W. Efficacy of 177Lu peptide receptor radionuclide therapy for the treatment of neuroendocrine tumors: A meta-analysis. Clin. Nucl. Med. 2019, 44, 719–727. [Google Scholar] [CrossRef]
- Wang, L.F.; Lin, L.; Wang, M.J.; Li, Y. The therapeutic efficacy of 177Lu-DOTATATE/DOTATOC in advanced neuroendocrine tumors: A meta-analysis. Medicine 2020, 99, e19304. [Google Scholar] [CrossRef]
- Zhang, J.; Song, Q.; Cai, L.; Xie, Y.; Chen, Y. The efficacy of 177Lu-DOTATATE peptide receptor radionuclide therapy (PRRT) in patients with metastatic neuroendocrine tumours: A systematic review and meta-analysis. J. Cancer Res. Clin. Oncol. 2020, 146, 1533–1543. [Google Scholar] [CrossRef]
- Ayala-Ramirez, M.; Feng, L.; Habra, M.A.; Rich, T.; Dickson, P.V.; Perrier, N.; Phan, A.; Waguespack, S.; Patel, S.; Jimenez, C. Clinical benefits of systemic chemotherapy for patients with metastatic pheochromocytomas or sympathetic extra-adrenal paragangliomas: Insights from the largest single-institutional experience. Cancer 2012, 118, 2804–2812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pryma, D.A.; Chin, B.B.; Noto, R.B.; Dillon, J.S.; Perkins, S.; Solnes, L.; Kostakoglu, L.; Serafini, A.N.; Pampaloni, M.H.; Jensen, J.; et al. Efficacy and Safety of High-Specific-Activity (131)I-MIBG Therapy in Patients with Advanced Pheochromocytoma or Paraganglioma. J. Nucl. Med. 2019, 60, 623–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fazio, N.; Buzzoni, R.; Delle Fave, G.; Tesselaar, M.E.; Wolin, E.; Van Cutsem, E.; Tomassetti, P.; Strosberg, J.; Voi, M.; Bubuteishvili-Pacaud, L.; et al. Everolimus in advanced, progressive, well-differentiated, non-functional neuroendocrine tumors: RADIANT-4 lung subgroup analysis. Cancer Sci. 2018, 109, 174–181. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Carnaghi, C.; Buzzoni, R.; Pommier, R.F.; Raderer, M.; Tomasek, J.; Lahner, H.; Valle, J.W.; Voi, M.; Bubuteishvili-Pacaud, L.; et al. Everolimus in Neuroendocrine Tumors of the Gastrointestinal Tract and Unknown Primary. Neuroendocrinology 2018, 106, 211–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maghsoomi, Z.; Emami, Z.; Malboosbaf, R.; Malek, M.; Khamseh, M.E. Efficacy and safety of peptide receptor radionuclide therapy in advanced radioiodine-refractory differentiated thyroid cancer and metastatic medullary thyroid cancer: A systematic review. BMC Cancer 2021, 21, 579. [Google Scholar] [CrossRef]
- Kim, M.; Kim, B.H. Current guidelines for management of medullary thyroid carcinoma. Endocrinol. Metab. 2021, 36, 514–524. [Google Scholar] [CrossRef]
- Elisei, R.; Schlumberger, M.J.; Müller, S.P.; Schöffski, P.; Brose, M.S.; Shah, M.H.; Licitra, L.; Jarzab, B.; Medvedev, V.; Kreissl, M.C.; et al. Cabozantinib in progressive medullary thyroid cancer. J. Clin. Oncol. 2013, 31, 3639–3646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, S.A., Jr.; Robinson, B.G.; Gagel, R.F.; Dralle, H.; Fagin, J.A.; Santoro, M.; Baudin, E.; Elisei, R.; Jarzab, B.; Vasselli, J.R.; et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: A randomized, double-blind phase III trial. J. Clin. Oncol. 2012, 30, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Schlumberger, M.; Elisei, R.; Müller, S.; Schöffski, P.; Brose, M.; Shah, M.; Licitra, L.; Krajewska, J.; Kreissl, M.C.; Niederle, B.; et al. Overall survival analysis of EXAM, a phase III trial of cabozantinib in patients with radiographically progressive medullary thyroid carcinoma. Ann. Oncol. 2017, 28, 2813–2819. [Google Scholar] [CrossRef] [PubMed]
- Stolniceanu, C.R.; Nistor, I.; Bilha, S.C.; Constantin, V.; Simona, V.; Matovic, M.; Stefanescu, C.; Covic, A. Nephrotoxicity/renal failure after therapy with 90Yttrium- and 177Lutetium-radiolabeled somatostatin analogs in different types of neuroendocrine tumors: A systematic review. Nucl. Med. Commun. 2020, 41, 601–617. [Google Scholar] [CrossRef]
- Sonbol, M.B.; Halfdanarson, T.R.; Hilal, T. Assessment of therapy-related myeloid neoplasms in patients with neuroendocrine tumors after peptide receptor radionuclide therapy: A systematic review. JAMA Oncol. 2020, 6, 1086–1092. [Google Scholar] [CrossRef]
- Adnan, A.; Kudachi, S.; Ramesh, S.; Prabhash, K.; Basu, S. Metastatic or locally advanced mediastinal neuroendocrine tumours: Outcome with 177Lu-DOTATATE-based peptide receptor radionuclide therapy and assessment of prognostic factors. Nucl. Med. Commun. 2019, 40, 947–957. [Google Scholar] [CrossRef] [PubMed]

| Study ID | Design and Country | Study Timeline | No. of Patients | Age, Years and Sex | Previous Treatment | NET Grade | NET Stage/Disease State at Baseline | SSTR Assessment Method and Grade | Per Cycle Activity of [177Lu]Lu-DOTA-TATE, GBq a (No. of Cycles) | Cumulative Administered Activity, GBq |
|---|---|---|---|---|---|---|---|---|---|---|
| PPGL | ||||||||||
| Van Essen 2006 [22] | Prospective study Netherlands | Not specified | N = 12 PGL: n = 12 | Mean 39.7 (range 22–55) M: n = 6 F: n = 6 | Surgery: n = 9 Radiotherapy: n = 7 Chemotherapy: n = 4 None: n = 2 | Not specified | Metastatic: n = 9 PD: n = 4 SD: n = 5 Unknown: n = 3 | SSTR scintigraphy (OctreoScan): Grade 2: n = 1 Grade 3: n = 7 Grade 4: n = 4 | 7.4 (max. 4 cycles) | Range 14.8–29.6 |
| Vyakaranam 2019 [23] | Retrospective cohort analysis (chart review) Sweden | 2005–2018 | N = 22 PCC: n = 9 PGL: n = 13 | Median 60 (range 24–80) M: n = 13 F: n = 9 | Surgery: n = 16 Radiotherapy: n = 14 [131I]I-MIBG: n = 6 Chemotherapy: n = 1 | Median Ki-67, 11% (range 1–30) n = 18 | Metastatic: n = 20 PD: n = 9 | SSTR scintigraphy Krenning score ≥ 3 | 7.4 (range 3–11 cycles including salvage therapy) | Median 29.6 Range 22.2–81.4 (including salvage therapy) |
| Zandee 2019 [24] | Retrospective case series Netherlands | From Jan 2000 | N = 30 PCC: n = 3 PGL: n = 27 | Median 47 (range 29–74) M: n = 10 F: n = 20 | Surgery: n = 19 Radiotherapy: n = 6 Chemotherapy: n = 5 [131I]I-MIBG: n = 3 SSA: n = 2 | Not specified | Metastatic: n = 17 Localized: n = 13 b PD: n = 20 SD: n = 7 Unknown: n = 3 | SSTR scintigraphy: Grade 2: n = 11 Grade 3: n = 13 Grade 4: n = 6 | 7.4 (range 1–4 cycles) | Range 14.8–29.6 |
| Jaiswal 2020 [25] | Retrospective case series India | Jan 2010–Dec 2019 | N = 14 PCC: n = 3 PGL: n = 10 PCC + PGL: n= 1 | Range 18–59 M: n = 6 c F: n = 8 c | Surgery: n = 9 EBRT: n = 3 | Not specified | Progressive, metastasis: n = 6 Progressive, inoperable: n = 1 Inoperable + metastasis: n = 4 Inoperable: n = 3 | PET/CT with 68Ga-labeled analog Krenning score ≥2: Score 2: n = 1 Score 3: n = 2 Score 4: n = 10 unknown: n = 1 | 5.55–7.4 (range 1–6 cycles) | Range 6–40 |
| Parghane 2021 [26] | Retrospective case series India | 2012–2019 | N = 9 PGL: n = 9 | Median 49 (range 33–61) M: n = 5 d F: n = 5 | Surgery: n = 6 EBRT: n = 6 Chemotherapy: n = 1 | Median Ki-67 19% (range 1–40): n = 5 | Metastatic: n = 9 PD: n = 7 | PET/CT with [68Ga]Ga-DOTA-TATE Krenning score ≥ 2 | 5.55–7.4 (range 1–6 cycles) | Average 24.42 Range 7.4–37 |
| Roll 2020 [27] | Retrospective case series Germany | May 2014–Oct 2016 | N = 6 PGL: n = 6 e | Range 50–84 M: n = 2 e F: n = 4 e | Surgery: n = 5 Embolization: n = 4 Fractionated photon irradiation: n = 1 | Not specified | Metastatic: n = 0 | PET/CT with [68Ga]Ga-DOTA-TATE Uptake higher than liver uptake on initial treatment | Mean 7.2 ± 0.4 (range 3–5 cycles) | Not specified |
| Pinato 2016 [21] | Retrospective case series UK | 2008–2014 | N = 4 PGL: n = 4 | Range 29–47 M: n = 3 F: n = 1 | Surgery: n = 3 Etoposide/cisplatin: n = 1 EBRT: n = 1 MIBG: n = 1 | Not specified | Metastatic: n = 4 PD: n = 4 | PET/CT with [68Ga]Ga-DOTA-TATE | 6.6–7.5 (1 patient unknown dose) (range 1–4 cycles) | Not specified |
| Bronchial NETs | ||||||||||
| Ianniello 2016 [28] | Prospective cohort study Italy | Apr 2008–Mar 2014 | N = 34 | Range 40–79 M: n = 17 F: n = 17 | SSA: n = 29 Surgery: n = 22 Chemotherapy: n = 13 PRRT: n = 9 Other treatments: n = 9 None: n = 2 | Typical: n = 15 Atypical: n = 19 | Metastatic: n = 34 PD: n = 34 | SSTR scintigraphy (OctreoScan) Krenning score ≥2: score 2: n = 14 score 3: n = 20 | 3.7 or 5.5 according to kidney and bone marrow toxicity risk factors (4 or 5 cycles) | Median 21.5 Range 12.9–27.8 |
| Van Essen 2007 [29] | Prospective cohort study Netherlands | Not specified | N = 9 | Median 62 (range 37–75) M: n = 6 F: n = 3 | Surgery: n = 8 Radiotherapy: n = 2 Chemotherapy: n = 1 | Typical: n = 4 Atypical: n = 5 | Metastatic: n = 9 PD: n = 2 SD: n = 2 | SSTR scintigraphy (OctreoScan): Grade 3: n = 8 Grade 4: n = 1 | 7.4 (3 or 4 cycles) | Intended cumulative dose: 22.2–29.6 |
| Garske-Román 2018 [30] | Prospective cohort study Sweden | Enrollment Sep 2010–Feb 2014; Sweden/Oslo patients’ survival data from health registries accessed until May 2016 | N = 6 | Median 69 (range 41–75) M: n = 5 F: n = 1 | Surgery: 33.3% Chemotherapy: 66.7% SSA: 20% Radiotherapy: 16.7% | Grade 1: n = 1 Grade 2: n = 5 | Metastatic: n = 6 PD: n = 4 Extensive disease: n = 2 | SSTR scintigraphy Krenning score ≥ 3 | 7.4 (5–8 cycles) | Not specified |
| Mariniello 2016 [31] | Retrospective cohort analysis Italy | Treated from 1997 to 2012 and followed until Oct 2014 | N = 48 | Mean (standard deviation): 61.5 (14.3) M: n = 32 F: n = 16 | Surgery: n = 34 Chemotherapy: n = 18 | Typical: n = 22, Atypical: n = 15 Not specified: n = 11 | Advanced (unresectable/metastatic; stage IIIb/IV) PD: n = 39 Stage IV disease: n = 44 | PET/CT with 68Ga-labeled analog or conventional OctreoScan | Planned cumulative dose of 27.75 (administered across 5 cycles) or 29.6 (8 cycles); 22.2 (6 cycles) if risk factors for delayed toxicity | Mean (standard deviation): 20.87 (7.78) |
| Mirvis 2020 [32] | Retrospective cohort analysis UK | 2009–2020 | N = 18 | Not specified for [177Lu]Lu-DOTA-TATE-only patients | Not specified for [177Lu]Lu-DOTA-TATE-only patients | Moderate to well differentiated: n = 18 | Advanced metastatic | [111In]In-DTPA- octreotide or 68Ga-SSA PET/CT Krenning score ≥ 2 | ~7.4 (range 2–4 cycles) | Median 29.8 Range 13.00–30.34 |
| Sabet 2017 [33] | Retrospective cohort analysis Germany/ Austria | Not specified | N = 22 | Mean 63 (range 42–74) M: n = 16 F: n = 6 | Biotherapy: n = 16 Surgery: n = 14 Chemotherapy: n = 7 Locoregional treatment: n = 1 | Ki-67 index: ≤2%: n = 9 3–20%: n = 13 Typical: n = 5 Atypical: n = 17 | Metastatic, unresectable stage IV disease: n = 22 PD: n = 17 | SSTR scintigraphy (e.g., OctreoScan) or PET imaging with 68Ga-SSA Uptake ≥ liver uptake | Mean 7.8 ± 0.68 (range 1–4 cycles) | Mean 27.2 ± 5.9 |
| NETs of unknown origin | ||||||||||
| Garske-Román 2018 [30] | Prospective cohort study Sweden | Enrollment Sep 2010–Feb 2014; Sweden/Oslo patients’ survival data from health registries accessed until May 2016 | N = 8 | Median 65 (range 54–80) M: n = 4 F: n = 4 | Surgery (any type—not primary): 12.5% SSA: 62.5% Radiotherapy: 37.5% Chemotherapy: 87.5% | Grade 1: n = 1 Grade 2: n = 7 | Metastatic: n = 8 PD: n = 6 Extensive disease: n = 7 | SSTR scintigraphy Krenning score ≥ 3 | 7.4 (2–7 cycles) | Not specified for NET type |
| MTC | ||||||||||
| Vaisman 2015 [34] | Prospective study Brazil | Jan 2011–Jul 2013 | N = 9 | Median 35.8 (range 20–54) M: n = 3 F: n = 6 | Not reported | Not specified | Progressive MTC: n = 9 | 111In-DTPA- octreotide scintigraphy Any uptake | 7.4 (up to 4 cycles) | Intended 29.6 |
| Parghane 2020 [35] | Retrospective case series India | Jan 2012–Jul 2018 | N = 43 | Median 48 (range 25–80) M: n = 35 F: n = 8 | Total thyroidectomy: n = 43 EBRT: n = 12 Chemotherapy (sorafenib): n = 1 | Not specified | Progressive c, metastatic MTC: ≥2 organ involvement: n = 34 Widespread metastatic disease: n = 17 | PET/CT with [68Ga]Ga-DOTA-TATE Krenning score ≥ 2 | 5.55 Average 3 cycles (range 1–6) | Average 18.5 Range 5.55–33.3 |
| Beukhof 2019 [36] | Retrospective case report or case series Netherlands | 2000–2017 | N = 10 | Median 62 (range 19–75) M: n = 4 F: n = 6 | Not specified | Not specified | Metastatic MTC: n = 10 PD: n = 8 | 111In-DTPA- octreotide scintigraphy and retrospective IHC | Not specified Average 4 cycles | Up to 27.8–29.6 |
| Study ID | No. of Patients Treated | Follow-Up, Months | Response Criteria | Imaging Method for Response Evaluation and Time Points | Tumor Response: n (%) | ORR a | DCR b | PFS/OS |
|---|---|---|---|---|---|---|---|---|
| PPGL | ||||||||
| van Essen 2006 [22] | 12 | Median 13 (range 4–30) | SWOG | CT or MRI Measured at 6–8 weeks, 3 mo and 6 mo after last treatment, within every 6 mo thereafter | PR: 1 (8) MR: 1 (8) SD: 6 (50) PD: 3 (25) No data: 1 (8) | 8% | 67% | TTP c: Median could not be determined (11 and 15 mo in 2 patients) OS: Not reported |
| Vyakaranam 2019 [23] | 22 | Median 32 (range 8–139) | RECIST 1.1 | CT/MRI Measured before every second treatment cycle, 3 mo after last treatment, at least every 6 mo thereafter | PR: 2 (9) SD: 20 (91) | 9% | 100% | Median PFS: 21.6 mo (range 6.7–138) Median OS: 49.6 mo (range 8.2–139) |
| Zandee 2019 [24] | 30 | Median 52.5 d (range 7–155) | RECIST 1.1 | Radiographic tumor assessment | All (N = 30) PR: 7 (23) SD: 20 (67) PD: 3 (10) pPGL (n = 17): PR: 2 (12) SD: 14 (82) PD: 1 (6) sPGL (n = 10) PR: 4 (40) SD: 5 (50) PD: 1 (10) PCC (n = 3): PR: 1 (33) SD: 1 (33) PD: 1 (33) | 23% 12% 40% 33% | 90% 94% 90% 67% | Median PFS: 30 mo d Median OS: NR d Median PFS: 91 mo d Median OS: NR d Median PFS: 18 mo d Median OS: 59 mo d Median PFS: 10 mo d Median OS: 17 mo d |
| Jaiswal 2020 [25] | 14 | Range 11–62 | RECIST 1.1 with MR | CeCT | All (N = 14) PR: 1 (7) MR: 4 (29) SD: 7 (50) e PD: 2 (14) PGL (n = 10): PR: 1 (10) MR: 4 (40) SD: 5 (50) PCC (n = 2): PD: 2 (100) PCC + pNET (n = 1): SD: 1 (100) PCC + pNET + PGL (n = 1): SD: 1 (100) | 7% 10% 0% 0% 0% | 86% 100% 0% 100% 100% | Median PFS: NR |
| Parghane 2021 [26] | 9 | Median 40 | RECIST 1.1 with MR | CeCT or diagnostic CT part of PET-CT scan Measured before each PRRT cycle (at 10–12 weeks) and then every 6 mo after completing cycles | CR: 0 PR: 1 (11) MR: 2 (22) SD: 3 (33) PD: 3 (33) | 11% | 67% | Median PFS: NR Median OS: NR PFS rate: 63% (95% CI 30–96) Estimated OS rate: 65% (95% CI 32–97) at 40 mo |
| Roll 2020 [27] | 6 | Median 39 (range 16–64) | RECIST 1.1 | [68Ga]Ga-DOTA-TATE PET and CeCT or MRI Measured 3 mo after the last treatment cycle | SD: 6 (100) | 0% | 100% | Not reported |
| Pinato 2016 [21] | 4 | Range 26–84 | Not specified | CT and PET Measured following each cycle | PR: 1 (25) SD: 2 (50) PD: 1 (25) | 25% | 75% | Median OS: NR (range 26–84 mo) Mean (standard deviation) OS: 53 (22.7) mo Mean (standard deviation) PFS: 36.4 (27.4) mo (range 1–78) |
| Bronchial NETs | ||||||||
| Ianniello 2016 [28] | 34 | Median 29 (range 7–69) | SWOG | Multiphase CT and/or MRI Measured at 3, 6, 12, 18, and 24 mo after treatment and every 6–12 mo thereafter | All (N = 34) CR: 1 (3) PR: 4 (12) SD: 16 (47) Typical (n = 15) CR: 1 (7) PR: 4 (27) SD: 7 (47) Atypical (n = 19) SD: 9 (47) | 15% 33% 0% | 62% 80% 47% | Median PFS: 18.5 mo (95% CI 12.9–26.4) Median OS: 48.6 mo (95% CI 26.4–68.9) Median PFS: 20.1 mo (95% CI 11.8–26.8) Median OS: 48.6 mo (95% CI 26.0–NR) Median PFS: 15.7 mo (95% CI 10.6–25.9) Median OS: 37 mo (95% CI 18.7–68.9) |
| van Essen 2007 [29] | 9 | Median 36 (range 23–76) | Modified SWOG | CT or MRI Measured at 6–8 weeks, 3 mo, and 6 mo after last treatment, and every 6 mo thereafter | PR: 5 (56) MR: 1 (11) SD: 2 (22) PD: 1 (11) | 56% | 89% | Median TTP b: 31 mo |
| Garske-Román 2018 [30] | 6 | Not specified | RECIST 1.1 | Radiological assessment Scintigraphy or ultrasonography used in clinically clear cases of progression when CT data were not available | PR: 1 (17) SD: 5 (83) | 17% | 100% | Median PFS: 18 mo (95% CI 12–43) Median OS: NR (19 mo–NR) |
| Mariniello 2016 [31] | 48 | Median 45.1 (range 3–191) | RECIST | CT, MRI Measured at 6–8 weeks after the second cycle and every 6 or 12 mo thereafter | PR: 6 (13) MR: 8 (17) SD: 22 (46) | 13% | 75% | Median PFS: 31.0 mo (IQR 21.0–49.1) PFS at 3 y after the start of PRRT: 39.8% (95% CI 0.25–0.54) 5-y OS: 61.4% (95% CI 41.5–77.0) Median OS: NR at 110 mo |
| Mirvis 2020 [32] | 18 | Not reported | RECIST 1.1 | CT | Not reported | NA | NA | Median PFS: 18 mo |
| Sabet 2017 [33] | 22 | Median 54 (range 5–75) | RECIST 1.1 | CT or MRI Measured at 3 mo after termination of PRRT and every 6 months thereafter | PR: 6 (27) SD: 9 (41) PD: 7 (32) | 27% | 68% | Median PFS: 27 mo (95% CI 9–45) Median OS: 42 mo (95% CI 25–59) |
| NETs of unknown origin | ||||||||
| Garske-Román 2018 [30] | 8 | Not specified | RECIST 1.1 | Radiological assessment Scintigraphy or ultrasonography used in clinically clear cases of progression when CT data were not available | PR: 3 (38) SD: 4 (50) PD: 1 (13) | 38% | 88% | Median PFS: 17.5 mo (95% CI 7–34) Median OS: 43 mo (95% CI 15–NR) |
| MTC | ||||||||
| Vaisman 2015 [34] | 7 f | Range 8–12 months | RECIST 1.1 | CT scans of the neck and chest and MRI of the liver and known bone metastasis Measured at 8–12 months after finishing 4th cycle | PR: 3 (43) SD: 3 (43) PD: 1 (14) | 43% | 86% | Not reported |
| Parghane 2020 [35] | 43 | Median (range) 20 (8–78) | RECIST 1.1 | CeCT or CT part of PET-CT scan | PR: 2 (5) SD: 25 (58) PD: 16 (37) | 5% | 63% | Median PFS: 24 mo (95% CI 15.1–32.9) Median OS: 26 mo (95% CI 16.6–35.3) |
| Beukhof 2019 [36] | 10 | Not specified | RECIST 1.1 | Not specified Measured at 3 months after completing treatment | SD: 4 (40) PD: 6 (60) | 0% | 40% | Median PFS: 0.7 y (range 0.3–12.0) Median OS: 1.14 y (range 0.4–12.0) Median OS in SD patients: 1.8 y (range 0.8–12.0) |
| Study ID | N | Indication | Acute Toxicity Per Treatment, % | AEs, n (%) | Renal Toxicity, n (%) | Hematologic Toxicity, n (%) | Additional Comments |
|---|---|---|---|---|---|---|---|
| Vyakaranam 2019 [23] | 22 | PPGL | Not reported | Not reported | None | None: 6 (27) a Any grade 1/2: 16 (73) a | |
| Zandee 2019 [24] | 30 | PPGL | Nausea: 34% Pain: 23% Vomiting: 13% | Cardiac failure: 1 (3) Pleural effusion and delirium: 1 (3) | Not reported | Anemia grade 3: 2 (7) b Thrombocytopenia grade 3: 4 (13) b Thrombocytopenia grade 4: 1 (3) b Leukopenia grade 3: 3 (10) b | MDS: 1 (considered to be related to treatment) Persistent thrombocytopenia limited treatment in 3 patients |
| Jaiswal 2020 [25] | 15 c | PPGL | Not reported | Nausea/vomiting: 3 (20) Weight loss: 2 (13) | None | Thrombocytopenia grade 2: 1 (7) d Anemia + thrombocytopenia grade 2: 1 (7) d | |
| Parghane 2021 [26] | 9 | PGL | Not reported | Nausea/vomiting: 2 (22) | None | Anemia grade 1: 1 (11) Thrombocytopenia: 0 Leukopenia: 0 | |
| Roll 2020 [27] | 7 e | PGL | Not reported | Not reported | Not reported | None: 4 (57) Leukopenia grade 1: 1 (14) Anemia grade 1: 1 (14) Leukopenia grade 2 + anemia grade 1: 1 (14) | |
| Pinato 2016 [21] | 5 f | PGL | None | Suspected pneumonitis: 1 (20) Reactionary swelling of metastases: 1 (20) | Not reported | Not reported | |
| Ianniello 2016 [28] | 34 | Bronchial NETs | No grade ≥3 b | Not reported | Not reported | Any grade ≥3: 0 b | |
| Mariniello 2016 [31] | 47 g | Bronchial NETs | Not reported | Not reported | Serum creatinine increase grade 0: 34 (74) a Serum creatinine increase grade 1: 11 (23) a Serum creatinine increase grade 2: 1 (2) a | Anemia grade 0: 12 (26) a Anemia grade 1: 32 (68) a Anemia grade 2: 3 (6) a Leukopenia Grade 0: 28 (60) a Leukopenia grade 1: 14 (30) a Leukopenia grade 2: 5 (11) a Thrombocytopenia grade 0: 28 (60) a Thrombocytopenia grade 1: 18 (38) a Thrombocytopenia grade 2: 1 (2) a | MDS: 0 AML: 0 |
| Mirvis 2020 [32] | 18 | Bronchial NETs | Not reported | Radiation pericarditis grade 3: 1 (6) | None | Thrombocytopenia grade 3: 1 (6) a | MDS: 0 Leukemia: 0 |
| Sabet 2017 [33] | 22 | Bronchial NETs | No serious events | Not reported | Any grade ≥3: 0 b | Any grade 3: 3 (14) b | |
| Vaisman 2015 [34] | 7 h | MTC | Not reported | Sexual dysfunction: 1 (14) Hair loss: 2 (29) Hypersensitivity: 1 (14) Any grade ≥3: 0 b | None | None | |
| Parghane 2020 [35] | 43 | MTC | Not reported | Nausea grade 1: 1 (2) Any grade ≥3: 0 | None | Any grade 1: 1 (2) | |
| Beukhof 2019 [36] | 10 | MTC | Not reported | Diarrhea Grade 2: 1 (10) a Hemoptysis Grade 3: 1 (10) a | Not reported | Not reported |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hertelendi, M.; Belguenani, O.; Cherfi, A.; Folitar, I.; Kollar, G.; Polack, B.D. Efficacy and Safety of [177Lu]Lu-DOTA-TATE in Adults with Inoperable or Metastatic Somatostatin Receptor-Positive Pheochromocytomas/Paragangliomas, Bronchial and Unknown Origin Neuroendocrine Tumors, and Medullary Thyroid Carcinoma: A Systematic Literature Review. Biomedicines 2023, 11, 1024. https://doi.org/10.3390/biomedicines11041024
Hertelendi M, Belguenani O, Cherfi A, Folitar I, Kollar G, Polack BD. Efficacy and Safety of [177Lu]Lu-DOTA-TATE in Adults with Inoperable or Metastatic Somatostatin Receptor-Positive Pheochromocytomas/Paragangliomas, Bronchial and Unknown Origin Neuroendocrine Tumors, and Medullary Thyroid Carcinoma: A Systematic Literature Review. Biomedicines. 2023; 11(4):1024. https://doi.org/10.3390/biomedicines11041024
Chicago/Turabian StyleHertelendi, Marianna, Oulaya Belguenani, Azzeddine Cherfi, Ilya Folitar, Gabor Kollar, and Berna Degirmenci Polack. 2023. "Efficacy and Safety of [177Lu]Lu-DOTA-TATE in Adults with Inoperable or Metastatic Somatostatin Receptor-Positive Pheochromocytomas/Paragangliomas, Bronchial and Unknown Origin Neuroendocrine Tumors, and Medullary Thyroid Carcinoma: A Systematic Literature Review" Biomedicines 11, no. 4: 1024. https://doi.org/10.3390/biomedicines11041024
APA StyleHertelendi, M., Belguenani, O., Cherfi, A., Folitar, I., Kollar, G., & Polack, B. D. (2023). Efficacy and Safety of [177Lu]Lu-DOTA-TATE in Adults with Inoperable or Metastatic Somatostatin Receptor-Positive Pheochromocytomas/Paragangliomas, Bronchial and Unknown Origin Neuroendocrine Tumors, and Medullary Thyroid Carcinoma: A Systematic Literature Review. Biomedicines, 11(4), 1024. https://doi.org/10.3390/biomedicines11041024