Dihydrotestosterone, and Not Testosterone, Enhances the LPS-Induced Inflammatory Cytokine Gene Expression in Human Adipocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Adipocytes Isolation and Differentiation
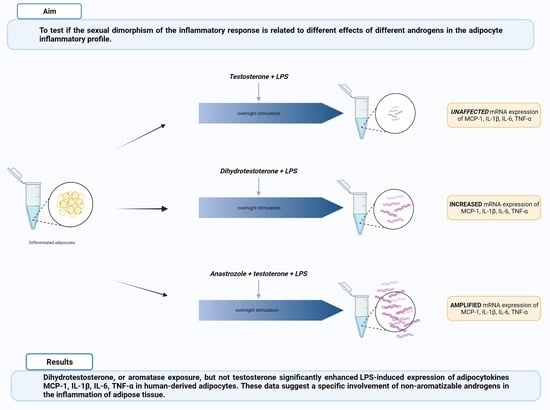
2.2. Grouping
2.3. RNA Extraction and Real-Time PCR Gene-Expression Analysis
2.4. Statistical Analysis
3. Results
3.1. DHT Exposure Reduces the Spontaneous Gene Expression of IL-6 and TNF-α in Adipocytes
3.2. DHT Increases the LPS-Induced Gene Expression of MCP-1, IL-6, and TNF-α in Human Adipocytes
3.3. Anastrozole Exposure Amplifies the LPS-Induced Adipocytokines Expression in Adipocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bays, H.E. Adiposopathy is “sick fat” a cardiovascular disease? J. Am. Coll. Cardiol. 2011, 57, 2461–2473. [Google Scholar] [CrossRef] [PubMed]
- Osborn, O.; Olefsky, J.M. The cellular and signaling networks linking the immune system and metabolism in disease. Nat. Med. 2012, 18, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Yudkin, J.S.; Stehouwer, C.D.; Emeis, J.J.; Coppack, S.W. C-reactive protein in healthy subjects: Associations with obesity, insulin resistance, and endothelial dysfunction: A potential role for cytokines originating from adipose tissue? Arterioscler. Thromb. Vasc. Biol. 1999, 19, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Arner, P.; Caro, J.F.; Atkinson, R.L.; Spiegelman, B.M. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J. Clin. Invest. 1995, 95, 2409–2415. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Tardif, J.C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef]
- Di Vincenzo, A.; Silvestrin, V.; Bertoli, E.; Foletto, M.; Pagano, C.; Fabris, R.; Vettor, R.; Busetto, L.; Rossato, M. Short-term effects of surgical weight loss after sleeve gastrectomy on sex steroids plasma levels and PSA concentration in men with severe obesity. Aging Male 2018, 17, 1–5. [Google Scholar] [CrossRef]
- Calderón, B.; Gómez-Martín, J.M.; Vega-Piñero, B.; Martín-Hidalgo, A.; Galindo, J.; Luque-Ramírez, M.; Escobar-Morreale, H.F.; Botella-Carretero, J.I. Prevalence of male secondary hypogonadism in moderate to severe obesity and its relationship with insulin resistance and excess body weight. Andrology 2016, 4, 62–67. [Google Scholar] [CrossRef]
- Escobar-Morreale, H.F.; Santacruz, E.; Luque-Ramírez, M.; Botella Carretero, J.I. Prevalence of ‘obesity-associated gonadal dysfunction’ in severely obese men and women and its resolution after bariatric surgery: A systematic review and meta-analysis. Hum. Reprod. Update 2017, 23, 390–408. [Google Scholar] [CrossRef]
- Wang, C.; Jackson, G.; Jones, T.H.; Matsumoto, A.M.; Nehra, A.; Perelman, M.A.; Swerdloff, R.S.; Traish, A.; Zitzmann, M.; Cunningham, G. Low testosterone associated with obesity and the metabolic syndrome contributes to sexual dysfunction and cardiovascular disease risk in men with type 2 diabetes. Diabetes Care 2011, 34, 1669–1675. [Google Scholar] [CrossRef]
- Haider, K.S.; Haider, A.; Saad, F.; Doros, G.; Hanefeld, M.; Dhindsa, S.; Dandona, P.; Traish, A. Remission of type 2 diabetes following long-term treatment with injectable testosterone undecanoate in patients with hypogonadism and type 2 diabetes: 11-year data from a real-world registry study. Diabetes ObesMetab. 2020, 22, 2055–2068. [Google Scholar] [CrossRef]
- Kapoor, D.; Clarke, S.; Stanworth, R.; Channer, K.S.; Jones, T.H. The effect of testosterone replacement therapy on adipocytokines and C-reactive protein in hypogonadal men with type 2 diabetes. Eur. J. Endocrinol. 2007, 156, 595–602. [Google Scholar] [CrossRef]
- Kalinchenko, S.Y.; Tishova, Y.A.; Mskhalaya, G.J.; Gooren, L.J.; Giltay, E.J.; Saad, F. Effects of testosterone supplementation on markers of the metabolic syndrome and inflammation in hypogonadal men with the metabolic syndrome: The double-blinded placebo-controlled Moscow study. Clin. Endocrinol. (Oxf.) 2010, 73, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Malkin, C.J.; Pugh, P.J.; Jones, R.D.; Kapoor, D.; Channer, K.S.; Jones, T.H. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J. Clin. Endocrinol. Metab. 2004, 89, 3313–3318. [Google Scholar] [CrossRef]
- Berk, K.A.; Oudshoorn, T.P.; Verhoeven, A.J.M.; Mulder, M.T.; Roks, A.J.M.; Dik, W.A.; Timman, R.; Sijbrands, E.J.G. Diet-induced weight loss and markers of endothelial dysfunction and inflammation in treated patients with type 2 diabetes. Clin. Nutr. ESPEN 2016, 15, 101–106. [Google Scholar] [CrossRef] [PubMed]
- McInnes, K.J.; Smith, L.B.; Hunger, N.I.; Saunders, P.T.; Andrew, R.; Walker, B.R. Deletion of the androgen receptor in adipose tissue in male mice elevates retinol binding protein 4 and reveals independent effects on visceral fat mass and on glucose homeostasis. Diabetes 2012, 61, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.E.; Neinast, M.D.; Sun, K.; Skiles, M.W.; Bills, D.J.; Zehr, A.J.; Zeve, D.; Hahner, D.L.; Cox, W.D.; Gent, M.L.; et al. The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion; inflammation; and fibrosis. Mol. Metab. 2013, 2, 227–242. [Google Scholar] [CrossRef]
- Wake, D.J.; Strand, M.; Rask, E.; Westerbacka, J.; Livingstone, D.E.; Soderberg, S.; Andrew, R.; Yki-Jarvinen, H.; Olsson, T.; Walker, B.R. Intra-adipose sex steroid metabolism and body fat distribution in idiopathic human obesity. Clin. Endocrinol. (Oxf.) 2007, 66, 440–446. [Google Scholar] [CrossRef]
- Bélanger, C.; Luu-The, V.; Dupont, P.; Tchernof, A. Adipose tissue intracrinology: Potential importance of local androgen/estrogen metabolism in the regulation of adiposity. Horm. Metab. Res. 2002, 34, 737–745. [Google Scholar] [CrossRef]
- Rossato, M.; Granzotto, M.; Macchi, V.; Porzionato, A.; Petrelli, L.; Calcagno, A.; Vencato, J.; De Stefani, D.; Silvestrin, V.; Rizzuto, R.; et al. Human white adipocytes express the cold receptor TRPM8 which activation induces UCP1 expression, mitochondrial activation and heat production. Mol. Cell Endocrinol. 2014, 383, 137–146. [Google Scholar] [CrossRef]
- Dieudonne, M.N.; Pecquery, R.; Leneveu, M.C.; Giudicelli, Y. Opposite effects of androgens and estrogens on adipogenesis in rat preadipocytes: Evidence for sex and site-related specificities and possible involvement of insulin-like growth factor 1 receptor and peroxisome proliferator-activated receptor gamma2. Endocrinology 2000, 141, 649–656. [Google Scholar] [CrossRef]
- Su, C.; Chen, M.; Huang, H.; Lin, J. Testosterone enhances lipopolysaccharide-induced interleukin-6 and macrophage chemotactic protein-1 expression by activating the extracellular signal-regulated kinase 1/2/nuclear factor-κBsignalling pathways in 3T3-L1 adipocytes. Mol. Med. Rep. 2015, 12, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Pierotti, S.; Lolli, F.; Lauretta, R.; Graziadio, C.; Di Dato, C.; Sbardella, E.; Tarsitano, M.G.; Isidori, A.; Bonifacio, V.; Lenzi, A.; et al. Androgen modulation of pro-inflammatory and anti-inflammatory cytokines during preadipocyte differentiation. Horm. Mol. Biol. Clin. Investig. 2010, 4, 483–488. [Google Scholar] [CrossRef]
- Camporez, J.P.; Lyu, K.; Goldberg, E.L.; Zhang, D.; Cline, G.W.; Jurczak, M.J.; Dixit, V.D.; Petersen, K.F.; Shulman, G.I. Anti-inflammatory effects of estrogen mediate the sexual dimorphic response to lipid-induced insulin resistance. J. Physiol. 2019, 597, 3885–3903. [Google Scholar] [CrossRef] [PubMed]
- Catalán, V.; Gómez-Ambrosi, J.; Ramirez, B.; Rotellar, F.; Pastor, C.; Silva, C.; Rodríguez, A.; Gil, M.J.; Cienfuegos, J.A.; Frühbeck, G. Proinflammatory cytokines in obesity: Impact of type 2 diabetes mellitus and gastric bypass. Obes Surg. 2007, 17, 1464–1474. [Google Scholar] [CrossRef] [PubMed]
- Koenen, T.B.; Stienstra, R.; van Tits, L.J.; Joosten, L.A.; van Velzen, J.F.; Hijmans, A.; Pol, J.A.; van der Vliet, J.A.; Netea, M.G.; Tack, C.J.; et al. The inflammasome and caspase-1 activation: A new mechanism underlying increased inflammatory activity in human visceral adipose tissue. Endocrinology 2011, 152, 3769–3778. [Google Scholar] [CrossRef] [PubMed]
- Wedell-Neergaard, A.-S.; Lehrskov, L.L.; Christensen, R.H.; Legaard, G.E.; Dorph, E.; Larsen, M.K.; Launbo, N.; Fagerlind, S.R.; Seide, S.K.; Nymand, S.; et al. Exercise-induced changes in visceral adipose tissue mass are regulated by IL-6 signaling: A randomized controlled trial. Cell Metab. 2019, 29, 844–855.e3. [Google Scholar] [CrossRef] [PubMed]
- Galbiati, F.F.; Goldman, A.L.; Gattu, A.; Guzelce, E.C.; Bhasin, S. Benefits and risks of testosterone treatment of older men with hypogonadism. Urol. Clin. N. Am. 2022, 49, 593–602. [Google Scholar] [CrossRef]
- Kang, J.; Chen, R.; Tharakan, T.; Minhas, S. Novel androgen therapies including selective androgen receptor modulators. Best Pract. Res. Clin. Endocrinol. Metab. 2022, 36, 101686. [Google Scholar] [CrossRef]
- Singh, R.; Artaza, J.N.; Taylor, W.E.; Gonzalez-Cadavid, N.F.; Bhasin, S. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology 2003, 144, 5081–5088. [Google Scholar] [CrossRef]
- DeVries, C.; Bratasz, A.; Wu, L.C.; Lustberg, M.; Reinbolt, R.; Jarjour, W.N. Aromatase-inhibitor-induced musculoskeletal inflammation is observed independent of oophorectomy in a novel mouse model. Pharmaceuticals 2022, 15, 1578. [Google Scholar] [CrossRef]
- Ohlsson, C.; Hammarstedt, A.; Vandenput, L.; Saarinen, N.; Ryberg, H.; Windahl, S.H.; Farman, H.H.; Jansson, J.O.; Movérare-Skrtic, S.; Smith, U.; et al. Increased adipose tissue aromatase activity improves insulin sensitivity and reduces adipose tissue inflammation in male mice. Am. J. Physiol. Endocrinol. Metab. 2017, 313, E450–E462. [Google Scholar] [CrossRef] [PubMed]
- Traish, A.M. Health risks associated with long-term finasteride and dutasteride use: It’s time to sound the alarm. World J. Men’s Health 2020, 38, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Pepper, D.J.; Sun, J.; Welsh, J.; Cui, X.; Suffredini, A.F.; Eichacker, P.Q. Increased body mass index and adjusted mortality in ICU patients with sepsis or septic shock: A systematic review and meta-analysis. Crit. Care 2016, 20, 181. [Google Scholar] [CrossRef]
- Gaulton, T.G.; Weiner, M.G.; Morales, K.H.; Gaieski, D.F.; Mehta, J.; Lautenbach, E. The effect of obesity on clinical outcomes in presumed sepsis: A retrospective cohort study. Intern. Emerg. Med. 2014, 9, 213–221. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Tsai, C.L.; Hwang, L.Y.; Lai, D.; Markham, C.; Patel, B. Obesity and mortality; length of stay and hospital cost among patients with sepsis: A nationwide inpatient retrospective cohort study. PLoS ONE 2016, 11, e0154599. [Google Scholar] [CrossRef] [PubMed]
- De La Rica, A.S.; Gilsanz, F.; Maseda, E. Epidemiologic trends of sepsis in western countries. Ann. Transl. Med. 2016, 4, 325. [Google Scholar] [CrossRef] [PubMed]
- Wacharasint, P.; Boyd, J.H.; Russell, J.A.; Walley, K.R. One size does not fit all in severe infection: Obesity alters outcome, susceptibility, treatment and inflammatory response. Crit. Care 2013, 17, R122. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Vincenzo, A.; Granzotto, M.; Crescenzi, M.; Vindigni, V.; Vettor, R.; Rossato, M. Dihydrotestosterone, and Not Testosterone, Enhances the LPS-Induced Inflammatory Cytokine Gene Expression in Human Adipocytes. Biomedicines 2023, 11, 1194. https://doi.org/10.3390/biomedicines11041194
Di Vincenzo A, Granzotto M, Crescenzi M, Vindigni V, Vettor R, Rossato M. Dihydrotestosterone, and Not Testosterone, Enhances the LPS-Induced Inflammatory Cytokine Gene Expression in Human Adipocytes. Biomedicines. 2023; 11(4):1194. https://doi.org/10.3390/biomedicines11041194
Chicago/Turabian StyleDi Vincenzo, Angelo, Marnie Granzotto, Marika Crescenzi, Vincenzo Vindigni, Roberto Vettor, and Marco Rossato. 2023. "Dihydrotestosterone, and Not Testosterone, Enhances the LPS-Induced Inflammatory Cytokine Gene Expression in Human Adipocytes" Biomedicines 11, no. 4: 1194. https://doi.org/10.3390/biomedicines11041194
APA StyleDi Vincenzo, A., Granzotto, M., Crescenzi, M., Vindigni, V., Vettor, R., & Rossato, M. (2023). Dihydrotestosterone, and Not Testosterone, Enhances the LPS-Induced Inflammatory Cytokine Gene Expression in Human Adipocytes. Biomedicines, 11(4), 1194. https://doi.org/10.3390/biomedicines11041194