The ST2/IL-33 Pathway in Adult and Paediatric Heart Disease and Transplantation
Abstract
:1. Introduction
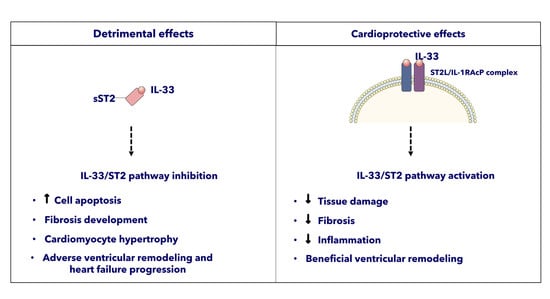
ST2 and the Cardiovascular System
2. ST2 Involvement in Cardiovascular Diseases
2.1. Ischemic Heart Disease
2.2. Heart Failure
2.3. Heart Transplantataion
2.4. Heart Valve Disease
2.5. Pulmonary Arterial Hypertension
2.6. Vascular Disease
2.7. Cardiovascular Interventions
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Klemenz, R.; Hoffmann, S.; Werenskiold, A.K. Serum-mediated and oncoprotein-mediated induction of a gene with sequence similarity to the gene encoding carcinoembryonic antigen. Proc. Natl. Acad. Sci. USA 1989, 86, 5708–5712. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Tzimas, M.N.; Griswold, D.E.; Young, P.R. Expression of St2, an interleukin-1 receptor homologue, is induced by proinflammatory stimuli. Biochem. Biophys. Res. Commun. 1997, 235, 474–478. [Google Scholar] [CrossRef]
- Tominaga, S.A. Putative Protein of a Growth Specific Cdna from Balb C-3t3 cells is highly similar to the extracellular portion of mouse interleukin-1 receptor. FEBS Lett. 1989, 258, 301–304. [Google Scholar] [CrossRef] [Green Version]
- Weinberg, E.O.; Shimpo, M.; De Keulenaer, G.W.; McGillivray, C.; Tominga, S.; Solomon, S.D.; Rouleau, J.L.; Lee, R.T. Expression and regulation of ST2 an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation 2002, 106, 2961–2966. [Google Scholar] [CrossRef] [Green Version]
- Pascual-Figal, D.A.; Januzzi, J.L. The biology of St2: The International St2 Consensus Panel. Am. J. Cardiol. 2015, 115, 3B–7B. [Google Scholar] [CrossRef]
- Iwahana, H.; Yanagisawa, K.; Ito-Kosaka, A.; Kuroiwa, K.; Tago, K.; Komatsu, N.; Katashima, R.; Itakura, M.; Tominaga, S. Different promoter usage and multiple transcription initiation sites of the interleukin-1 receptor-related Human St2 Gene in Uf7 and Tm12 Cells. Eur. J. Biochem. 1999, 264, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tago, K.; Io, K.; Kuroiwa, K.; Arai, T.; Iwahana, H.; Tominaga, S.; Yanagisawa, K. The cloning and nucleotide sequence of human ST2L cDNA. Genomics 2000, 67, 284–290. [Google Scholar] [CrossRef]
- Löhning, M.; Stroehmann, A.; Coyle, A.J.; Grogan, J.L.; Lin, S.; Gutierrez-Ramos, J.C.; Levinson, D.; Radbruch, A.; Kamradt, T. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proc. Natl. Acad. Sci. USA 1998, 95, 6930–6935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turnquist, H.R.; Thomson, A.W. Il-33 broadens its repertoire to affect Dc. Eur. J. Immunol. 2009, 39, 3292–3295. [Google Scholar] [CrossRef] [PubMed]
- Mueller, T.; Jaffe, A.S. Soluble St2-analytical considerations. Am. J. Cardiol. 2015, 115, 8B–21B. [Google Scholar] [CrossRef] [PubMed]
- Bergers, G.; Reikerstorfer, A.; Braselmann, S.; Graninger, P.; Busslinger, M. Alternative promoter usage of the fos-responsive gene fit-1 generates messenger-rna isoforms coding for either secreted or membrane-bound proteins related to the il-1 receptor. EMBO J. 1994, 13, 1176–1188. [Google Scholar] [CrossRef]
- Tominaga, S.; Kuroiwa, K.; Tago, K.; Iwahana, H.; Yanagisawa, K.; Komatsu, N. Presence and expression of a novel variant form of St2 gene product in human leukemic cell line Ut-7/Gm. Biochem. Biophys. Res. Commun. 1999, 264, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Griesenauer, B.; Paczesny, S. The St2/Il-33 axis in immune cells during inflammatory diseases. Front. Immunol. 2017, 8, 475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tago, K.; Noda, T.; Hayakawa, M.; Iwahana, H.; Yanagisawa, K.; Yashiro, T.; Tominaga, S. Tissue distribution and subcellular localization of a variant form of the human ST2 gene product, ST2V. Biochem. Biophys. Res. Commun. 2001, 285, 1377–1383. [Google Scholar] [CrossRef]
- Iwahana, H.; Hayakawa, M.; Kuroiwa, K.; Tago, K.; Yanagisawa, K.; Noji, S.; Tominaga, S. Molecular cloning of the chicken ST2 gene and a novel variant form of the ST2 gene product, ST2LV. Biochim. Biophys. Acta 2004, 1681, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, D.; Jiang, H.R.; Kewin, P.; Li, Y.; Mu, R.; Fraser, A.R.; Pitman, N.; Kurowska-Stolarska, M.; McKenzie, A.N.; McInnes, I.B.; et al. IL-33 exacerbates antigen-induced arthritis by activating mast cells. Proc. Natl. Acad. Sci. USA 2008, 105, 10913–10918. [Google Scholar] [CrossRef] [Green Version]
- Carriere, V.; Roussel, L.; Ortega, N.; Lacorre, D.A.; Americh, L.; Aguilar, L.; Bouche, G.; Girard, J.P. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc. Natl. Acad. Sci. USA 2007, 104, 282–287. [Google Scholar] [CrossRef] [Green Version]
- Miller, A.M.; Liew, F.Y. The Il-33/St2 Pathway—A new therapeutic target in cardiovascular disease. Pharmacol. Ther. 2011, 131, 179–186. [Google Scholar] [CrossRef]
- Cayrol, C.; Girard, J.P. Interleukin-33 (Il-33): A nuclear cytokine from the Il-1 Family. Immunol. Rev. 2018, 281, 154–168. [Google Scholar] [CrossRef]
- Zhang, L.L.; Lu, R.; Zhao, G.Q.; Pflugfelder, S.C.; Li, D.Q. Tlr-mediated induction of pro-allergic cytokine il-33 in ocular mucosal epithelium. Int. J. Biochem. Cell Biol. 2011, 43, 1383–1391. [Google Scholar] [CrossRef] [Green Version]
- Uematsu, S.; Akira, S. Toll-like receptors and innate immunity. J. Mol. Med. 2006, 84, 712–725. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Suzuki, S.; Duncan, G.S.; Millar, D.G.; Wada, T.; Mirtsos, C.; Takada, H.; Wakeham, A.; Itie, A.; Li, S.; et al. Severe impairment of interleukin-1 and Toll-like receptor signalling in mice lacking IRAK-4. Nature 2002, 416, 750–756. [Google Scholar] [CrossRef]
- Sato, S.; Sanjo, H.; Takeda, K.; Ninomiya-Tsuji, J.; Yamamoto, M.; Kawai, T.; Matsumoto, K.; Takeuchi, O.; Akira, S. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat. Immunol. 2005, 6, 1087–1095. [Google Scholar] [CrossRef]
- Tan, Q.; Huang, Q.; Ma, Y.L.; Mao, K.; Yang, G.; Luo, P.; Ma, G.; Mei, P.; Jin, Y. Potential roles of IL-1 subfamily members in glycolysis in disease. Cytokine Growth Factor Rev. 2018, 44, 18–27. [Google Scholar] [CrossRef]
- Hernandez-Santana, Y.E.; Giannoudaki, E.; Leon, G.; Lucitt, M.B.; Walsh, P.T. Current perspectives on the interleukin-1 family as targets for inflammatory disease. Eur. J. Immunol. 2019, 49, 1306–1320. [Google Scholar] [CrossRef]
- Sanada, S.; Hakuno, D.; Higgins, L.J.; Schreiter, E.R.; McKenzie, A.N.; Lee, R.T. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J. Clin. Investig. 2007, 117, 1538–1549. [Google Scholar] [CrossRef] [Green Version]
- Daniels, L.B.; Bayes-Genis, A. Using ST2 in cardiovascular patients: A review. Future Cardiol. 2014, 10, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Da la Fuente, M.; MacDonald, T.T.; Hemoso, M.A. The IL-33/ST2 axis: Role in health and disease. Cytokine Growth Factor Rev. 2015, 26, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Seki, K.; Sanada, S.; Kudinova, A.Y.; Steinhauser, M.L.; Handa, V.; Lee, R.T. Gannon, Interleukin- prevents apoptosis and improves survival after experimental myocardial infarction through ST2 signaling. Circ. Heart Fail 2009, 2, 684–691. [Google Scholar] [CrossRef] [Green Version]
- Gruson, D.; Ahn, S.A.; Rousseau, M.F. Biomarkers of inflamation and cardiac remodeling: The quest of relevant companions for the risk stratification of heart failure patients is still ongoing. Biochem. Med. 2011, 21, 254–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Drazner, M.H., Jr.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013, 128, e240–e327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beetler, D.J.; Bruno, K.A.; Di Florio, D.N.; Douglass, E.J.; Shrestha, S.; Tschöpe, C.; Cunningham, M.W.; Krejčí, J.; Bienertová-Vašků, J.; Pankuweit, S.; et al. Sex and age differences in sST2 in cardiovascular disease. Front. Cardiovasc. Med. 2023, 9, 1073814. [Google Scholar] [CrossRef]
- Heusch, G. Myocardial ischemia: Lack of coronary blood flow, myocardial oxygen supply-demand imbalance, or what? Am. J. Physiol. Circ. Physiol. 2019, 316, H1439–H1446. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galeone, A.; Grano, M.; Brunetti, G. Tumor Necrosis Factor Family Members and Myocardial Ischemia-Reperfusion Injury: State of the Art and Therapeutic Implications. Int. J. Mol. Sci. 2023, 24, 4606. [Google Scholar] [CrossRef]
- Dorn, G.W. Novel pharmacotherapies to abrogate postinfarction ventricular remodeling. Nat. Rev. Cardiol. 2009, 6, 283–291. [Google Scholar] [CrossRef]
- Yousef, Z.R.; Redwood, S.R.; Marber, M.S. Postinfarction left ventricular remodelling: Where are the theories and trials leading us? Heart 2000, 83, 76–80. [Google Scholar] [CrossRef] [Green Version]
- Ghali, R.; Habeichi, N.J.; Kaplan, A.; Tannous, C.; Abidi, E.; Bekdash, A.; Farhat, R.; Itani, H.; Jurjus, A.; Booz, G.W.; et al. IL-33 induces type-2-cytokine phenotype but exacerbates cardiac remodeling post-myocardial infarction with eosinophil recruitment, worsened systolic dysfunction, and ventricular wall rupture. Clin. Sci. 2020, 134, 1191–1218. [Google Scholar] [CrossRef] [PubMed]
- Shimpo, M.; Morrow, D.A.; Weinberg, E.O.; Sabatine, M.S.; Murphy, S.A.; Antman, E.M.; Lee, R.T. Serum levels of the interleukin-1 receptor family member ST2 predict mortality and clinical outcome in acute myocardial infarction. Circulation 2004, 109, 2186–2190. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, W.S.; Roger, V.L.; Jaffe, A.S.; Weston, S.A.; AbouEzzeddine, O.F.; Jiang, R.; Manemann, S.M.; Enriquez-Sarano, M. Prognostic Value of Soluble ST2 After Myocardial Infarction: A Community Perspective. Am. J. Med. 2017, 130, 1112.e9–1112.e15. [Google Scholar] [CrossRef] [Green Version]
- Sabatine, M.S.; Morrow, D.A.; Higgins, L.J.; MacGillivray, C.; Guo, W.; Bode, C.; Rifai, N.; Cannon, C.P.; Gerszten, R.E.; Lee, R.T. Complementary roles for biomarkers of biomechanical strain ST2 and N-terminal prohormone B-type natriuretic peptide in patients with ST-elevation myocardial infarction. Circulation 2008, 117, 1936–1944. [Google Scholar] [CrossRef] [Green Version]
- Weir, R.A.; Miller, A.M.; Murphy, G.E.; Clements, S.; Steedman, T.; Connell, J.M.; McInnes, I.B.; Dargie, H.J.; McMurray, J.J. Serum soluble ST2: A potential novel mediator in left ventricular and infarct remodeling after acute myocardial infarction. J. Am. Coll. Cardiol. 2010, 55, 243–250. [Google Scholar] [CrossRef] [Green Version]
- Eggers, K.M.; Armstrong, P.W.; Califf, R.M.; Simoons, M.L.; Venge, P.; Wallentin, L.; James, S.K. ST2 and mortality in non–ST-segment elevation acute coronary syndrome. Am. Heart J. 2010, 159, 788–794. [Google Scholar] [CrossRef]
- Dhillon, O.S.; Narayan, H.K.; Quinn, P.A.; Squire, I.B.; Davies, J.E.; Ng, L.L. Interleukin 33 and ST2 in non- ST-elevation myocardial infarction: Comparison with Global Registry of Acute Coronary Events Risk Scoring and NT-proBNP. Am. Heart J. 2011, 161, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Kohli, P.; Bonaca, M.P.; Kakkar, R.; Kudinova, A.Y.; Scirica, B.M.; Sabatine, M.S.; Murphy, S.A.; Braunwald, E.; Lee, R.T.; Morrow, D.A. Role of ST2 in non-ST-elevation acute coronary syndrome in the MERLIN-TIMI 36 trial. Clin. Chem. 2012, 58, 257–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kercheva, M.; Ryabova, T.; Gusakova, A.; Suslova, T.E.; Ryabov, V.; Karpov, R.S. Serum soluble ST2 and adverse left ventricular remodeling in patients with ST-segment elevation myocardial infarction. Clin. Med. Insights Cardiol. 2019, 13, 1179546819842804. [Google Scholar] [CrossRef]
- Biere, L.; Garcia, G.; Guillou, S.; Larcher, F.; Furber, A.; Willoteaux, S.; Mirebeau-Prunier, D.; Prunier, F. ST2 as a predictor of late ventricular remodeling after myocardial infarction. Int. J. Cardiol. 2018, 259, 40–42. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, E.O.; Shimpo, M.; Hurwitz, S.; Tominaga, S.; Rouleau, J.L.; Lee, R.T. Identification of serum soluble ST2 receptor as a novel heart failure biomarker. Circulation 2003, 107, 721–726. [Google Scholar] [CrossRef] [Green Version]
- Januzzi, J.L., Jr.; Peacock, W.F.; Maisel, A.S.; Chae, C.U.; Jesse, R.L.; Baggish, A.L.; O’Donoghue, M.; Sakhuja, R.; Chen, A.A.; van Kimmenade, R.R.; et al. Measurement of the interleukin family member ST2 in patients with acute dyspnea: Results from the PRIDE (Pro-Brain Natriuretic Peptide Investigation of Dyspnea in the Emergency Department) study. J. Am. Coll. Cardiol. 2007, 50, 607–613. [Google Scholar] [CrossRef] [Green Version]
- Rehman, S.U.; Martinez-Rumayor, A.; Mueller, T.; Januzzi, J.L., Jr. Independent and incremental prognostic value of multimarker testing in acute dyspnea: Results from the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) study. Clin. Chim. Acta 2008, 392, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Mueller, T.; Dieplinger, B.; Gegenhuber, A.; Poelz, W.; Pacher, R.; Haltmayer, M. Increased plasma concentrations of soluble ST2 are predictive for 1-year mortality in patients with acute destabilized heart failure. Clin. Chem. 2008, 54, 752–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boisot, S.; Beede, J.; Isakson, S.; Chiu, A.; Clopton, P.; Januzzi, J.; Maisel, A.S.; Fitzgerald, R.L. Serial sampling of ST2 predicts 90-day mortality following destabilized heart failure. J. Card. Fail. 2008, 14, 732–738. [Google Scholar] [CrossRef]
- Bartunek, J.; Delrue, L.; Van Durme, F.; Muller, O.; Casselman, F.; De Wiest, B.; Croes, R.; Verstreken, S.; Goethals, M.; de Raedt, H.; et al. Non myocardial production of ST2 protein in human hypertrophy and failure is related to diastolic load. J. Am. Coll. Cardiol. 2008, 52, 2166–2174. [Google Scholar] [CrossRef] [Green Version]
- Demyanets, S.; Kaun, C.; Pentz, R.; Krychtiuk, K.A.; Rauscher, S.; Pfaffenberger, S.; Zuckermann, A.; Aliabadi, A.; Gröger, M.; Maurer, G.; et al. Components of the interleukin-33/ST2 system are differentially expressed and regulated in human cardiac cells and in cells of the cardiac vasculature. J. Mol. Cell Cardiol. 2013, 60, 16–26. [Google Scholar] [CrossRef] [Green Version]
- Pascual-Figal, D.A.; Pérez-Martínez, M.T.; Asensio-Lopez, M.C.; Sanchez-Más, J.; García-García, M.E.; Martinez, C.M.; Lencina, M.; Jara, R.; Januzzi, J.L.; Lax, A. Pulmonary Production of Soluble ST2 in Heart Failure. Circ. Heart Fail. 2018, 11, e005488. [Google Scholar] [CrossRef]
- O’Meara, E.; Prescott, M.F.; Claggett, B.; Rouleau, J.L.; Chiang, L.M.; Solomon, S.D.; Packer, M.; McMurray, J.J.V.; Zile, M.R. Independent Prognostic Value of Serum Soluble ST2 Measurements in Patients with Heart Failure and a Reduced Ejection Fraction in the PARADIGM-HF Trial (Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure). Circ. Heart Fail. 2018, 11, e004446. [Google Scholar] [CrossRef]
- Tseng, C.C.S.; Huibers, M.M.H.; Gaykema, L.H.; Siera-de Koning, E.; Ramjankhan, F.Z.; Maisel, A.S.; de Jonge, N. Soluble ST2 in end-stage heart failure, before and after support with a left ventricular assist device. Eur. J. Clin. Investig. 2018, 48, e12886. [Google Scholar] [CrossRef] [Green Version]
- You, H.; Jiang, W.; Jiao, M.; Wang, X.; Jia, L.; You, S.; Li, Y.; Wen, H.; Jiang, H.; Yuan, H.; et al. Association of Soluble ST2 Serum Levels with Outcomes in Pediatric Dilated Cardiomyopathy. Can. J. Cardiol. 2019, 35, 727–735. [Google Scholar] [CrossRef]
- Hauser, J.A.; Demyanets, S.; Rusai, K.; Goritschan, C.; Weber, M.; Panesar, D.; Rindler, L.; Taylor, A.M.; Marculescu, R.; Burch, M.; et al. Diagnostic performance and reference values of novel biomarkers of paediatric heart failure. Heart 2016, 102, 1633–1639. [Google Scholar] [CrossRef]
- Laqqan, M.; Schwaighofer, C.; Graeber, S.; Raedle-Hurst, T. Predictive value of soluble ST2 in adolescent and adult patients with complex congenital heart disease. PLoS ONE 2018, 13, e0202406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geenen, L.W.; Baggen, V.J.M.; van den Bosch, A.E.; Eindhoven, J.A.; Cuypers, J.A.A.E.; Witsenburg, M.; Boersma, E.; Roos-Hesselink, J.W. Prognostic value of soluble ST2 in adults with congenital heart disease. Heart 2019, 105, 999–1006. [Google Scholar] [CrossRef]
- Wang, J.; He, M.; Li, H.; Chen, Y.; Nie, X.; Cai, Y.; Xie, R.; Li, L.; Chen, P.; Sun, Y.; et al. Soluble ST2 Is a Sensitive and Specific Biomarker for Fulminant Myocarditis. J. Am. Heart Assoc. 2022, 11, e024417. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, D.M.; Büttner, P.; Rommel, K.P.; Blazek, S.; Loncar, G.; von Haehling, S.; von Roeder, M.; Lücke, C.; Gutberlet, M.; Thiele, H.; et al. Soluble ST2 Receptor: Biomarker of Left Ventricular Impairment and Functional Status in Patients with Inflammatory Cardiomyopathy. Cells 2022, 11, 414. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.; Seol, H.; Gordish-Dressman, H.; Hathout, Y.; Spurney, C.F.; CINRG Investigators. Interleukin 1 Receptor-Like 1 Protein (ST2) is a Potential Biomarker for Cardiomyopathy in Duchenne Muscular Dystrophy. Pediatr. Cardiol. 2017, 38, 1606–1612. [Google Scholar] [CrossRef]
- Pascual-Figal, D.A.; Garrido, I.P.; Blanco, R.; Minguela, A.; Lax, A.; Ordoñez-Llanos, J.; Bayes-Genis, A.; Valdés, M.; Moore, S.A.; Januzzi, J.L. Soluble ST2 is a marker for acute cardiac allograft rejection. Ann. Thorac. Surg. 2011, 92, 2118–2124. [Google Scholar] [CrossRef]
- Januzzi, J.L.; Horne, B.D.; Moore, S.A.; Galenko, O.; Snow, G.L.; Brunisholz, K.D.; Muhlestein, J.B.; Alharethi, R.; Carlquist, J.F.; Budge, D.; et al. Interleukin receptor family member ST2 concentrations in patients following heart transplantation. Biomarkers 2013, 18, 250–256. [Google Scholar] [CrossRef]
- Lee, G.Y.; Choi, J.O.; Ju, E.S.; Lee, Y.J.; Jeon, E.S. Role of Soluble ST2 as a Marker for Rejection after Heart Transplant. Korean Circ. J. 2016, 46, 811–820. [Google Scholar] [CrossRef] [Green Version]
- Mathews, L.R.; Lott, J.M.; Isse, K.; Lesniak, A.; Landsittel, D.; Demetris, A.J.; Sun, Y.; Mercer, D.F.; Webber, S.A.; Zeevi, A.; et al. Elevated ST2 Distinguishes Incidences of Pediatric Heart and Small Bowel Transplant Rejection. Am. J. Transplant. 2016, 16, 938–950. [Google Scholar] [CrossRef] [Green Version]
- Grupper, A.; AbouEzzeddine, O.F.; Maleszewski, J.J.; Grupper, A.; Geske, J.R.; Kremers, W.K.; Kushwaha, S.S.; Pereira, N.L. Elevated ST2 levels are associated with antibody-mediated rejection in heart transplant recipients. Clin. Transplant. 2018, 32, e13349. [Google Scholar] [CrossRef]
- Tay, S.S.; Plain, K.M.; Bishop, G.A. Role of IL-4 and Th2 responses in allograft rejection and tolerance. Curr. Opin. Organ. Transplant 2009, 14, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Li, X.Y.; Jin, X.B.; Zhang, B.B.; Gong, Q.; Yang, H.; Zheng, F.; Gong, F.L.; Zhu, J.Y. IL-33 prolongs murine cardiac allograft survival through induction of TH2-type immune deviation. Transplantation 2010, 89, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Turnquist, H.R.; Zhao, Z.; Rosborough, B.R.; Liu, Q.; Castellaneta, A.; Isse, K.; Wang, Z.; Lang, M.; Stolz, D.B.; Zheng, X.X.; et al. IL-33 expands suppressive CD11b+ Gr-1(int) and regulatory T cells, including ST2L+ Foxp3+ cells, and mediates regulatory T cell-dependent promotion of cardiac allograft survival. J. Immunol. 2011, 187, 4598–4610. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zhang, N.; Shi, J.; Dai, C.; Wu, S.; Jiao, M.; Tang, X.; Liu, Y.; Li, X.; Xu, Y.; et al. Allograft or Recipient ST2 Deficiency Oppositely Affected Cardiac Allograft Vasculopathy via Differentially Altering Immune Cells Infiltration. Front. Immunol. 2021, 18, 657803. [Google Scholar] [CrossRef] [PubMed]
- Galeone, A.; Salem, J.E.; Lebreton, G.; Coutance, G.; Nguyen, L.; Hulot, J.S.; Atassi, F.; Bega, M.; Leprince, P.; Varnous, S. Suppression of tumorigenicity-2 (ST2) is a promising biomarker in heart transplantation. Clin. Transplant. 2022, 36, e14616. [Google Scholar] [CrossRef]
- Galeone, A.; Lebreton, G.; Coutance, G.; Demondion, P.; Schmidt, M.; Amour, J.; Varnous, S.; Leprince, P. A single-center long-term experience with marginal donor utilization for heart transplantation. Clin. Transplant. 2020, 34, e14057. [Google Scholar] [CrossRef]
- Sawada, H.; Naito, Y.; Hirotani, S.; Akahori, H.; Iwasaku, T.; Okuhara, Y.; Miki, K.; Eguchi, A.; Mitsuno, M.; Miyamoto, Y.; et al. Expression of interleukin-33 and ST2 in nonrheumatic aortic valve stenosis. Int. J. Cardiol. 2013, 168, 529–531. [Google Scholar] [CrossRef]
- Cai, A.; Miyazawa, A.; Sunderland, N.; Piper, S.E.; Gibbs, T.G.J.; Wang, D.; Redding, S.; Amin-Youseff, G.; Wendler, O.; Byrne, J.; et al. ST2 in patients with severe aortic stenosis and heart failure. Cardiol. J. 2021, 28, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Lancellotti, P.; Dulgheru, R.; Magne, J.; Henri, C.; Servais, L.; Bouznad, N.; Ancion, A.; Martinez, C.; Davin, L.; Le Goff, C.; et al. Elevated Plasma Soluble ST2 Is Associated with Heart Failure Symptoms and Outcome in Aortic Stenosis. PLoS ONE 2015, 10, e0138940. [Google Scholar] [CrossRef]
- Arrieta, V.; Jover, E.; Navarro, A.; Martín-Núñez, E.; Garaikoetxea, M.; Matilla, L.; García-Peña, A.; Fernández-Celis, A.; Gainza, A.; Álvarez, V.; et al. Soluble ST2 levels are related to replacement myocardial fibrosis in severe aortic stenosis. Rev. Esp. Cardiol. (Engl. Ed.) 2022, 21, S1885-5857(22)00328-0. [Google Scholar] [CrossRef]
- Matilla, L.; Ibarrola, J.; Arrieta, V.; Garcia-Peña, A.; Martinez-Martinez, E.; Sádaba, R.; Alvarez, V.; Navarro, A.; Fernández-Celis, A.; Gainza, A.; et al. Soluble ST2 promotes oxidative stress and inflammation in cardiac fibroblasts: An in vitro and in vivo study in aortic stenosis. Clin. Sci. 2019, 133, 1537–1548. [Google Scholar] [CrossRef] [PubMed]
- Galeone, A.; Lessana, A.; Mascolo, E.; Di Serio, F.; Marraudino, N.; Laborde, F.; Paparella, D. Interleukin-1 Receptor-Related Protein ST2 and Mitral Valve Repair Outcome in Patients with Chronic Degenerative Mitral Regurgitation. Thorac. Cardiovasc. Surg. 2014, 62, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pena, A.; Ibarrola, J.; Navarro, A.; Sadaba, A.; Tiraplegui, C.; Garaikoetxea, M.; Arrieta, V.; Matilla, L.; Fernández-Celis, A.; Sadaba, R.; et al. Activation of the Interleukin-33/ST2 Pathway Exerts Deleterious Effects in Myxomatous Mitral Valve Disease. Int. J. Mol. Sci. 2021, 22, 2310. [Google Scholar] [CrossRef]
- Farber, H.W.; Loscalzo, J. Pulmonary arterial hypertension. N. Engl. J. Med. 2004, 351, 1655–1665. [Google Scholar] [CrossRef]
- Groth, A.; Vrugt, B.; Brock, M.; Speich, R.; Ulrich, S.; Huber, L.C. Inflammatory cytokines in pulmonary hypertension. Respir. Res. 2014, 15, 47. [Google Scholar] [CrossRef] [Green Version]
- Shao, D.; Perros, F.; Caramori, G.; Meng, C.; Dormuller, P.; Chou, P.C.; Church, C.; Papi, A.; Casolari, P.; Welsh, D.; et al. Nuclear il-33 regulates soluble st2 receptor and il-6 expression in primary human arterial endothelial cells and is decreased in idiopathic pulmonary arterial hypertension. Biochem. Biophys. Res. Commun. 2014, 451, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Carlomagno, G.; Messalli, G.; Melillo, R.M.; Stanziola, A.A.; Visciano, C.; Mercurio, V.; Imbriaco, M.; Ghio, S.; Sofia, M.; Bonaduce, D.; et al. Serum soluble st2 and interleukin-33 levels in patients with pulmonary arterial hypertension. Int. J. Cardiol. 2013, 168, 1545–1547. [Google Scholar] [CrossRef]
- Keranov, S.; Widmann, L.; Jafari, L.; Liebetrau, C.; Keller, T.; Troidl, C.; Kriechbaum, S.; Voss, S.; Bauer, P.; Richter, M.J.; et al. GDF-15 and soluble ST2 as biomarkers of right ventricular dysfunction in pulmonary hypertension. Biomark. Med. 2022, 16, 1193–1207. [Google Scholar] [CrossRef]
- Kriechbaum, S.D.; Wiedenroth, C.B.; Peters, K.; Barde, M.A.; Ajnwojner, R.; Wolter, J.S.; Haas, M.; Roller, F.C.; Guth, S.; Rieth, A.J.; et al. Galectin-3, GDF-15, and sST2 for the assessment of disease severity and therapy response in patients suffering from inoperable chronic thromboembolic pulmonary hypertension. Biomarkers 2020, 25, 578–586. [Google Scholar] [CrossRef]
- Zheng, Y.G.; Yang, T.; He, J.G.; Chen, G.; Liu, Z.H.; Xiong, C.M.; Gu, Q.; Ni, X.H.; Zhao, Z.H. Plasma soluble st2 levels correlate with disease severity and predict clinical worsening in patients with pulmonary arterial hypertension. Clin. Cardiol. 2014, 37, 365–370. [Google Scholar] [CrossRef]
- Placido, R.; Cortez-Dias, N.; Martins, S.R.; Almeida, A.G.; Calisto, C.; Goncalves, S.; Sadoune, M.; Diogo, A.N.; Mebazaa, A.; Pinto, F.J. Prognostic stratification in pulmonary hypertension: A multi-biomarker approach estratificacao prognostica na hipertensao pulmonar: Valor acrescido da abordagem multibiomarcadores. Rev. Port. Cardiol. 2017, 36, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Geenen, L.W.; Baggen, V.J.M.; Kauling, R.M.; Koudstaal, T.; Boomars, K.A.; Boersma, E.; Roos-Hesselink, J.W.; van den Bosch, A.E. The Prognostic Value of Soluble ST2 in Adults with Pulmonary Hypertension. J. Clin. Med. 2019, 8, 1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffiths, M.; Yang, J.; Simpson, C.E.; Vaidya, D.; Nies, M.; Brandal, S.; Damico, R.; Ivy, D.D.; Austin, E.D.; Pauciulo, M.W.; et al. ST2 Is a Biomarker of Pediatric Pulmonary Arterial Hypertension Severity and Clinical Worsening. Chest 2021, 160, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Coglianese, E.E.; Larson, M.G.; Vasan, R.S.; Ho, J.E.; Ghorbani, A.; McCabe, E.L.; Cheng, S.; Fradley, M.G.; Retschman, D.; Gao, W.; et al. Distribution and clinical correlates of the interleukin receptor family member soluble ST2 in the Framingham heart study. Clin. Chem. 2012, 58, 1673–1681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, J.E.; Larson, M.G.; Ghorbani, A.; Cheng, S.; Vasan, R.S.; Wang, T.J.; Januzzi, J.L., Jr. Soluble ST2 predicts elevated SBP in the community. J. Hypertens. 2013, 31, 1431–1436. [Google Scholar] [CrossRef] [Green Version]
- Ojji, D.B.; Opie, L.H.; Lecour, S.; Lacerda, L.; Adeyemi, O.; Sliwa, K. Relationship between left ventricular geometry and soluble ST2 in a cohort of hypertensive patients. J. Clin. Hypertens. 2013, 15, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Ojji, D.B.; Opie, L.H.; Lecour, S.; Lacerda, L.; Adeyemi, O.M.; Sliwa, K. The effect of left ventricular remodelling on soluble ST2 in a cohort of hypertensive subjects. J. Hum. Hypertens. 2014, 28, 432–437. [Google Scholar] [CrossRef]
- Miller, A.M.; Xu, D.; Asquith, D.L.; Denby, L.; Li, Y.; Sattar, N.; Baker, A.H.; McInnes, I.B.; Liew, F.Y. IL-33 reduces the development of atherosclerosis. J. Exp. Med. 2008, 205, 339–346. [Google Scholar] [CrossRef]
- Willems, S.; Quax, P.H.; de Borst, G.J.; de Vries, J.P.; Moll, F.L.; de Kleijn, D.P.; Hoefer, I.E.; Pasterkamp, G. Soluble ST2 levels are not associated with secondary cardiovascular events and vulnerable plaque phenotype in patients with carotid artery stenosis. Atherosclerosis 2013, 231, 48–53. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Tan, X.; Gao, H.; Yuan, H.; Hu, R.; Jia, L.; Zhu, J.; Sun, L.; Zhang, H.; Huang, L.; et al. Magnitude of Soluble ST2 as a Novel Biomarker for Acute Aortic Dissection. Circulation 2018, 137, 259–269. [Google Scholar] [CrossRef]
- Squiccimarro, E.; Stasi, A.; Lorusso, R.; Paparella, D. Narrative review of the systemic inflammatory reaction to cardiac surgery and cardiopulmonary bypass. Artif. Organs 2022, 46, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Galeone, A.; Brunetti, G.; Rotunno, C.; Oranger, A.; Colucci, S.; de Luca Tupputi Schinosa, L.; Zallone, A.; Grano, M.; Paprella, D. Activation of the receptor activator of the nuclear factor-κB ligand pathway during coronary bypass surgery: Comparison between on- and off-pump coronary artery bypass surgery procedures. Eur. J. Cardiothorac. Surg. 2013, 44, e141–e147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szerafin, T.; Niederpold, T.; Mangold, A.; Hoetzenecker, K.; Hacker, S.; Roth, G.; Lichtenauer, M.; Dworschak, M.; Wolner, E.; Ankersmit, H.J. Secretion of soluble ST2—Possible explanation for systemic immunosuppression after heart surgery. Thorac. Cardiovasc. Surg. 2009, 57, 25–29. [Google Scholar] [CrossRef]
- Lobdell, K.W.; Parker, D.M.; Likosky, D.S.; Rezaee, M.; von Ballmoos, M.W.; Alam, S.S.; Owens, S.; Thiessen-Philbrook, H.; MacKenzie, T.; Brown, J.R. Preoperative serum ST2 level predicts acute kidney injury after adult cardiac surgery. J. Thorac. Cardiovasc. Surg. 2018, 156, 1114–1123. [Google Scholar] [CrossRef]
- Stabler, M.E.; Rezaee, M.E.; Parker, D.M.; MacKenzie, T.A.; Bohm, A.R.; DiScipio, A.W.; Malenka, D.J.; Brown, J.R. sST2 as a novel biomarker for the prediction of in-hospital mortality after coronary artery bypass grafting. Biomarkers 2019, 24, 268–276. [Google Scholar] [CrossRef]
- Patel, D.M.; Thiessen-Philbrook, H.; Brown, J.R.; McArthur, E.; Moledina, D.G.; Mansour, S.G.; Shlipak, M.G.; Koyner, J.L.; Kavsak, P.; Whitlock, R.P.; et al. Association of plasma-soluble ST2 and galectin-3 with cardiovascular events and mortality following cardiac surgery. Am. Heart J. 2020, 220, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Cao, Y.; Wang, Z.; Wang, X.; Li, C.; Hao, X.; Wang, L.; Du, Z.; Yang, F.; Jiang, C.; et al. Soluble ST2 predicts continuous renal replacement therapy in patients receiving venoarterial extracorporeal membrane oxygenation. Perfusion 2023, 13, 2676591231169410. [Google Scholar] [CrossRef]
- Parker, D.M.; Everett, A.D.; Stabler, M.E.; Jacobs, M.L.; Jacobs, J.P.; Vricella, L.; Thiessen-Philbrook, H.; Parikh, C.R.; Manlhiot, C.; Brown, J.R. ST2 Predicts Risk of Unplanned Readmission Within 1 Year After Pediatric Congenital Heart Surgery. Ann. Thorac. Surg. 2020, 110, 2070–2075. [Google Scholar] [CrossRef]
- Willems, S.; Sels, J.W.; Flier, S.; Versteeg, D.; Buhre, W.F.; de Kleijn, D.P.; Hoefer, I.E.; Pasterkamp, G. Temporal changes of soluble ST2 after cardiovascular interventions. Eur. J. Clin. Investig. 2013, 43, 113–120. [Google Scholar] [CrossRef]
- Søndergaard, F.T.; Beske, R.P.; Frydland, M.; Møller, J.E.; Helgestad, O.K.L.; Jensen, L.O.; Holmvang, L.; Goetze, J.P.; Engstrøm, T.; Hassager, C. Soluble ST2 in plasma is associated with post-procedural no-or-slow reflow after primary percutaneous coronary intervention in ST-elevation myocardial infarction. Eur. Heart J. Acute Cardiovasc. Care 2023, 12, 48–52. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, M.; Ma, S. Association of Soluble Suppression of Tumorigenicity with No-Reflow Phenomenon and Long-Term Prognosis in Patients with Non-ST-Segment Elevation Acute Coronary Syndrome after Percutaneous Coronary Intervention. J. Atheroscler. Thromb. 2021, 28, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Dworakowski, R.; Wendler, O.; Bhan, A.; Smith, L.; Pearson, P.; Alcock, E.; Rajagopal, K.; Brickham, B.; Dew, T.; Byrne, J.; et al. Successful transcatheter aortic valve implantation (TAVI) is associated with transient left ventricular dysfunction. Heart 2012, 98, 1641–1646. [Google Scholar] [CrossRef] [PubMed]
- Stundl, A.; Lünstedt, N.S.; Courtz, F.; Freitag-Wolf, S.; Frey, N.; Holdenrieder, S.; Zur, B.; Grube, E.; Nickenig, G.; Werner, N.; et al. Soluble ST2 for Risk Stratification and the Prediction of Mortality in Patients Undergoing Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2017, 120, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Schmid, J.; Stojakovic, T.; Zweiker, D.; Scharnagl, H.; Maderthaner, R.D.; Scherr, D.; Maier, R.; Schmidt, A.; März, W.; Binder, J.S.; et al. ST2 predicts survival in patients undergoing transcatheter aortic valve implantation. Int. J. Cardiol. 2017, 244, 87–92. [Google Scholar] [CrossRef]
- Wernly, B.; Lichtenauer, M.; Jirak, P.; Eder, S.; Reiter, C.; Kammler, J.; Kypta, A.; Jung, C.; Franz, M.; Hoppe, U.C.; et al. Soluble ST2 predicts 1-year outcome in patients undergoing transcatheter aortic valve implantation. Eur. J. Clin. Investig. 2017, 47, 149–157. [Google Scholar] [CrossRef]
- Dörr, O.; Walther, C.; Liebetrau, C.; Keller, T.; Sommer, T.; Boeder, N.; Bayer, M.; Bauer, P.; Möllmann, H.; Gaede, L.; et al. Galectin-3 and ST2 as predictors of therapeutic success in high-risk patients undergoing percutaneous mitral valve repair (MitraClip). Clin. Cardiol. 2018, 41, 1164–1169. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunetti, G.; Barile, B.; Nicchia, G.P.; Onorati, F.; Luciani, G.B.; Galeone, A. The ST2/IL-33 Pathway in Adult and Paediatric Heart Disease and Transplantation. Biomedicines 2023, 11, 1676. https://doi.org/10.3390/biomedicines11061676
Brunetti G, Barile B, Nicchia GP, Onorati F, Luciani GB, Galeone A. The ST2/IL-33 Pathway in Adult and Paediatric Heart Disease and Transplantation. Biomedicines. 2023; 11(6):1676. https://doi.org/10.3390/biomedicines11061676
Chicago/Turabian StyleBrunetti, Giacomina, Barbara Barile, Grazia Paola Nicchia, Francesco Onorati, Giovanni Battista Luciani, and Antonella Galeone. 2023. "The ST2/IL-33 Pathway in Adult and Paediatric Heart Disease and Transplantation" Biomedicines 11, no. 6: 1676. https://doi.org/10.3390/biomedicines11061676
APA StyleBrunetti, G., Barile, B., Nicchia, G. P., Onorati, F., Luciani, G. B., & Galeone, A. (2023). The ST2/IL-33 Pathway in Adult and Paediatric Heart Disease and Transplantation. Biomedicines, 11(6), 1676. https://doi.org/10.3390/biomedicines11061676