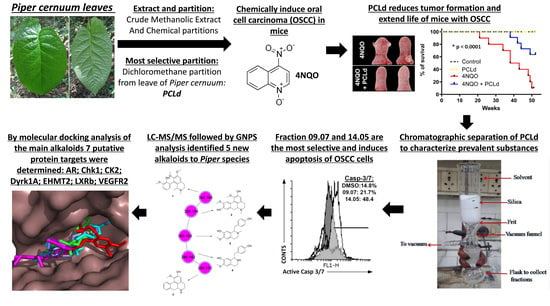
Anticancer Activity and Molecular Targets of Piper cernuum Substances in Oral Squamous Cell Carcinoma Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Plant and Crude Extract Preparation
2.3. PCLd Fractionation
2.4. LC–DAD–MS/MS Analyses
2.5. Analysis of Mass Spectra and Annotation Using GNPS
2.6. NMR Analysis
2.7. Mouse Husbandry
2.8. Chronic Toxicity, and Therapeutical Properties of PCLd and Survival Analysis
2.9. Macroscopic and Histopathological Analysis of the Tongue
2.10. Chronic Toxicity of PCLd
2.11. Survival Analysis
2.12. Cell Viability Assay (Cytotoxicity)
2.13. Statistical Analysis, Calculation of IC50, and Selectivity Index (SI)
2.14. ROS Production
2.15. Hemolysis Assay
2.16. Cell Cycle and Analysis of SubG1
2.17. Analysis of Exposure to Phosphatidylserine (Apoptosis)
2.18. Active Caspase 3/7 Assay
2.19. Prediction of the Mechanism of Action of the Main Compounds of the 14.05 Fraction
3. Results and Discussion
3.1. Piper cernuum Partition Is Nontoxic, Reduces Cancer Foci Numbers and Size, and Increases the Survival of Mice with Chemically Induced OSCC
3.2. Cytotoxicity and Selectivity Determination of PCLd of Fractions
3.3. Cell Death Pathway Investigation
3.4. Phytochemical Analysis
3.5. Prediction of the Molecular Targets of Annotated Alkaloids in PCLd 14.05 Fraction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grobe, A.; Blessmann, M.; Hanken, H.; Friedrich, R.E.; Schon, G.; Wikner, J.; Effenberger, K.E.; Kluwe, L.; Heiland, M.; Pantel, K.; et al. Prognostic relevance of circulating tumor cells in blood and disseminated tumor cells in bone marrow of patients with squamous cell carcinoma of the oral cavity. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2014, 20, 425–433. [Google Scholar] [CrossRef]
- Panzarella, V.; Pizzo, G.; Calvino, F.; Compilato, D.; Colella, G.; Campisi, G. Diagnostic delay in oral squamous cell carcinoma: The role of cognitive and psychological variables. Int. J. Oral Sci. 2014, 6, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Rivera, C. Essentials of oral cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 11884–11894. [Google Scholar] [PubMed]
- Cristaldi, M.; Mauceri, R.; Di Fede, O.; Giuliana, G.; Campisi, G.; Panzarella, V. Salivary Biomarkers for Oral Squamous Cell Carcinoma Diagnosis and Follow-Up: Current Status and Perspectives. Front. Physiol. 2019, 10, 1476. [Google Scholar] [CrossRef]
- Thomson, P.J. Perspectives on oral squamous cell carcinoma prevention-proliferation, position, progression and prediction. J. Oral Pathol. Med. 2018, 47, 803–807. [Google Scholar] [CrossRef]
- Cai, H.; Li, J.; Zhang, Y.; Liao, Y.; Zhu, Y.; Wang, C.; Hou, J. LDHA Promotes Oral Squamous Cell Carcinoma Progression Through Facilitating Glycolysis and Epithelial-Mesenchymal Transition. Front. Oncol. 2019, 9, 1446. [Google Scholar] [CrossRef] [PubMed]
- Massano, J.; Regateiro, F.S.; Januario, G.; Ferreira, A. Oral squamous cell carcinoma: Review of prognostic and predictive factors. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2006, 102, 67–76. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, Y.; Li, H.; Zhou, Y.; Zhang, Q.; Chen, R.; Jin, T.; Hu, K.; Li, S.; Wang, Y.; et al. Correction: Vitamin D promotes the cisplatin sensitivity of oral squamous cell carcinoma by inhibiting LCN2-modulated NF-kappaB pathway activation through RPS3. Cell Death Dis. 2020, 11, 190. [Google Scholar] [CrossRef]
- Salehi, B.; Zakaria, Z.A.; Gyawali, R.; Ibrahim, S.A.; Rajkovic, J.; Shinwari, Z.K.; Khan, T.; Sharifi-Rad, J.; Ozleyen, A.; Turkdonmez, E.; et al. Piper Species: A Comprehensive Review on Their Phytochemistry, Biological Activities and Applications. Molecules 2019, 24, 1364. [Google Scholar] [CrossRef]
- Rasmussen, T.D.; Guerra, B.; Issinger, O.-G. Screening of DTP Compound Libraries for CK2 Inhibitors with Focus on Natural Products. In Protein Kinase CK2 Cellular Function in Normal and Disease States; Ahmed, K., Issinger, O.-G., Szyszka, R., Eds.; Springer International Publishing: Cham, Germany, 2015; pp. 319–340. [Google Scholar]
- Macedo, A.L.; da Silva, D.P.D.; Moreira, D.L.; de Queiroz, L.N.; Vasconcelos, T.R.A.; Araujo, G.F.; Kaplan, M.A.C.; Pereira, S.S.C.; de Almeida, E.C.P.; Valverde, A.L.; et al. Cytotoxicity and selectiveness of Brazilian Piper species towards oral carcinoma cells. Biomed. Pharmacother. 2019, 110, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Coll, J.C.; Bowden, B.F. The Application of Vacuum Liquid Chromatography to the Separation of Terpene Mixtures. J. Nat. Prod. 1986, 49, 934–936. [Google Scholar] [CrossRef]
- Rokosik, E.; Dwiecki, K.; Rudzińska, M.; Siger, A.; Polewski, K. Column chromatography as a method for minor components removal from rapeseed oil. Grasas Y Aceites 2019, 70, e316. [Google Scholar] [CrossRef]
- Macedo, A.L.; Boaretto, A.G.; Silva, A.N.d.; Maia, D.S.; Siqueira, J.M.d.; Silva, D.B.; Carollo, C.A. Evaluation of the Effect of Brazilian Savanna (Cerrado) Seasons in Flavonoids and Alkaloids Accumulation: The Case of Duguetia furfuracea. J. Braz. Chem. Soc. 2021, 32, 1840–1850. [Google Scholar] [CrossRef]
- Chambers, M.C.; Maclean, B.; Burke, R.; Amodei, D.; Ruderman, D.L.; Neumann, S.; Gatto, L.; Fischer, B.; Pratt, B.; Egertson, J.; et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.H.; Knudsen, B.; Bemis, D.; Tickoo, S.; Gudas, L.J. Oral cavity and esophageal carcinogenesis modeled in carcinogen-treated mice. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2004, 10 Pt 1, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Zorzanelli, B.C.; Ouverney, G.; Pauli, F.P.; da Fonseca, A.C.C.; de Almeida, E.C.P.; de Carvalho, D.G.; Possik, P.A.; Rabelo, V.W.; Abreu, P.A.; Pontes, B.; et al. Pro-Apoptotic Antitumoral Effect of Novel Acridine-Core Naphthoquinone Compounds against Oral Squamous Cell Carcinoma. Molecules 2022, 27, 5148. [Google Scholar] [CrossRef]
- Fonseca, A.; de Queiroz, L.N.; Sales Felisberto, J.; Jesse Ramos, Y.; Mesquita Marques, A.; Wermelinger, G.F.; Pontes, B.; de Lima Moreira, D.; Robbs, B.K. Cytotoxic effect of pure compounds from Piper rivinoides Kunth against oral squamous cell carcinoma. Nat. Prod. Res. 2021, 35, 6163–6167. [Google Scholar] [CrossRef]
- Faget, D.V.; Lucena, P.I.; Robbs, B.K.; Viola, J.P. NFAT1 C-terminal domains are necessary but not sufficient for inducing cell death. PLoS ONE 2012, 7, e47868. [Google Scholar] [CrossRef]
- Borges, A.A.; de Souza, M.P.; da Fonseca, A.C.C.; Wermelinger, G.F.; Ribeiro, R.C.B.; Amaral, A.A.P.; de Carvalho, C.J.C.; Abreu, L.S.; de Queiroz, L.N.; de Almeida, E.C.P.; et al. Chemoselective Synthesis of Mannich Adducts from 1,4-Naphthoquinones and Profile as Autophagic Inducers in Oral Squamous Cell Carcinoma. Molecules 2022, 28, 309. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Aprahamian, M.L.; Lindert, S. Improving inverse docking target identification with Z-score selection. Chem. Biol. Drug Des. 2019, 93, 1105–1116. [Google Scholar] [CrossRef]
- Sun, Z.; Guan, X.; Li, N.; Liu, X.; Chen, X. Chemoprevention of oral cancer in animal models, and effect on leukoplakias in human patients with ZengShengPing, a mixture of medicinal herbs. Oral Oncol. 2010, 46, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Shrotriya, S.; Tyagi, A.; Deep, G.; Orlicky, D.J.; Wisell, J.; Wang, X.J.; Sclafani, R.A.; Agarwal, R.; Agarwal, C. Grape seed extract and resveratrol prevent 4-nitroquinoline 1-oxide induced oral tumorigenesis in mice by modulating AMPK activation and associated biological responses. Mol. Carcinog. 2015, 54, 291–300. [Google Scholar] [CrossRef]
- Wilkey, J.F.; Buchberger, G.; Saucier, K.; Patel, S.M.; Eisenberg, E.; Nakagawa, H.; Michaylira, C.Z.; Rustgi, A.K.; Mallya, S.M. Cyclin D1 overexpression increases susceptibility to 4-nitroquinoline-1-oxide-induced dysplasia and neoplasia in murine squamous oral epithelium. Mol. Carcinog. 2009, 48, 853–861. [Google Scholar] [CrossRef]
- Yuan, B.; Heniford, B.W.; Ackermann, D.M.; Hawkins, B.L.; Hendler, F.J. Harvey ras (H-ras) point mutations are induced by 4-nitroquinoline-1-oxide in murine oral squamous epithelia, while squamous cell carcinomas and loss of heterozygosity occur without additional exposure. Cancer Res. 1994, 54, 5310–5317. [Google Scholar] [PubMed]
- Hawkins, B.L.; Heniford, B.W.; Ackermann, D.M.; Leonberger, M.; Martinez, S.A.; Hendler, F.J. 4NQO carcinogenesis: A mouse model of oral cavity squamous cell carcinoma. Head Neck 1994, 16, 424–432. [Google Scholar] [CrossRef]
- Panigrahi, G.B.; Walker, I.G. The N2-guanine adduct but not the C8-guanine or N6-adenine adducts formed by 4-nitroquinoline 1-oxide blocks the 3′-5′ exonuclease action of T4 DNA polymerase. Biochemistry 1990, 29, 2122–2126. [Google Scholar] [CrossRef]
- Parasuraman, S. Toxicological screening. J. Pharmacol. Pharmacother. 2011, 2, 74–79. [Google Scholar]
- Silveira, E.J.; Godoy, G.P.; Lins, R.D.; Arruda Mde, L.; Ramos, C.C.; Freitas Rde, A.; Queiroz, L.M. Correlation of clinical, histological, and cytokeratin profiles of squamous cell carcinoma of the oral tongue with prognosis. Int. J. Surg. Pathol. 2007, 15, 376–383. [Google Scholar] [CrossRef]
- Pereira, M.C.; Oliveira, D.T.; Landman, G.; Kowalski, L.P. Histologic subtypes of oral squamous cell carcinoma: Prognostic relevance. J Can Dent Assoc 2007, 73, 339–344. [Google Scholar]
- Soyele, O.O.; Aborisade, A.; Adesina, O.M.; Olatunji, A.; Adedigba, M.; Ladeji, A.M.; Adeola, H.A. Concordance between clinical and histopathologic diagnosis and an audit of oral histopathology service at a Nigerian tertiary hospital. Pan Afr. Med. J. 2019, 34, 100. [Google Scholar] [CrossRef] [PubMed]
- Girola, N.; Figueiredo, C.R.; Farias, C.F.; Azevedo, R.A.; Ferreira, A.K.; Teixeira, S.F.; Capello, T.M.; Martins, E.G.; Matsuo, A.L.; Travassos, L.R.; et al. Camphene isolated from essential oil of Piper cernuum (Piperaceae) induces intrinsic apoptosis in melanoma cells and displays antitumor activity in vivo. Biochem. Biophys. Res. Commun. 2015, 467, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Ricci, M.S.; Zong, W.X. Chemotherapeutic approaches for targeting cell death pathways. Oncologist 2006, 11, 342–357. [Google Scholar] [CrossRef]
- Paranjpe, R.; Gundala, S.R.; Lakshminarayana, N.; Sagwal, A.; Asif, G.; Pandey, A.; Aneja, R. Piper betel leaf extract: Anticancer benefits and bio-guided fractionation to identify active principles for prostate cancer management. Carcinogenesis 2013, 34, 1558–1566. [Google Scholar] [CrossRef]
- Grinevicius, V.M.A.S.; Andrade, K.S.; Ourique, F.; Micke, G.A.; Ferreira, S.R.S.; Pedrosa, R.C. Antitumor activity of conventional and supercritical extracts from Piper nigrum L. cultivar Bragantina through cell cycle arrest and apoptosis induction. J. Supercrit. Fluids 2017, 128, 94–101. [Google Scholar] [CrossRef]
- Stevigny, C.; Jiwan, J.L.; Rozenberg, R.; de Hoffmann, E.; Quetin-Leclercq, J. Key fragmentation patterns of aporphine alkaloids by electrospray ionization with multistage mass spectrometry. Rapid Commun. Mass Spectrom. RCM 2004, 18, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Qing, Z.; Xu, Y.; Yu, L.; Liu, J.; Huang, X.; Tang, Z.; Cheng, P.; Zeng, J. Investigation of fragmentation behaviours of isoquinoline alkaloids by mass spectrometry combined with computational chemistry. Sci. Rep. 2020, 10, 733. [Google Scholar] [CrossRef]
- Castro-Saavedra, S.; Fuentes-Barros, G.; Tirapegui, C.; Acevedo-Fuentes, W.; Cassels, B.K.; Barriga, A.; Vilches-Herrera, M. Phytochemical Analysis of Alkaloids from the Chilean Endemic Tree Cryptocarya Alba. J. Chil. Chem. Soc. 2016, 61, 5. [Google Scholar] [CrossRef]
- Costa, E.V.; Sampaio, M.F.C.; Salvador, M.J.; Nepel, A.; Barison, A. Chemical constituents from the stem bark of Annona pickelii (Annonaceae). Quim. Nova 2015, 38, 769–776. [Google Scholar]
- Santos, M.d.F.C.; Fontes, J.E.N.; Dutra, L.M.; Bomfim, L.M.; Costa, C.O.D.; Moraes, V.R.S.; Barison, A.; Soares, M.B.P.; Silva, F.M.A.d.; Almeida, J.R.G.d.S.; et al. Alkaloids from Leaves of Guatteria Pogonopus (Annonaceae) and their Cytotoxicities. Quim. Nova 2018, 41, 884–890. [Google Scholar] [CrossRef]
- Liu, X.; Tian, H.; Li, H.; Ge, C.; Zhao, F.; Yao, M.; Li, J. Derivate Isocorydine (d-ICD) Suppresses Migration and Invasion of Hepatocellular Carcinoma Cell by Downregulating ITGA1 Expression. Int. J. Mol. Sci. 2017, 18, 514. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Li, R.; Xin, A.; Han, Y.; Zhang, Y.; Liu, J.; Li, W.; Di, D. Design, synthesis, and anticancer properties of isocorydine derivatives. Bioorganic Med. Chem. 2017, 25, 6542–6553. [Google Scholar] [CrossRef]
- Sun, H.; Hou, H.; Lu, P.; Zhang, L.; Zhao, F.; Ge, C.; Wang, T.; Yao, M.; Li, J. Isocorydine inhibits cell proliferation in hepatocellular carcinoma cell lines by inducing G2/m cell cycle arrest and apoptosis. PLoS ONE 2012, 7, e36808. [Google Scholar] [CrossRef]
- Kazemi Noureini, S.; Tanavar, F. Boldine, a natural aporphine alkaloid, inhibits telomerase at non-toxic concentrations. Chem. -Biol. Interact. 2015, 231, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Kazemi Noureini, S.; Kheirabadi, M.; Masoumi, F.; Khosrogerdi, F.; Zarei, Y.; Suarez-Rozas, C.; Salas-Norambuena, J.; Kennedy Cassels, B. Telomerase Inhibition by a New Synthetic Derivative of the Aporphine Alkaloid Boldine. Int. J. Mol. Sci. 2018, 19, 1239. [Google Scholar] [CrossRef]
- Paydar, M.; Kamalidehghan, B.; Wong, Y.L.; Wong, W.F.; Looi, C.Y.; Mustafa, M.R. Evaluation of cytotoxic and chemotherapeutic properties of boldine in breast cancer using in vitro and in vivo models. Drug Des. Dev. Ther. 2014, 8, 719–733. [Google Scholar]
- Ahmad, N.; Jie, C.J.; Majid, N.A. Investigation of Boldine as a Potential Telomerase Inhibitor by Downregulation of hTERT/hTERC in HCT 116 Human Colon Carcinoma Cells. Sains Malays. 2019, 48, 8. [Google Scholar] [CrossRef]
- D’Souza, S.L.; Deshmukh, B.; Bhamore, J.R.; Rawat, K.A.; Lenka, N.; Kailasa, S.K. Synthesis of fluorescent nitrogen-doped carbon dots from dried shrimps for cell imaging and boldine drug delivery system. RSC Adv. 2016, 6, 12169–12179. [Google Scholar] [CrossRef]
- Liu, W.N.; Leung, K.N. Apoptosis- and differentiation-inducing activities of jacaric acid, a conjugated linolenic acid isomer, on human eosinophilic leukemia EoL-1 cells. Oncol. Rep. 2014, 32, 1881–1888. [Google Scholar] [CrossRef]
- Al-Ghazzawi, A.M. Anti-cancer activity of new benzyl isoquinoline alkaloid from Saudi plant Annona squamosa. BMC Chem. 2019, 13, 13. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.Z.; Zhang, Q.H.; Lu, D. Studies on the chemical components of Nelumbinis Plumula and the inhibitory activity on protein disulfide isomerase. Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China J. Chin. Mater. Med. 2017, 42, 3004–3010. [Google Scholar]
- Liu, X.; Qing, S.; Che, K.; Li, L.; Liao, X. Androgen receptor promotes oral squamous cell carcinoma cell migration by increasing EGFR phosphorylation. OncoTargets Ther. 2019, 12, 4245–4252. [Google Scholar] [CrossRef]
- Yang, C.Y.; Liu, C.R.; Chang, I.Y.; OuYang, C.N.; Hsieh, C.H.; Huang, Y.L.; Wang, C.I.; Jan, F.W.; Wang, W.L.; Tsai, T.L.; et al. Cotargeting CHK1 and PI3K Synergistically Suppresses Tumor Growth of Oral Cavity Squamous Cell Carcinoma in Patient-Derived Xenografts. Cancers 2020, 12, 1726. [Google Scholar] [CrossRef]
- Battistutta, R.; Lolli, G. Inhibitory Properties of ATP-Competitive Coumestrol and Boldine are Correlated to Different Modulations of CK2 Flexibility. J. Nat. Prod. 2019, 82, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.E.; Nguyen, A.; Kang, M.K.; Kim, R.H.; Park, N.H.; Shin, K.H. DYRK1A is required for maintenance of cancer stemness, contributing to tumorigenic potential in oral/oropharyngeal squamous cell carcinoma. Exp. Cell Res. 2021, 405, 112656. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Bioassay Record for AID 588345. Available online: https://pubchem.ncbi.nlm.nih.gov/bioassay/588345 (accessed on 11 May 2023).
- National Center for Biotechnology Information. PubChem Bioassay Record for AID 504332. Available online: https://pubchem.ncbi.nlm.nih.gov/bioassay/504332 (accessed on 11 May 2023).
- Guo, Y.; Zhao, Y.R.; Liu, H.; Xin, Y.; Yu, J.Z.; Zang, Y.J.; Xu, Q.G. EHMT2 promotes the pathogenesis of hepatocellular carcinoma by epigenetically silencing APC expression. Cell Biosci. 2021, 11, 152. [Google Scholar] [CrossRef]
- Kaneko, T.; Kanno, C.; Ichikawa-Tomikawa, N.; Kashiwagi, K.; Yaginuma, N.; Ohkoshi, C.; Tanaka, M.; Sugino, T.; Imura, T.; Hasegawa, H.; et al. Liver X receptor reduces proliferation of human oral cancer cells by promoting cholesterol efflux via up-regulation of ABCA1 expression. Oncotarget 2015, 6, 33345–33357. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, T.; Xiao, Q.; Mei, B.; Zhang, X. Procyanidin B2 inhibits angiogenesis and cell growth in oral squamous cell carcinoma cells through the vascular endothelial growth factor (VEGF)/VEGF receptor 2 (VEGFR2) pathway. Bioengineered 2022, 13, 6500–6508. [Google Scholar] [CrossRef]
- Makhoba, X.H.; Viegas, C., Jr.; Mosa, R.A.; Viegas, F.P.D.; Pooe, O.J. Potential Impact of the Multi-Target Drug Approach in the Treatment of Some Complex Diseases. Drug Des. Dev. Ther. 2020, 14, 3235–3249. [Google Scholar] [CrossRef]
- Petrelli, A.; Giordano, S. From single- to multi-target drugs in cancer therapy: When aspecificity becomes an advantage. Curr. Med. Chem. 2008, 15, 422–432. [Google Scholar] [PubMed]
- Foloppe, N.; Fisher, L.M.; Howes, R.; Potter, A.; Robertson, A.G.; Surgenor, A.E. Identification of chemically diverse Chk1 inhibitors by receptor-based virtual screening. Bioorganic Med. Chem. 2006, 14, 4792–4802. [Google Scholar] [CrossRef]
- Jan, S.; Dar, M.I.; Wani, R.; Sandey, J.; Mushtaq, I.; Lateef, S.; Syed, S.H. Targeting EHMT2/ G9a for cancer therapy: Progress and perspective. Eur. J. Pharmacol. 2021, 893, 173827. [Google Scholar] [CrossRef] [PubMed]
- Takase, S.; Hiroyama, T.; Shirai, F.; Maemoto, Y.; Nakata, A.; Arata, M.; Matsuoka, S.; Sonoda, T.; Niwa, H.; Sato, S.; et al. A specific G9a inhibitor unveils BGLT3 lncRNA as a universal mediator of chemically induced fetal globin gene expression. Nat. Commun. 2023, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Falke, H.; Chaikuad, A.; Becker, A.; Loaec, N.; Lozach, O.; Abu Jhaisha, S.; Becker, W.; Jones, P.G.; Preu, L.; Baumann, K.; et al. 10-iodo-11H-indolo[3,2-c]quinoline-6-carboxylic acids are selective inhibitors of DYRK1A. J. Med. Chem. 2015, 58, 3131–3143. [Google Scholar] [CrossRef]
- Farnegardh, M.; Bonn, T.; Sun, S.; Ljunggren, J.; Ahola, H.; Wilhelmsson, A.; Gustafsson, J.A.; Carlquist, M. The three-dimensional structure of the liver X receptor beta reveals a flexible ligand-binding pocket that can accommodate fundamentally different ligands. J. Biol. Chem. 2003, 278, 38821–38828. [Google Scholar] [CrossRef]
- Harris, P.A.; Boloor, A.; Cheung, M.; Kumar, R.; Crosby, R.M.; Davis-Ward, R.G.; Epperly, A.H.; Hinkle, K.W.; Hunter, R.N., 3rd; Johnson, J.H.; et al. Discovery of 5-[[4-[(2,3-dimethyl-2H-indazol-6-yl)methylamino]-2-pyrimidinyl]amino]-2-methyl-benzenesulfonamide (Pazopanib), a novel and potent vascular endothelial growth factor receptor inhibitor. J. Med. Chem. 2008, 51, 4632–4640. [Google Scholar] [CrossRef]
- Estebanez-Perpina, E.; Arnold, L.A.; Nguyen, P.; Rodrigues, E.D.; Mar, E.; Bateman, R.; Pallai, P.; Shokat, K.M.; Baxter, J.D.; Guy, R.K.; et al. A surface on the androgen receptor that allosterically regulates coactivator binding. Proc. Natl. Acad. Sci. USA 2007, 104, 16074–16079. [Google Scholar] [CrossRef]
- Uhlen, M.; Zhang, C.; Lee, S.; Sjostedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A pathology atlas of the human cancer transcriptome. Science 2017, 357, 2507. [Google Scholar] [CrossRef]







| Treatment | Dose | Change in Body Weight | Change in Food Consumption | Morbidity a | Mortality | Gross Necropsy b | Histologyc |
|---|---|---|---|---|---|---|---|
| Control | 0 | Absent | Absent | Absent | Absent | No alteration | Normal. |
| PCLd | 480 mg/kg | Absent | Absent | Absent | Absent | No alteration | No significant alteration in comparison to the control. |
| 4NQO | 100 mg/mL | Absent | Absent | Absent | Absent | No alteration | Moderate/severe pulmonary arterial and venous hyperemia. Moderate portal hyperemia. Perivascular and periportal lymphocyte focus. |
| 4NQO + PCLd | 100 mg/mL + 480 mg/kg | Absent | Absent | Absent | Absent | No alteration | Moderate/severe pulmonary arterial and venous hyperemia. Moderate portal hyperemia. |
| Fraction | SCC9—Oral Cancer | Primary Gingival Fibroblast | Selective Index (SI) | ||
|---|---|---|---|---|---|
| IC50 (µM) | SD | IC50 (µM) | SD | ||
| 3 | 76.35 | 0.03 | 38.84 | 0.81 | 0.51 |
| 5 | 79.64 | 0.04 | 111.2 | 0.79 | 1.39 |
| 6 | 71.96 | 0.04 | 73.73 | 0.87 | 1.02 |
| 9 a | 40.25 | 0.06 | 107.50 | 0.03 | 2.67 |
| 12 | 45.08 | 0.04 | 14.00 | 0.03 | 0.31 |
| 14 a | 77.63 | 0.05 | >600 | ND | >7.7 |
| 09.01 | 71.3 | 0.05 | 103.9 | 0.02 | 1.46 |
| 09.03 | 46.26 | 0.04 | 81.94 | 0.02 | 1.77 |
| 09.05 | 47.45 | 0.02 | 74.02 | 0.02 | 1.56 |
| 09.07 | 36.87 | 0.01 | 74.71 | 0.02 | 2.03 |
| 09.09 | 52.18 | 0.03 | 74.2 | 0.03 | 1.42 |
| 14.03 | 74.8 | 0.02 | 63.77 | 0.02 | 0.85 |
| 14.05 | 64.2 | 0.04 | 162.6 | 0.07 | 2.53 |
| 14.07 | >600 | ND | 239.6 | 0.06 | ND |
| 14.09 | >600 | ND | >600 | ND | ND |
| 14.10 | >600 | ND | >600 | ND | ND |
| Carboplatin | 322.30 | 0.04 | 320.50 | 0.07 | 0.99 |
| Protein | PDB ID | Source | Target Association with Cancer and/or OSCC | Median FPKM a | Patients with High or Medium Protein Expression Level (%) a | Ref. |
|---|---|---|---|---|---|---|
| Androgen receptor (AR) | 2PIP | PharmMapper | This receptor is expressed in different OSCC cell lines and is required for cell migration, | 0.1 | 25 | [54] |
| Serine/threonine-protein Checkpoint kinase 1 (CHK1) | 2CGW | PharmMapper | Targeting CHK1 results in in vitro and in vivo antiproliferative activity against OSCC, | 3.3 | N/A | [55] |
| Casein kinase 2 alpha (CK2) | 6HNY | ChEMBL/PBD | Boldine inhibits CK2 and induces a proapoptotic effect. Also, this protein has been validated as an anticancer target for OSCC in in vivo models, | 17.5 | 100 | [56] |
| Dual specificity tyrosine phosphorylation regulated kinase 1A (DYRK1A) | 4YLK | PharmMapper | DYRK1A is upregulated in OSCC and is required for tumor growth and stemness, | 5.6 | 75 | [57,58] |
| Histone-lysine N-methyltransferase 2 (EHMT2) | 7 × 73 | ChEMBL | EHMT2 levels are increased in several cancer types. Consequently, it is considered an epigenetic target that is inhibited by coclaurine, | 8.1 | 75 | [59,60] |
| Oxysterols receptor Liver X receptor-β (LXRβ) | 1PQ9 | PharmMapper | Activation of this receptor reduces OSCC cells proliferation and tumor growth, | N/A | N/A | [61] |
| Vascular endothelial growth factor receptor 2 (VEGFR 2) | 3CJF | PharmMapper | Inhibition of the VEGFR2 pathway induced apoptosis and suppressed angiogenesis in OSCC. In addition, a VEGFR2 inhibitor is safe and effective against OSCC in clinical trials, | 2.1 | 25 | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, T.Q.; Lima, M.E.D.; da Silva, R.C.; Macedo, A.L.; de Queiroz, L.N.; Angrisani, B.R.P.; da Fonseca, A.C.C.; Câmara, P.R.; Rabelo, V.V.-H.; Carollo, C.A.; et al. Anticancer Activity and Molecular Targets of Piper cernuum Substances in Oral Squamous Cell Carcinoma Models. Biomedicines 2023, 11, 1914. https://doi.org/10.3390/biomedicines11071914
Machado TQ, Lima MED, da Silva RC, Macedo AL, de Queiroz LN, Angrisani BRP, da Fonseca ACC, Câmara PR, Rabelo VV-H, Carollo CA, et al. Anticancer Activity and Molecular Targets of Piper cernuum Substances in Oral Squamous Cell Carcinoma Models. Biomedicines. 2023; 11(7):1914. https://doi.org/10.3390/biomedicines11071914
Chicago/Turabian StyleMachado, Thaíssa Queiróz, Maria Emanuelle Damazio Lima, Rafael Carriello da Silva, Arthur Ladeira Macedo, Lucas Nicolau de Queiroz, Bianca Roberta Peres Angrisani, Anna Carolina Carvalho da Fonseca, Priscilla Rodrigues Câmara, Vitor Von-Held Rabelo, Carlos Alexandre Carollo, and et al. 2023. "Anticancer Activity and Molecular Targets of Piper cernuum Substances in Oral Squamous Cell Carcinoma Models" Biomedicines 11, no. 7: 1914. https://doi.org/10.3390/biomedicines11071914
APA StyleMachado, T. Q., Lima, M. E. D., da Silva, R. C., Macedo, A. L., de Queiroz, L. N., Angrisani, B. R. P., da Fonseca, A. C. C., Câmara, P. R., Rabelo, V. V. -H., Carollo, C. A., de Lima Moreira, D., de Almeida, E. C. P., Vasconcelos, T. R. A., Abreu, P. A., Valverde, A. L., & Robbs, B. K. (2023). Anticancer Activity and Molecular Targets of Piper cernuum Substances in Oral Squamous Cell Carcinoma Models. Biomedicines, 11(7), 1914. https://doi.org/10.3390/biomedicines11071914