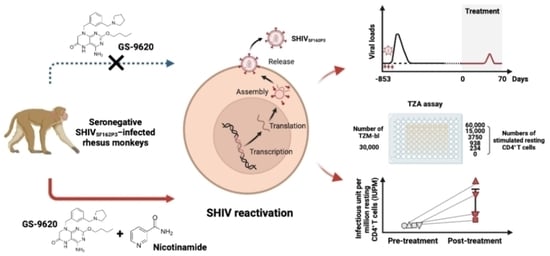
TLR7 Agonist GS–9620 Combined with Nicotinamide Generate Viral Reactivation in Seronegative SHIVSF162P3-Infected Rhesus Monkeys
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Collection and Processing
2.3. TaqMan PCR
2.4. TZA Assay
2.5. PMA and Ionomycin Stimulation Assay
2.6. Flow Cytometry
2.7. Luminex Detection
2.8. Statistical Analysis
3. Results
3.1. Viral Reactivation Generated by Dual NAM and GS–9620 Therapy in Seronegative SHIVSF162P3-Infected Rhesus Macaques

3.2. CD8+ T Cell Responses Induced by Administration of GS–9620 and NAM during Short-Term Therapy
3.3. High-Frequency GS–9620 Administration Combined with NAM Therapy Enhances the Reactivation of Replication-Competent Latent Virus in Seronegative SHIVSF162P3-Infected Rhesus Macaques
3.4. Increased TNF-α Secretion of CD8+ T Cells Induced by Treating with High-Frequency GS–9620 Administration Combined with NAM Therapy in Seronegative SHIVSF162P3-Infected Rhesus Macaques
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, Y.; Anderson, J.L.; Lewin, S.R. Getting the “Kill” into “Shock and Kill”: Strategies to Eliminate Latent HIV. Cell Host Microbe 2018, 23, 14–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menendez-Arias, L.; Delgado, R. Update and latest advances in antiretroviral therapy. Trends Pharmacol. Sci. 2022, 43, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, I.; Hashemi, F.B. Strategies to eradicate HIV from infected patients: Elimination of latent provirus reservoirs. Cell Mol. Life Sci. 2019, 76, 3583–3600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Churchill, M.J.; Deeks, S.G.; Margolis, D.M.; Siliciano, R.F.; Swanstrom, R. HIV reservoirs: What, where and how to target them. Nat. Rev. Microbiol. 2016, 14, 55–60. [Google Scholar] [CrossRef]
- Barton, K.; Winckelmann, A.; Palmer, S. HIV-1 Reservoirs During Suppressive Therapy. Trends Microbiol. 2016, 24, 345–355. [Google Scholar] [CrossRef] [Green Version]
- Abner, E.; Jordan, A. HIV “shock and kill” therapy: In need of revision. Antiviral Res. 2019, 166, 19–34. [Google Scholar] [CrossRef]
- Kula-Pacurar, A.; Rodari, A.; Darcis, G.; Van Lint, C. Shocking HIV-1 with immunomodulatory latency reversing agents. Semin. Immunol. 2021, 51, 101478. [Google Scholar] [CrossRef]
- Martinsen, J.T.; Gunst, J.D.; Hojen, J.F.; Tolstrup, M.; Sogaard, O.S. The Use of Toll-Like Receptor Agonists in HIV-1 Cure Strategies. Front. Immunol. 2020, 11, 1112. [Google Scholar] [CrossRef]
- Macedo, A.B.; Novis, C.L.; Bosque, A. Targeting Cellular and Tissue HIV Reservoirs With Toll-Like Receptor Agonists. Front. Immunol. 2019, 10, 2450. [Google Scholar] [CrossRef] [Green Version]
- Tsai, A.; Irrinki, A.; Kaur, J.; Cihlar, T.; Kukolj, G.; Sloan, D.D.; Murry, J.P. Toll-Like Receptor 7 Agonist GS-9620 Induces HIV Expression and HIV-Specific Immunity in Cells from HIV-Infected Individuals on Suppressive Antiretroviral Therapy. J. Virol. 2017, 91, e02166-16. [Google Scholar] [CrossRef] [Green Version]
- SenGupta, D.; Brinson, C.; DeJesus, E.; Mills, A.; Shalit, P.; Guo, S.; Cai, Y.; Wallin, J.J.; Zhang, L.; Humeniuk, R.; et al. The TLR7 agonist vesatolimod induced a modest delay in viral rebound in HIV controllers after cessation of antiretroviral therapy. Sci. Transl. Med. 2021, 13, eabg3071. [Google Scholar] [CrossRef]
- Lim, S.Y.; Osuna, C.E.; Hraber, P.T.; Hesselgesser, J.; Gerold, J.M.; Barnes, T.L.; Sanisetty, S.; Seaman, M.S.; Lewis, M.G.; Geleziunas, R.; et al. TLR7 agonists induce transient viremia and reduce the viral reservoir in SIV-infected rhesus macaques on antiretroviral therapy. Sci. Transl. Med. 2018, 10, eaao4521. [Google Scholar] [CrossRef] [Green Version]
- Bam, R.A.; Hansen, D.; Irrinki, A.; Mulato, A.; Jones, G.S.; Hesselgesser, J.; Frey, C.R.; Cihlar, T.; Yant, S.R. TLR7 Agonist GS-9620 Is a Potent Inhibitor of Acute HIV-1 Infection in Human Peripheral Blood Mononuclear Cells. Antimicrob. Agents Chemother. 2017, 61, e01369-16. [Google Scholar] [CrossRef] [Green Version]
- Walker-Sperling, V.E.K.; Mercado, N.B.; Chandrashekar, A.; Borducchi, E.N.; Liu, J.; Nkolola, J.P.; Lewis, M.; Murry, J.P.; Yang, Y.; Geleziunas, R.; et al. Therapeutic efficacy of combined active and passive immunization in ART-suppressed, SHIV-infected rhesus macaques. Nat. Commun. 2022, 13, 3463. [Google Scholar] [CrossRef]
- Borducchi, E.N.; Liu, J.; Nkolola, J.P.; Cadena, A.M.; Yu, W.H.; Fischinger, S.; Broge, T.; Abbink, P.; Mercado, N.B.; Chandrashekar, A.; et al. Antibody and TLR7 agonist delay viral rebound in SHIV-infected monkeys. Nature 2018, 563, 360–364. [Google Scholar] [CrossRef]
- Ventura, J.D.; Nkolola, J.P.; Chandrashekar, A.; Borducchi, E.N.; Liu, J.; Mercado, N.B.; Hope, D.L.; Giffin, V.M.; McMahan, K.; Geleziunas, R.; et al. Therapeutic efficacy of an Ad26/MVA vaccine with SIV gp140 protein and vesatolimod in ART-suppressed rhesus macaques. NPJ Vaccines 2022, 7, 53. [Google Scholar] [CrossRef]
- Moldt, B.; Chandrashekar, A.; Borducchi, E.N.; Nkolola, J.P.; Stephenson, H.; Nagel, M.; Hung, M.; Goldsmith, J.; Pace, C.S.; Carr, B.; et al. HIV envelope antibodies and TLR7 agonist partially prevent viral rebound in chronically SHIV-infected monkeys. PLoS Pathog. 2022, 18, e1010467. [Google Scholar] [CrossRef]
- Samer, S.; Arif, M.S.; Giron, L.B.; Zukurov, J.P.L.; Hunter, J.; Santillo, B.T.; Namiyama, G.; Galinskas, J.; Komninakis, S.V.; Oshiro, T.M.; et al. Nicotinamide activates latent HIV-1 ex vivo in ART suppressed individuals, revealing higher potency than the association of two methyltransferase inhibitors, chaetocin and BIX01294. Braz. J. Infect. Dis. 2020, 24, 150–159. [Google Scholar] [CrossRef]
- Bricker, K.M.; Chahroudi, A.; Mavigner, M. New Latency Reversing Agents for HIV-1 Cure: Insights from Nonhuman Primate Models. Viruses 2021, 13, 1560. [Google Scholar] [CrossRef]
- Delagreverie, H.M.; Delaugerre, C.; Lewin, S.R.; Deeks, S.G.; Li, J.Z. Ongoing Clinical Trials of Human Immunodeficiency Virus Latency-Reversing and Immunomodulatory Agents. Open. Forum Infect. Dis. 2016, 3, ofw189. [Google Scholar] [CrossRef] [Green Version]
- Chong, H.; Xue, J.; Zhu, Y.; Cong, Z.; Chen, T.; Wei, Q.; Qin, C.; He, Y. Monotherapy with a low-dose lipopeptide HIV fusion inhibitor maintains long-term viral suppression in rhesus macaques. PLoS Pathog. 2019, 15, e1007552. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Chong, H.; Zhu, Y.; Zhang, J.; Tong, L.; Lu, J.; Chen, T.; Cong, Z.; Wei, Q.; He, Y. Efficient treatment and pre-exposure prophylaxis in rhesus macaques by an HIV fusion-inhibitory lipopeptide. Cell 2022, 185, 131–144 e18. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Siliciano, R.F. Targeting the Latent Reservoir for HIV-1. Immunity 2018, 48, 872–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fosdick, A.; Zheng, J.; Pflanz, S.; Frey, C.R.; Hesselgesser, J.; Halcomb, R.L.; Wolfgang, G.; Tumas, D.B. Pharmacokinetic and pharmacodynamic properties of GS-9620, a novel Toll-like receptor 7 agonist, demonstrate interferon-stimulated gene induction without detectable serum interferon at low oral doses. J. Pharmacol. Exp. Ther. 2014, 348, 96–105. [Google Scholar] [CrossRef] [Green Version]
- Roethle, P.A.; McFadden, R.M.; Yang, H.; Hrvatin, P.; Hui, H.; Graupe, M.; Gallagher, B.; Chao, J.; Hesselgesser, J.; Duatschek, P.; et al. Identification and optimization of pteridinone Toll-like receptor 7 (TLR7) agonists for the oral treatment of viral hepatitis. J. Med. Chem. 2013, 56, 7324–7333. [Google Scholar] [CrossRef]
- Macedo, A.B.; Novis, C.L.; De Assis, C.M.; Sorensen, E.S.; Moszczynski, P.; Huang, S.H.; Ren, Y.; Spivak, A.M.; Jones, R.B.; Planelles, V.; et al. Dual TLR2 and TLR7 agonists as HIV latency-reversing agents. JCI Insight 2018, 3, e122673. [Google Scholar] [CrossRef] [Green Version]
- Khan, J.A.; Forouhar, F.; Tao, X.; Tong, L. Nicotinamide adenine dinucleotide metabolism as an attractive target for drug discovery. Expert. Opin. Ther. Targets 2007, 11, 695–705. [Google Scholar] [CrossRef]
- Kwon, H.S.; Brent, M.M.; Getachew, R.; Jayakumar, P.; Chen, L.F.; Schnolzer, M.; McBurney, M.W.; Marmorstein, R.; Greene, W.C.; Ott, M. Human immunodeficiency virus type 1 Tat protein inhibits the SIRT1 deacetylase and induces T cell hyperactivation. Cell Host Microbe 2008, 3, 158–167. [Google Scholar] [CrossRef] [Green Version]
- Pagans, S.; Pedal, A.; North, B.J.; Kaehlcke, K.; Marshall, B.L.; Dorr, A.; Hetzer-Egger, C.; Henklein, P.; Frye, R.; McBurney, M.W.; et al. SIRT1 regulates HIV transcription via Tat deacetylation. PLoS Biol. 2005, 3, e41. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [Green Version]
- Ram, R.R.; Duatschek, P.; Margot, N.; Abram, M.; Geleziunas, R.; Hesselgesser, J.; Callebaut, C. Activation of HIV-specific CD8(+) T-cells from HIV+ donors by vesatolimod. Antivir. Ther. 2020, 25, 163–169. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004, 5, 987–995. [Google Scholar] [CrossRef]
- Luchner, M.; Reinke, S.; Milicic, A. TLR Agonists as Vaccine Adjuvants Targeting Cancer and Infectious Diseases. Pharmaceutics 2021, 13, 142. [Google Scholar] [CrossRef]




| Group | GS–9620 | NAM+GS–9620 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Animal No. in this study | GS-1 | GS-2 | GS-3 | GS-4 | GSNAM-1 | GSNAM-2 | GSNAM-3 | GSNAM-4 | |
| Gender | Male | Male | Male | Male | Male | Male | Male | Male | |
| Weight (kg) | 11.90 | 8.10 | 9.20 | 7.80 | 10.80 | 8.10 | 9.50 | 9.50 | |
| Alleles associated with the control of SIV/SHIV replication | MHC-Ⅰ Mamu-A*01 | NEG | NEG | NEG | NEG | NEG | NEG | NEG | NEG |
| MHC-Ⅰ Mamu-A*02 | NEG | NEG | NEG | NEG | NEG | NEG | NEG | NEG | |
| MHC-Ⅰ Mamu-B*08 | NEG | NEG | NEG | NEG | NEG | NEG | NEG | NEG | |
| MHC-Ⅰ Mamu-B*12 | NEG | NEG | NEG | NEG | NEG | NEG | NEG | NEG | |
| Pathogen | Simian immunodeficiency virus | NEG | NEG | NEG | NEG | NEG | NEG | NEG | NEG |
| Simian type D retrovirus | NEG | NEG | NEG | NEG | NEG | NEG | NEG | NEG | |
| Simian T-lymphotropic virus | NEG | NEG | NEG | NEG | NEG | NEG | NEG | NEG | |
| Herpes B virus | NEG | NEG | NEG | NEG | NEG | NEG | NEG | NEG | |
| SHIVSF162P3 Challenge | Route | I.R. | I.R. | I.R. | I.R. | I.R. | I.R. | I.R. | I.R. |
| Inoculum (TCID50) | 500 | 500 | 500 | 500 | 500 | 500 | 500 | 500 | |
| Challenge strategy | Mucosal exposure with SHIVSF162P3 twice-weekly for three weeks | Mucosal exposure with SHIVSF162P3 twice-weekly for three weeks | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cong, Z.; Sun, Y.; Dang, C.; Yang, C.; Zhang, J.; Lu, J.; Chen, T.; Wei, Q.; Wang, W.; Xue, J. TLR7 Agonist GS–9620 Combined with Nicotinamide Generate Viral Reactivation in Seronegative SHIVSF162P3-Infected Rhesus Monkeys. Biomedicines 2023, 11, 1707. https://doi.org/10.3390/biomedicines11061707
Cong Z, Sun Y, Dang C, Yang C, Zhang J, Lu J, Chen T, Wei Q, Wang W, Xue J. TLR7 Agonist GS–9620 Combined with Nicotinamide Generate Viral Reactivation in Seronegative SHIVSF162P3-Infected Rhesus Monkeys. Biomedicines. 2023; 11(6):1707. https://doi.org/10.3390/biomedicines11061707
Chicago/Turabian StyleCong, Zhe, Yuting Sun, Cui Dang, Chenbo Yang, Jingjing Zhang, Jiahan Lu, Ting Chen, Qiang Wei, Wei Wang, and Jing Xue. 2023. "TLR7 Agonist GS–9620 Combined with Nicotinamide Generate Viral Reactivation in Seronegative SHIVSF162P3-Infected Rhesus Monkeys" Biomedicines 11, no. 6: 1707. https://doi.org/10.3390/biomedicines11061707
APA StyleCong, Z., Sun, Y., Dang, C., Yang, C., Zhang, J., Lu, J., Chen, T., Wei, Q., Wang, W., & Xue, J. (2023). TLR7 Agonist GS–9620 Combined with Nicotinamide Generate Viral Reactivation in Seronegative SHIVSF162P3-Infected Rhesus Monkeys. Biomedicines, 11(6), 1707. https://doi.org/10.3390/biomedicines11061707