Serotonergic Modulation of Neurovascular Transmission: A Focus on Prejunctional 5-HT Receptors/Mechanisms
Abstract
:1. Introduction
1.1. A Summary on 5-HT Receptors
1.2. An Overview of the Effects of 5-HT on the Cardiovascular System
1.3. The Specific Interactions of 5-HT at Peripheral and Central Levels to Induce Cardiovascular Effects
1.3.1. Sensory Afferents
1.3.2. Sympathetic Ganglia
1.3.3. Cardiac Effects of 5-HT
Bradycardia
Tachycardia
1.3.4. Vascular and Blood Pressure Effects of 5-HT
Initial Transient Vasodepressor Effect
Vasopressor Effect
Late Long-Lasting Vasodepressor Effect
1.3.5. Receptor-Independent Actions of 5-HT
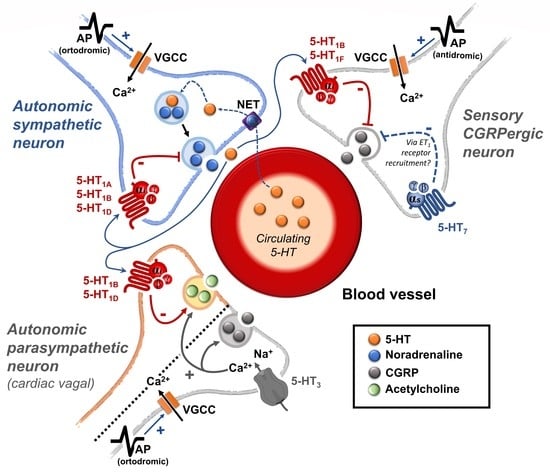
2. Peripheral Autonomic Nervous System and Prejunctional 5-HT Receptors
2.1. An Overview of the Peripheral Actions of 5-HT Regulating the Vascular Function
2.2. The Role of Prejunctional 5-HT Receptors
2.2.1. The 5-HT Receptors Inhibiting the Autonomic Outflow
2.2.2. The 5-HT Receptors as Facilitators of the Autonomic Outflow
2.3. Clinical Relevance and Therapeutic Potential
3. Sensory CGRPergic Perivascular Nerves and Prejunctional 5-HT Receptors
3.1. The Sensory Perivascular CGRPergic Neurons as an Intrinsic Modulator of Vascular Tone
3.2. Prejunctional 5-HT Receptors as Inhibitors of the Perivascular Sensory CGRPergic Outflow
3.3. Clinical Relevance
4. Perspectives and Some Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barnes, N.M.; Ahern, G.P.; Becamel, C.; Bockaert, J.; Camilleri, M.; Chaumont-Dubel, S.; Claeysen, S.; Cunningham, K.A.; Fone, K.C.; Gershon, M.; et al. International Union of Basic and Clinical Pharmacology. CX. Classification of Receptors for 5-hydroxytryptamine; Pharmacology and Function. Pharmacol. Rev. 2021, 73, 310–520. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Liu, F. The Role of Serotonin beyond the Central Nervous System during Embryogenesis. Front. Cell Neurosci. 2017, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Villalón, C.M. The role of serotonin receptors in the control of cardiovascular function. In The Serotonin System; Tricklebank, M.D., Daly, E., Eds.; Academic Press: Cambridge, MA, USA, 2019; Chapter 3; pp. 45–61. [Google Scholar]
- Hoyer, D.; Clarke, D.E.; Fozard, J.R.; Hartig, P.R.; Martin, G.R.; Mylecharane, E.J.; Saxena, P.R.; Humphrey, P.P. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol. Rev. 1994, 46, 157–203. [Google Scholar] [PubMed]
- Hoyer, D.; Hannon, J.P.; Martin, G.R. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol. Biochem. Behav. 2002, 71, 533–554. [Google Scholar] [CrossRef]
- Kaumann, A.J.; Levy, F.O. 5-hydroxytryptamine receptors in the human cardiovascular system. Pharmacol. Ther. 2006, 111, 674–706. [Google Scholar] [CrossRef]
- Watts, S.W.; Davis, R.P. 5-hydroxtryptamine receptors in systemic hypertension: An arterial focus. Cardiovasc. Ther. 2011, 29, 54–67. [Google Scholar] [CrossRef]
- Watts, S.W.; Morrison, S.F.; Davis, R.P.; Barman, S.M. Serotonin and blood pressure regulation. Pharmacol. Rev. 2012, 64, 359–388. [Google Scholar] [CrossRef]
- González-Hernández, A.; Marichal-Cancino, B.A.; Lozano-Cuenca, J.; López-Canales, J.S.; Muñoz-Islas, E.; Ramírez-Rosas, M.B.; Villalón, C.M. Heteroreceptors Modulating CGRP Release at Neurovascular Junction: Potential Therapeutic Implications on Some Vascular-Related Diseases. Biomed. Res. Int. 2016, 2016, 2056786. [Google Scholar] [CrossRef]
- Ramage, A.G. Influence of 5-HT1A receptor agonists on sympathetic and parasympathetic nerve activity. J. Cardiovasc. Pharmacol. 1990, 15 (Suppl. S7), S75–S85. [Google Scholar] [CrossRef]
- Ramage, A.G. Central cardiovascular regulation and 5-hydroxytryptamine receptors. Brain Res. Bull. 2001, 56, 425–439. [Google Scholar] [CrossRef]
- Sánchez-Lopez, A.; Centurión, D.; Vázquez, E.; Arulmani, U.; Saxena, P.R.; Villalón, C.M. Pharmacological profile of the 5-HT-induced inhibition of cardioaccelerator sympathetic outflow in pithed rats: Correlation with 5-HT1 and putative 5-ht5A/5B receptors. Br. J. Pharmacol. 2003, 140, 725–735. [Google Scholar] [CrossRef]
- García-Pedraza, J.; Hernández-Abreu, O.; García, M.; Morán, A.; Villalón, C.M. Chronic 5-HT(2) receptor blockade unmasks the role of 5-HT(1F) receptors in the inhibition of rat cardioaccelerator sympathetic outflow. Can. J. Physiol. Pharmacol. 2018, 96, 328–336. [Google Scholar] [CrossRef]
- García-Pedraza, J.; García, M.; Martín, M.L.; Gómez-Escudero, J.; Rodríguez-Barbero, A.; Román, L.S.; Morán, A. Peripheral 5-HT₁D and 5-HT₇ serotonergic receptors modulate sympathetic neurotransmission in chronic sarpogrelate treated rats. Eur. J. Pharmacol. 2013, 714, 65–73. [Google Scholar] [CrossRef]
- Dabiré, H. Central 5-hydroxytryptamine (5-HT) receptors in blood pressure regulation. Therapie 1991, 46, 421–429. [Google Scholar]
- Bedi, U.S.; Arora, R. Cardiovascular manifestations of posttraumatic stress disorder. J. Natl. Med. Assoc. 2007, 99, 642–649. [Google Scholar]
- Tania, V.; Catherine, V. Roles of the Serotoninergic System in Coping with Traumatic Stress. In Serotonin and the CNS; Berend, O., Ed.; IntechOpen: Rijeka, Croatia, 2021; pp. 1–5. [Google Scholar]
- Paine, N.J.; Watkins, L.L.; Blumenthal, J.A.; Kuhn, C.M.; Sherwood, A. Association of depressive and anxiety symptoms with 24-hour urinary catecholamines in individuals with untreated high blood pressure. Psychosom. Med. 2015, 77, 136–144. [Google Scholar] [CrossRef]
- Brindley, R.L.; Bauer, M.B.; Blakely, R.D.; Currie, K.P.M. An interplay between the serotonin transporter (SERT) and 5-HT receptors controls stimulus-secretion coupling in sympathoadrenal chromaffin cells. Neuropharmacology 2016, 110, 438–448. [Google Scholar] [CrossRef]
- Nakatani, Y.; Sato-Suzuki, I.; Tsujino, N.; Nakasato, A.; Seki, Y.; Fumoto, M.; Arita, H. Augmented brain 5-HT crosses the blood-brain barrier through the 5-HT transporter in rat. Eur. J. Neurosci. 2008, 27, 2466–2472. [Google Scholar] [CrossRef]
- Wang, H.M.; Wang, Y.; Liu, M.; Bai, Y.; Zhang, X.H.; Sun, Y.X.; Wang, H.L. Fluoxetine inhibits monocrotaline-induced pulmonary arterial remodeling involved in inhibition of RhoA-Rho kinase and Akt signalling pathways in rats. Can. J. Physiol. Pharmacol. 2012, 90, 1506–1515. [Google Scholar] [CrossRef]
- Lin, J.C.; Chou, C.C.; Tu, Z.; Yeh, L.F.; Wu, S.C.; Khoo, K.H.; Lin, C.H. Characterization of protein serotonylation via bioorthogonal labeling and enrichment. J. Proteome Res. 2014, 13, 3523–3529. [Google Scholar] [CrossRef]
- Penumatsa, K.C.; Fanburg, B.L. Transglutaminase 2-mediated serotonylation in pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 306, L309–L315. [Google Scholar] [CrossRef] [PubMed]
- Tjurmina, O.A.; Armando, I.; Saavedra, J.M.; Goldstein, D.S.; Murphy, D.L. Exaggerated adrenomedullary response to immobilization in mice with targeted disruption of the serotonin transporter gene. Endocrinology 2002, 143, 4520–4526. [Google Scholar] [CrossRef] [PubMed]
- Tiradentes, R.V.; Pires, J.G.; Silva, N.F.; Ramage, A.G.; Santuzzi, C.H.; Futuro Neto, H.A. Effects of acute administration of selective serotonin reuptake inhibitors on sympathetic nerve activity. Braz. J. Med. Biol. Res. 2014, 47, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, S.; Levey, A.I.; Blakely, R.D. Polarized expression of the antidepressant-sensitive serotonin transporter in epinephrine-synthesizing chromaffin cells of the rat adrenal gland. Mol. Cell. Neurosci. 1997, 9, 170–184. [Google Scholar] [CrossRef]
- Furlan, A.; Dyachuk, V.; Kastriti, M.E.; Calvo-Enrique, L.; Abdo, H.; Hadjab, S.; Chontorotzea, T.; Akkuratova, N.; Usoskin, D.; Kamenev, D.; et al. Multipotent peripheral glial cells generate neuroendocrine cells of the adrenal medulla. Science 2017, 357, eaal3753. [Google Scholar] [CrossRef]
- Kameneva, P.; Melnikova, V.I.; Kastriti, M.E.; Kurtova, A.; Kryukov, E.; Murtazina, A.; Faure, L.; Poverennaya, I.; Artemov, A.V.; Kalinina, T.S.; et al. Serotonin limits generation of chromaffin cells during adrenal organ development. Nat. Commun. 2022, 13, 2901. [Google Scholar] [CrossRef]
- Carbone, E.; Borges, R.; Eiden, L.E.; García, A.G.; Hernández-Cruz, A. Chromaffin Cells of the Adrenal Medulla: Physiology, Pharmacology, and Disease. Compr. Physiol. 2019, 9, 1443–1502. [Google Scholar] [CrossRef]
- Brindley, R.L.; Bauer, M.B.; Blakely, R.D.; Currie, K.P.M. Serotonin and Serotonin Transporters in the Adrenal Medulla: A Potential Hub for Modulation of the Sympathetic Stress Response. ACS Chem. Neurosci. 2017, 8, 943–954. [Google Scholar] [CrossRef]
- Linder, A.E.; Beggs, K.M.; Burnett, R.J.; Watts, S.W. Body distribution of infused serotonin in rats. Clin. Exp. Pharmacol. Physiol. 2009, 36, 599–601. [Google Scholar] [CrossRef]
- Kobayashi, S.; Tsukahara, S.; Sugita, K.; Nagata, T. Adrenergic and cholinergic innervation of rat cerebral arteries. Consecutive demonstration on whole mount preparations. Histochemistry 1981, 70, 129–138. [Google Scholar] [CrossRef]
- Sheng, Y.; Zhu, L. The crosstalk between autonomic nervous system and blood vessels. Int. J. Physiol. Pathophysiol. Pharmacol. 2018, 10, 17–28. [Google Scholar]
- Koep, J.L.; Taylor, C.E.; Coombes, J.S.; Bond, B.; Ainslie, P.N.; Bailey, T.G. Autonomic control of cerebral blood flow: Fundamental comparisons between peripheral and cerebrovascular circulations in humans. J. Physiol. 2022, 600, 15–39. [Google Scholar] [CrossRef]
- Suzuki, N.; Hardebo, J.E. The cerebrovascular parasympathetic innervation. Cerebrovasc. Brain Metab. Rev. 1993, 5, 33–46. [Google Scholar]
- Roloff, E.V.L.; Tomiak-Baquero, A.M.; Kasparov, S.; Paton, J.F.R. Parasympathetic innervation of vertebrobasilar arteries: Is this a potential clinical target? J. Physiol. 2016, 594, 6463–6485. [Google Scholar] [CrossRef]
- Miller, K.E.; Salvatierra, A.T. Apposition of enkephalin- and neurotensin-immunoreactive neurons by serotonin-immunoreactive varicosities in the rat spinal cord. Neuroscience 1998, 85, 837–846. [Google Scholar] [CrossRef]
- Villalón, C.M.; Centurión, D.; Rabelo, G.; de Vries, P.; Saxena, P.R.; Sánchez-López, A. The 5-HT1-like receptors mediating inhibition of sympathetic vasopressor outflow in the pithed rat: Operational correlation with the 5-HT1A, 5-HT1B and 5-HT1D subtypes. Br. J. Pharmacol. 1998, 124, 1001–1011. [Google Scholar] [CrossRef]
- Rapport, M.M.; Green, A.A.; Page, I.H. Serum vasoconstrictor, serotonin; isolation and characterization. J. Biol. Chem. 1948, 176, 1243–1251. [Google Scholar] [CrossRef]
- Rapport, M.M.; Green, A.A.; Page, I.H. Partial purification of the vasoconstrictor in beef serum. J. Biol. Chem. 1948, 174, 735–741. [Google Scholar] [CrossRef]
- Herr, N.; Bode, C.; Duerschmied, D. The Effects of Serotonin in Immune Cells. Front. Cardiovasc. Med. 2017, 4, 48. [Google Scholar] [CrossRef]
- Palermo, A.; del Rosso, G.; Costantini, C.; Bertalero, P.; Rizzi, S.; Libretti, A. Platelet content of serotonin and response to stress. J. Hypertens. Suppl. 1986, 4, S43–S45. [Google Scholar]
- Teff, K.L.; Young, S.N. Effects of carbohydrate and protein administration on rat tryptophan and 5-hydroxytryptamine: Differential effects on the brain, intestine, pineal, and pancreas. Can. J. Physiol. Pharmacol. 1988, 66, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Edvinsson, J.C.A.; Maddahi, A.; Christiansen, I.M.; Reducha, P.V.; Warfvinge, K.; Sheykhzade, M.; Edvinsson, L.; Haanes, K.A. Lasmiditan and 5-Hydroxytryptamine in the rat trigeminal system; expression, release and interactions with 5-HT1 receptors. J. Headache Pain 2022, 23, 26. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Sugiura, Y.; Magome, T.; Kamakura, T.; Takimoto, Y.; Hanada, Y.; Kitayama, K.; Nakamura, Y.; Shimada, S.; Ohta, N.; et al. Expression Analysis of Serotonin Receptors, Serotonin Transporter and l-Amino Acid Decarboxylase in the Mouse Sphenopalatine Ganglion by RT-PCR, Northern Blot Analysis and In Situ Hybridization. Neuroscience 2019, 411, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Punda, H.; Mardesic, S.; Filipovic, N.; Kosovic, I.; Benzon, B.; Ogorevc, M.; Bocina, I.; Kolic, K.; Vukojevic, K.; Saraga-Babic, M. Expression Pattern of 5-HT (Serotonin) Receptors during Normal Development of the Human Spinal Cord and Ganglia and in Fetus with Cervical Spina Bifida. Int. J. Mol. Sci. 2021, 22, 7320. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Satoh, S. Presynaptic inhibition by serotonin of cardiac sympathetic transmission in dogs. Clin. Exp. Pharmacol. Physiol. 1983, 10, 535–542. [Google Scholar] [CrossRef]
- García-Pedraza, J.; Hernández-Abreu, O.; Morán, A.; Carretero, J.; García-Domingo, M.; Villalón, C.M. Role of peripheral 5-HT(5A) receptors in 5-HT-induced cardiac sympatho-inhibition in type 1 diabetic rats. Sci. Rep. 2020, 10, 19358. [Google Scholar] [CrossRef]
- Morán, A.; Fernández, M.M.; Velasco, C.; Martín, M.L.; San Román, L. Characterization of prejunctional 5-HT1 receptors that mediate the inhibition of pressor effects elicited by sympathetic stimulation in the pithed rat. Br. J. Pharmacol. 1998, 123, 1205–1213. [Google Scholar] [CrossRef]
- Pilowsky, P.M. Chapter 16—Serotonin in Central Cardiovascular Regulation: Ex Uno Plura (From One Comes Many). In Serotonin; Pilowsky, P.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 335–347. [Google Scholar]
- Morán, A.; Velasco, C.; Salvador, T.; Martín, M.L.; San Román, L. Inhibitory 5-hydroxytryptamine receptors involved in pressor effects obtained by stimulation of sympathetic outflow from spinal cord in pithed rats. Br. J. Pharmacol. 1994, 113, 1358–1362. [Google Scholar] [CrossRef]
- Villamil-Hernández, M.T.; Alcántara-Vázquez, O.; Sánchez-López, A.; Gutiérrez-Lara, E.J.; Centurión, D. Pharmacological evidence that 5-HT1A/1B/1D, α2-adrenoceptors and D2-like receptors mediate ergotamine-induced inhibition of the vasopressor sympathetic outflow in pithed rats. Eur. J. Pharmacol. 2014, 740, 512–521. [Google Scholar] [CrossRef]
- Molderings, G.J.; Frölich, D.; Likungu, J.; Göthert, M. Inhibition of noradrenaline release via presynaptic 5-HT1D alpha receptors in human atrium. Naunyn Schmiedebergs Arch. Pharmacol. 1996, 353, 272–280. [Google Scholar] [CrossRef]
- Morán, A.; Velasco, C.; Martín, M.L.; San Román, L. Pharmacological characterization of 5-HT receptors in parasympathetic innervation of rat heart. Eur. J. Pharmacol. 1994, 252, 161–166. [Google Scholar] [CrossRef]
- Lee, T.J.; Liu, J.; Evans, M.S. Cholinergic-nitrergic transmitter mechanisms in the cerebral circulation. Microsc. Res. Tech. 2001, 53, 119–128. [Google Scholar] [CrossRef]
- Jackowski, A.; Crockard, A.; Burnstock, G. 5-Hydroxytryptamine demonstrated immunohistochemically in rat cerebrovascular nerves largely represents 5-hydroxytryptamine uptake into sympathetic nerve fibres. Neuroscience 1989, 29, 453–462. [Google Scholar] [CrossRef]
- Boyle, S.H.; Brummett, B.H.; Kuhn, C.M.; Barefoot, J.C.; Siegler, I.C.; Williams, R.B.; Georgiades, A. The Effects of Tryptophan Enhancement and Depletion on Plasma Catecholamine Levels in Healthy Individuals. Psychosom. Med. 2019, 81, 34–40. [Google Scholar] [CrossRef]
- Švec, J.; Švec, P.; Bencová, V.; Krčméry, V. Anxio-depressive Syndrome—Biopsychosocial Model of Supportive Care. Klin. Onkol. 2015, 28, 177–182. [Google Scholar] [CrossRef]
- Razzaque, Z.; Pickard, J.D.; Ma, Q.P.; Shaw, D.; Morrison, K.; Wang, T.; Longmore, J. 5-HT1B-receptors and vascular reactivity in human isolated blood vessels: Assessment of the potential craniovascular selectivity of sumatriptan. Br. J. Clin. Pharmacol. 2002, 53, 266–274. [Google Scholar] [CrossRef]
- Gonzalez-Hernandez, A.; Marichal-Cancino, B.A.; MaassenVanDenBrink, A.; Villalon, C.M. Side effects associated with current and prospective antimigraine pharmacotherapies. Expert. Opin. Drug. Metab. Toxicol. 2018, 14, 25–41. [Google Scholar] [CrossRef]
- Marichal-Cancino, B.A.; González-Hernández, A.; Guerrero-Alba, R.; Medina-Santillán, R.; Villalón, C.M. A critical review of the neurovascular nature of migraine and the main mechanisms of action of prophylactic antimigraine medications. Expert. Rev. Neurother. 2021, 21, 1035–1050. [Google Scholar] [CrossRef]
- Villalón, C.M.; Sánchez-López, A.; Centurión, D. Operational characteristics of the 5-HT1-like receptors mediating external carotid vasoconstriction in vagosympathectomized dogs. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1996, 354, 550–556. [Google Scholar] [CrossRef]
- Zaidi, M.; Bevis, P.J.; Girgis, S.I.; Lynch, C.; Stevenson, J.C.; MacIntyre, I. Circulating CGRP comes from the perivascular nerves. Eur. J. Pharmacol. 1985, 117, 283–284. [Google Scholar] [CrossRef]
- Escott, K.J.; Connor, H.E.; Brain, S.D.; Beattie, D.T. The involvement of calcitonin gene-related peptide (CGRP) and substance P in feline pial artery diameter responses evoked by capsaicin. Neuropeptides 1995, 29, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Van der Schueren, B.J.; de Hoon, J.N.; Vanmolkot, F.H.; Van Hecken, A.; Depre, M.; Kane, S.A.; De Lepeleire, I.; Sinclair, S.R. Reproducibility of the capsaicin-induced dermal blood flow response as assessed by laser Doppler perfusion imaging. Br. J. Clin. Pharmacol. 2007, 64, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Dux, M.; Rosta, J.; Messlinger, K. TRP Channels in the Focus of Trigeminal Nociceptor Sensitization Contributing to Primary Headaches. Int. J. Mol. Sci. 2020, 21, 342. [Google Scholar] [CrossRef]
- Julius, D.; Basbaum, A.I. Molecular mechanisms of nociception. Nature 2001, 413, 203–210. [Google Scholar] [CrossRef]
- Kawasaki, H.; Nuki, C.; Saito, A.; Takasaki, K. Adrenergic modulation of calcitonin gene-related peptide (CGRP)-containing nerve-mediated vasodilation in the rat mesenteric resistance vessel. Brain Res. 1990, 506, 287–290. [Google Scholar] [CrossRef]
- Kawasaki, H.; Takasaki, K.; Saito, A.; Goto, K. Calcitonin gene-related peptide acts as a novel vasodilator neurotransmitter in mesenteric resistance vessels of the rat. Nature 1988, 335, 164–167. [Google Scholar] [CrossRef]
- Taguchi, T.; Kawasaki, H.; Imamura, T.; Takasaki, K. Endogenous calcitonin gene-related peptide mediates nonadrenergic noncholinergic depressor response to spinal cord stimulation in the pithed rat. Circ. Res. 1992, 71, 357–364. [Google Scholar] [CrossRef]
- Kawasaki, H.; Saito, A.; Takasaki, K. Age-related decrease of calcitonin gene-related peptide-containing vasodilator innervation in the mesenteric resistance vessel of the spontaneously hypertensive rat. Circ. Res. 1990, 67, 733–743. [Google Scholar] [CrossRef]
- Avilés-Rosas, V.H.; Rivera-Mancilla, E.; Marichal-Cancino, B.A.; Manrique-Maldonado, G.; Altamirano-Espinoza, A.H.; Maassen Van Den Brink, A.; Villalón, C.M. Olcegepant blocks neurogenic and non-neurogenic CGRPergic vasodepressor responses and facilitates noradrenergic vasopressor responses in pithed rats. Br. J. Pharmacol. 2017, 174, 2001–2014. [Google Scholar] [CrossRef]
- Russell, F.A.; King, R.; Smillie, S.J.; Kodji, X.; Brain, S.D. Calcitonin gene-related peptide: Physiology and pathophysiology. Physiol. Rev. 2014, 94, 1099–1142. [Google Scholar] [CrossRef]
- Russo, A.F.; Hay, D.L. CGRP physiology, pharmacology, and therapeutic targets: Migraine and beyond. Physiol. Rev. 2023, 103, 1565–1644. [Google Scholar] [CrossRef]
- Villalón, C.M.; Albarrán-Juárez, J.A.; Lozano-Cuenca, J.; Pertz, H.H.; Görnemann, T.; Centurión, D. Pharmacological profile of the clonidine-induced inhibition of vasodepressor sensory outflow in pithed rats: Correlation with alpha(2A/2C)-adrenoceptors. Br. J. Pharmacol. 2008, 154, 51–59. [Google Scholar] [CrossRef]
- González-Hernández, A.; Manrique-Maldonado, G.; Lozano-Cuenca, J.; Muñoz-Islas, E.; Centurión, D.; Maassen VanDenBrink, A.; Villalón, C.M. The 5-HT(1) receptors inhibiting the rat vasodepressor sensory CGRPergic outflow: Further involvement of 5-HT(1F), but not 5-HT(1A) or 5-HT(1D), subtypes. Eur. J. Pharmacol. 2011, 659, 233–243. [Google Scholar] [CrossRef]
- González-Hernández, A.; Muñoz-Islas, E.; Lozano-Cuenca, J.; Ramírez-Rosas, M.B.; Sánchez-López, A.; Centurión, D.; Ramírez-San Juan, E.; Villalón, C.M. Activation of 5-HT1B receptors inhibits the vasodepressor sensory CGRPergic outflow in pithed rats. Eur. J. Pharmacol. 2010, 637, 131–137. [Google Scholar] [CrossRef]
- Ibrahimi, K.; Danser, A.; Terwindt, G.M.; van den Meiracker, A.H.; MaassenVanDenBrink, A. A human trigeminovascular biomarker for antimigraine drugs: A randomised, double-blind, placebo-controlled, crossover trial with sumatriptan. Cephalalgia 2017, 37, 94–98. [Google Scholar] [CrossRef]
- Benemei, S.; Cortese, F.; Labastida-Ramírez, A.; Marchese, F.; Pellesi, L.; Romoli, M.; Vollesen, A.L.; Lampl, C.; Ashina, M. Triptans and CGRP blockade—Impact on the cranial vasculature. J. Headache Pain 2017, 18, 103. [Google Scholar] [CrossRef]
- Labastida-Ramírez, A.; Rubio-Beltrán, E.; Haanes, K.A.; Chan, K.Y.; Garrelds, I.M.; Johnson, K.W.; Danser, A.H.J.; Villalón, C.M.; MaassenVanDenBrink, A. Lasmiditan inhibits calcitonin gene-related peptide release in the rodent trigeminovascular system. Pain 2020, 161, 1092–1099. [Google Scholar] [CrossRef]
- Buzzi, M.G.; Carter, W.B.; Shimizu, T.; Heath, H., 3rd; Moskowitz, M.A. Dihydroergotamine and sumatriptan attenuate levels of CGRP in plasma in rat superior sagittal sinus during electrical stimulation of the trigeminal ganglion. Neuropharmacology 1991, 30, 1193–1200. [Google Scholar] [CrossRef]
- Gupta, S.; Akerman, S.; van den Maagdenberg, A.M.; Saxena, P.R.; Goadsby, P.J.; van den Brink, A.M. Intravital microscopy on a closed cranial window in mice: A model to study trigeminovascular mechanisms involved in migraine. Cephalalgia 2006, 26, 1294–1303. [Google Scholar] [CrossRef]
- Limmroth, V.; Katsarava, Z.; Liedert, B.; Guehring, H.; Schmitz, K.; Diener, H.C.; Michel, M.C. An in vivo rat model to study calcitonin gene related peptide release following activation of the trigeminal vascular system. Pain 2001, 92, 101–106. [Google Scholar] [CrossRef]
- Williamson, D.J.; Hargreaves, R.J.; Hill, R.G.; Shepheard, S.L. Sumatriptan inhibits neurogenic vasodilation of dural blood vessels in the anaesthetized rat-intravital microscope studies. Cephalalgia 1997, 17, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Ashina, M.; Hansen, J.M.; Do, T.P.; Melo-Carrillo, A.; Burstein, R.; Moskowitz, M.A. Migraine and the trigeminovascular system-40 years and counting. Lancet Neurol. 2019, 18, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.J.; Holland, P.R.; Martins-Oliveira, M.; Hoffmann, J.; Schankin, C.; Akerman, S. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol. Rev. 2017, 97, 553–622. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Ajona, D.; Chan, C.; Villar-Martínez, M.D.; Goadsby, P.J. Targeting CGRP and 5-HT(1F) Receptors for the Acute Therapy of Migraine: A Literature Review. Headache 2019, 59 (Suppl. S2), 3–19. [Google Scholar] [CrossRef]
- Lozano-Cuenca, J.; González-Hernández, A.; Muñoz-Islas, E.; Sánchez-López, A.; Centurión, D.; Cobos-Puc, L.E.; Villalón, C.M. Effect of some acute and prophylactic antimigraine drugs on the vasodepressor sensory CGRPergic outflow in pithed rats. Life Sci. 2009, 84, 125–131. [Google Scholar] [CrossRef]
- Nicholson, R.; Small, J.; Dixon, A.K.; Spanswick, D.; Lee, K. Serotonin receptor mRNA expression in rat dorsal root ganglion neurons. Neurosci. Lett. 2003, 337, 119–122. [Google Scholar] [CrossRef]
- Muñoz-Islas, E.; Gupta, S.; Jiménez-Mena, L.R.; Lozano-Cuenca, J.; Sánchez-López, A.; Centurión, D.; Mehrotra, S.; MaassenVanDenBrink, A.; Villalón, C.M. Donitriptan, but not sumatriptan, inhibits capsaicin-induced canine external carotid vasodilatation via 5-HT1B rather than 5-HT1D receptors. Br. J. Pharmacol. 2006, 149, 82–91. [Google Scholar] [CrossRef]
- Muñoz-Islas, E.; Lozano-Cuenca, J.; González-Hernández, A.; Ramírez-Rosas, M.B.; Sánchez-López, A.; Centurión, D.; Maassenvandenbrink, A.; Villalón, C.M. Spinal sumatriptan inhibits capsaicin-induced canine external carotid vasodilatation via 5-HT1B rather than 5-HT1D receptors. Eur. J. Pharmacol. 2009, 615, 133–138. [Google Scholar] [CrossRef]
- Rubio-Beltrán, E.; Labastida-Ramírez, A.; Villalón, C.M.; MaassenVanDenBrink, A. Is selective 5-HT(1F) receptor agonism an entity apart from that of the triptans in antimigraine therapy? Pharmacol. Ther. 2018, 186, 88–97. [Google Scholar] [CrossRef]
- Fujii, H.; Takatori, S.; Zamami, Y.; Hashikawa-Hobara, N.; Miyake, N.; Tangsucharit, P.; Mio, M.; Kawasaki, H. Adrenergic stimulation-released 5-HT stored in adrenergic nerves inhibits CGRPergic nerve-mediated vasodilatation in rat mesenteric resistance arteries. Br. J. Pharmacol. 2012, 166, 2084–2094. [Google Scholar] [CrossRef]
- Gomez-Mancilla, B.; Cutler, N.R.; Leibowitz, M.T.; Spierings, E.L.; Klapper, J.A.; Diamond, S.; Goldstein, J.; Smith, T.; Couch, J.R.; Fleishaker, J.; et al. Safety and efficacy of PNU-142633, a selective 5-HT1D agonist, in patients with acute migraine. Cephalalgia 2001, 21, 727–732. [Google Scholar] [CrossRef]
- González-Hernández, A.; Marichal-Cancino, B.A.; Lozano-Cuenca, J.; MaassenVanDenBrink, A.; Villalón, C.M. Functional Characterization of the Prejunctional Receptors Mediating the Inhibition by Ergotamine of the Rat Perivascular Sensory Peptidergic Drive. ACS Chem. Neurosci. 2019, 10, 3173–3182. [Google Scholar] [CrossRef]
- de Jong, A.P.; Verhage, M. Presynaptic signal transduction pathways that modulate synaptic transmission. Curr. Opin. Neurobiol. 2009, 19, 245–253. [Google Scholar] [CrossRef]
- Cuesta, C.; García-Pedraza, J.; García, M.; Villalón, C.M.; Morán, A. Role of 5-HT7 receptors in the inhibition of the vasodepressor sensory CGRPergic outflow in pithed rats. Vasc. Pharmacol. 2014, 63, 4–12. [Google Scholar] [CrossRef]
- Chan, A.K.; von der Weid, P.Y. 5-HT decreases contractile and electrical activities in lymphatic vessels of the guinea-pig mesentery: Role of 5-HT 7-receptors. Br. J. Pharmacol. 2003, 139, 243–254. [Google Scholar] [CrossRef]
- Chan, M.F.; Okun, I.; Stavros, F.L.; Hwang, E.; Wolff, M.E.; Balaji, V.N. Identification of a new class of ETA selective endothelin antagonists by pharmacophore directed screening. Biochem. Biophys. Res. Commun. 1994, 201, 228–234. [Google Scholar] [CrossRef]
- Wiklund, N.P.; Wiklund, C.U.; Cederqvist, B.; Ohlén, A.; Hedqvist, P.; Gustafsson, L.E. Endothelin modulation of neuroeffector transmission in smooth muscle. J. Cardiovasc. Pharmacol. 1991, 17 (Suppl. S7), S335–S339. [Google Scholar] [CrossRef]
- Filippelli, A.; Falciani, M.; Piucci, B.; D’Amico, M.; D’Agostino, B.; Filippelli, W.; Rossi, F. Endothelin-1 affects capsaicin-evoked release of neuropeptides from rat vas deferens. Eur. J. Pharmacol. 1999, 364, 183–191. [Google Scholar] [CrossRef]
- Brenchat, A.; Zamanillo, D.; Hamon, M.; Romero, L.; Vela, J.M. Role of peripheral versus spinal 5-HT(7) receptors in the modulation of pain undersensitizing conditions. Eur. J. Pain. 2012, 16, 72–81. [Google Scholar] [CrossRef]
- Chen, J.J.; Vasko, M.R.; Wu, X.; Staeva, T.P.; Baez, M.; Zgombick, J.M.; Nelson, D.L. Multiple subtypes of serotonin receptors are expressed in rat sensory neurons in culture. J. Pharmacol. Exp. Ther. 1998, 287, 1119–1127. [Google Scholar]
- Pierce, P.A.; Xie, G.X.; Meuser, T.; Peroutka, S.J. 5-Hydroxytryptamine receptor subtype messenger RNAs in human dorsal root ganglia: A polymerase chain reaction study. Neuroscience 1997, 81, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Sugiuar, T.; Bielefeldt, K.; Gebhart, G.F. TRPV1 function in mouse colon sensory neurons is enhanced by metabotropic 5-hydroxytryptamine receptor activation. J. Neurosci. 2004, 24, 9521–9530. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chen, T.; Gao, Y.; Quirion, R.; Hong, Y. Inhibition of SNL-induced upregulation of CGRP and NPY in the spinal cord and dorsal root ganglia by the 5-HT(2A) receptor antagonist ketanserin in rats. Pharmacol. Biochem. Behav. 2012, 101, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Islas, E.; Vidal-Cantú, G.C.; Bravo-Hernández, M.; Cervantes-Durán, C.; Quiñonez-Bastidas, G.N.; Pineda-Farias, J.B.; Barragán-Iglesias, P.; Granados-Soto, V. Spinal 5-HT₅A receptors mediate 5-HT-induced antinociception in several pain models in rats. Pharmacol. Biochem. Behav. 2014, 120, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Tramontana, M.; Giuliani, S.; Del Bianco, E.; Lecci, A.; Maggi, C.A.; Evangelista, S.; Geppetti, P. Effects of capsaicin and 5-HT3 antagonists on 5-hydroxytryptamine-evoked release of calcitonin gene-related peptide in the guinea-pig heart. Br. J. Pharmacol. 1993, 108, 431–435. [Google Scholar] [CrossRef]
- Smillie, S.J.; Brain, S.D. Calcitonin gene-related peptide (CGRP) and its role in hypertension. Neuropeptides 2011, 45, 93–104. [Google Scholar] [CrossRef]
- Lu, J.T.; Son, Y.J.; Lee, J.; Jetton, T.L.; Shiota, M.; Moscoso, L.; Niswender, K.D.; Loewy, A.D.; Magnuson, M.A.; Sanes, J.R.; et al. Mice lacking alpha-calcitonin gene-related peptide exhibit normal cardiovascular regulation and neuromuscular development. Mol. Cell. Neurosci. 1999, 14, 99–120. [Google Scholar] [CrossRef]
- Mai, T.H.; Wu, J.; Diedrich, A.; Garland, E.M.; Robertson, D. Calcitonin gene-related peptide (CGRP) in autonomic cardiovascular regulation and vascular structure. J. Am. Soc. Hypertens. JASH 2014, 8, 286–296. [Google Scholar] [CrossRef]
- Kudrow, D.; Pascual, J.; Winner, P.K.; Dodick, D.W.; Tepper, S.J.; Reuter, U.; Hong, F.; Klatt, J.; Zhang, F.; Cheng, S.; et al. Vascular safety of erenumab for migraine prevention. Neurology 2020, 94, e497–e510. [Google Scholar] [CrossRef]
- de Vries Lentsch, S.; van der Arend, B.W.H.; Maassen VanDenBrink, A.; Terwindt, G.M. Blood Pressure in Patients with Migraine Treated with Monoclonal Anti-CGRP (Receptor) Antibodies: A Prospective Follow-up Study. Neurology 2022, 99, e1897–e1904. [Google Scholar] [CrossRef]
- Kumar, A.; Potts, J.D.; DiPette, D.J. Protective Role of α-Calcitonin Gene-Related Peptide in Cardiovascular Diseases. Front. Physiol. 2019, 10, 821. [Google Scholar] [CrossRef]
- Kumar, A.; Williamson, M.; Hess, A.; DiPette, D.J.; Potts, J.D. Alpha-Calcitonin Gene Related Peptide: New Therapeutic Strategies for the Treatment and Prevention of Cardiovascular Disease and Migraine. Front. Physiol. 2022, 13, 826122. [Google Scholar] [CrossRef]
- MaassenVanDenBrink, A.; Terwindt, G.M.; van den Maagdenberg, A. Calcitonin gene-related peptide (receptor) antibodies: An exciting avenue for migraine treatment. Genome Med. 2018, 10, 10. [Google Scholar] [CrossRef]
- Shi, X.Y.; Yang, Y.; Zhao, Y.T. Plasma calcitonin gene-related peptide (CGRP) level in patients with essential hypertension. Zhonghua Nei Ke Za Zhi 1990, 29, 616–618. [Google Scholar]
- Xu, D.; Wang, X.A.; Wang, J.P. Calcitonin gene-related peptide (CGRP) in normotensive and spontaneously hypertensive rats. Peptides 1989, 17, 174–177. [Google Scholar] [CrossRef]
- Watson, R.E.; Supowit, S.C.; Zhao, H.; Katki, K.A.; Dipette, D.J. Role of sensory nervous system vasoactive peptides in hypertension. Braz. J. Med. Biol. Res. 2002, 35, 1033–1045. [Google Scholar] [CrossRef]
- Guyton, A.C.; Coleman, T.G.; Cowley, A.V., Jr.; Scheel, K.W.; Manning, R.D., Jr.; Norman, R.A., Jr. Arterial pressure regulation. Overriding dominance of the kidneys in long-term regulation and in hypertension. Am. J. Med. 1972, 52, 584–594. [Google Scholar] [CrossRef]
- Hoffman, B.B. Catecholamines, sympathomimetic drugs, and adrenergic receptor antagonists. In Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 10th ed.; Hardman, J.G., Laurence, L., Goodman Gilman, A., Eds.; McGraw-Hill: New York, NY, USA, 2001; pp. 215–268. [Google Scholar]
- Gardiner, S.M.; Compton, A.M.; Bennett, T. Regional hemodynamic effects of calcitonin gene-related peptide. Am. J. Physiol. 1989, 256, R332–R338. [Google Scholar] [CrossRef]
- Han, S.P.; Naes, L.; Westfall, T.C. Calcitonin gene-related peptide is the endogenous mediator of nonadrenergic-noncholinergic vasodilation in rat mesentery. J. Pharmacol. Exp. Ther. 1990, 255, 423–428. [Google Scholar]
- Han, S.P.; Naes, L.; Westfall, T.C. Inhibition of periarterial nerve stimulation-induced vasodilation of the mesenteric arterial bed by CGRP (8–37) and CGRP receptor desensitization. Biochem. Biophys. Res. Commun. 1990, 168, 786–791. [Google Scholar] [CrossRef]
- Holzer, P. Local effector functions of capsaicin-sensitive sensory nerve endings: Involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience 1988, 24, 739–768. [Google Scholar] [CrossRef] [PubMed]
- Machida, T.; Iizuka, K.; Hirafuji, M. 5-hydroxytryptamine and its receptors in systemic vascular walls. Biol. Pharm. Bull. 2013, 36, 1416–1419. [Google Scholar] [CrossRef] [PubMed]
- Sumner, M.J. Characterization of the 5-HT receptor mediating endothelium-dependent relaxation in porcine vena cava. Br. J. Pharmacol. 1991, 102, 938–942. [Google Scholar] [CrossRef] [PubMed]
- Rivasi, G.; Menale, S.; Turrin, G.; Coscarelli, A.; Giordano, A.; Ungar, A. The Effects of Pain and Analgesic Medications on Blood Pressure. Curr. Hypertens. Rep. 2022, 24, 385–394. [Google Scholar] [CrossRef]
- Sánchez, M.G.; Morissette, M.; Di Paolo, T. Oestradiol Modulation of Serotonin Reuptake Transporter and Serotonin Metabolism in the Brain of Monkeys. J. Neuroendocrinol. 2013, 25, 560–569. [Google Scholar] [CrossRef]
- Soslau, G. Cardiovascular serotonergic system: Evolution, receptors, transporter, and function. J. Exp. Zool. A Ecol. Integr. Physiol. 2022, 337, 115–127. [Google Scholar] [CrossRef]
- Erspamer, V.; Asero, B. Identification of enteramine, the specific hormone of the enterochromaffin cell system, as 5-hydroxytryptamine. Nature 1952, 169, 800–801. [Google Scholar] [CrossRef]
- Fetkovska, N.; Pletscher, A.; Ferracin, F.; Amstein, R.; Buhler, F.R. Impaired uptake of 5 hydroxytryptamine platelet in essential hypertension: Clinical relevance. Cardiovasc. Drugs Ther. 1990, 4 (Suppl. S1), 105–109. [Google Scholar] [CrossRef]
- Diaz, J.; Ni, W.; Thompson, J.; King, A.; Fink, G.D.; Watts, S.W. 5-Hydroxytryptamine lowers blood pressure in normotensive and hypertensive rats. J. Pharmacol. Exp. Ther. 2008, 325, 1031–1038. [Google Scholar] [CrossRef]
- Patrick Davis, R.; Linder, A.E.; Watts, S.W. Lack of the serotonin transporter (SERT) reduces the ability of 5-hydroxytryptamine to lower blood pressure. Naunyn Schmiedebergs Arch. Pharmacol. 2011, 383, 543–546. [Google Scholar] [CrossRef]
- Karlsen, H.R.; Løchen, M.L.; Langvik, E. Antidepressant Use and Risk of Myocardial Infarction: A Longitudinal Investigation of Sex-Specific Associations in the HUNT Study. Psychosom. Med. 2023, 85, 26–33. [Google Scholar] [CrossRef]
- Niazi, S.K.; Memon, S.H.; Lesser, E.R.; Brennan, E.; Aslam, N. Assessment of psychiatric comorbidities and serotonergic or noradrenergic medication use on blood pressure using 24-hour ambulatory blood pressure monitoring. J. Clin. Hypertens. 2021, 23, 1599–1607. [Google Scholar] [CrossRef]
- Muñoz-Islas, E.; González-Hernández, A.; Lozano-Cuenca, J.; Ramírez-Rosas, M.B.; Medina-Santillán, R.; Centurión, D.; MaassenVanDenBrink, A.; Villalón, C.M. Inhibitory effect of chronic oral treatment with fluoxetine on capsaicin-induced external carotid vasodilatation in anaesthetised dogs. Cephalalgia 2015, 35, 1041–1053. [Google Scholar] [CrossRef]
- Pak, K.; Kim, K.; Seo, S.; Lee, M.J.; Kim, I.J. Serotonin transporter is negatively associated with body mass index after glucose loading in humans. Brain Imaging Behav. 2022, 16, 1246–1251. [Google Scholar] [CrossRef]
- Garrett, S.M.; Whitaker, R.M.; Beeson, C.C.; Schnellmann, R.G. Agonism of the 5-hydroxytryptamine 1F receptor promotes mitochondrial biogenesis and recovery from acute kidney injury. J. Pharmacol. Exp. Ther. 2014, 350, 257–264. [Google Scholar] [CrossRef]
- Simmons, E.C.; Scholpa, N.E.; Cleveland, K.H.; Schnellmann, R.G. 5-hydroxytryptamine 1F Receptor Agonist Induces Mitochondrial Biogenesis and Promotes Recovery from Spinal Cord Injury. J. Pharmacol. Exp. Ther. 2020, 372, 216–223. [Google Scholar] [CrossRef]
- Newman-Tancredi, A.; Depoortère, R.Y.; Kleven, M.S.; Kołaczkowski, M.; Zimmer, L. Translating biased agonists from molecules to medications: Serotonin 5-HT(1A) receptor functional selectivity for CNS disorders. Pharmacol. Ther. 2022, 229, 107937. [Google Scholar] [CrossRef]

| 5-HT Receptor | Receptor Subtype | Agonists | Antagonists | Some Functions | Canonical Transduction System |
|---|---|---|---|---|---|
| 5-HT1 | 5-HT1A | 8-OH-DPAT | WAY 100635 | Central hypotension | G-protein coupled receptor (Gi) |
| 5-HT1B | Sumatriptan CP-93,129 (rodents) | SB224289 | Vasoconstriction, sympatho-inhibition | ||
| 5-HT1D | PNU-109291 PNU-142633 | BRL15572 | Autoreceptor, sympatho-inhibition | ||
| 5-HT1e * | 5-HT >> 5-CT, LY334370 | Methiothepin (non-selective) | Unknown | ||
| 5-HTF | LY344864, lasmiditan, LY334370 | Methysergide (non-selective) | (−) Trigeminal system | ||
| 5-HT5 | 5-HT5A | 5-HT, ergotamine | SB699551 | Cardiac sympatho-inhibition in rats | |
| 5-HT5b * | 5-CT (non-selective) | Unknown | Unknown | ||
| 5-HT4 | - | Renzapride, BIMU8, ML10302, SC53116 | GR 113808, SB204070 | (+) Neuronal activity, vasodilatation, tachycardia in pigs and humans |  G-protein coupled receptor (Gs) |
| 5-HT6 | - | 5-MeO-T ≥ 5-HT SB357134, SB271046 | Ro 630563 | Memory, not involved in cardiovascular regulation | |
| 5-HT7 | - | 5-CT>>5-HT AS-19 | SB269970, SB258719 | Circadian rhythm, vasodila- tation, tachycardia in cats | |
| 5-HT2 | 5-HT2A | DOI, DOB α-methyl-5-HT | MDL100907 Ketanserin | Vasoconstriction, plateletaggregation |  G-protein coupled receptor (Gq) |
| 5-HT2B | DOI, BW723C86 α-methyl-5-HT | SB204741 RS-127445 | Vasoconstriction, release of NO | ||
| 5-HT2C | DOI, Ro 60-0175 α-methyl-5-HT | SB242084 RS-102221 | CSF production | ||
| 5-HT3 | Pentameric ion channel ** | Phenylbiguanide 2-methyl-5-HT | Tropisetron, Granisetron MDL-72222 | (+) Neuronal activity, reflex bradycardia | Ligand-gated ion channel |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Hernández, A.; Marichal-Cancino, B.A.; MaassenVanDenBrink, A.; Villalón, C.M. Serotonergic Modulation of Neurovascular Transmission: A Focus on Prejunctional 5-HT Receptors/Mechanisms. Biomedicines 2023, 11, 1864. https://doi.org/10.3390/biomedicines11071864
González-Hernández A, Marichal-Cancino BA, MaassenVanDenBrink A, Villalón CM. Serotonergic Modulation of Neurovascular Transmission: A Focus on Prejunctional 5-HT Receptors/Mechanisms. Biomedicines. 2023; 11(7):1864. https://doi.org/10.3390/biomedicines11071864
Chicago/Turabian StyleGonzález-Hernández, Abimael, Bruno A. Marichal-Cancino, Antoinette MaassenVanDenBrink, and Carlos M. Villalón. 2023. "Serotonergic Modulation of Neurovascular Transmission: A Focus on Prejunctional 5-HT Receptors/Mechanisms" Biomedicines 11, no. 7: 1864. https://doi.org/10.3390/biomedicines11071864
APA StyleGonzález-Hernández, A., Marichal-Cancino, B. A., MaassenVanDenBrink, A., & Villalón, C. M. (2023). Serotonergic Modulation of Neurovascular Transmission: A Focus on Prejunctional 5-HT Receptors/Mechanisms. Biomedicines, 11(7), 1864. https://doi.org/10.3390/biomedicines11071864