Cell-Penetrating Peptide-Based Delivery of Macromolecular Drugs: Development, Strategies, and Progress
Abstract
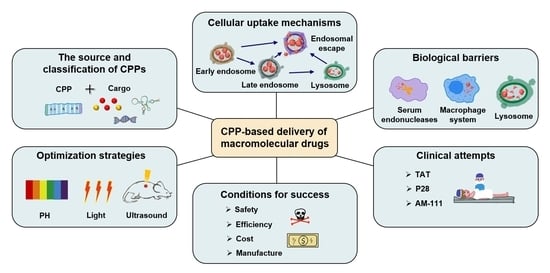
:1. Introduction
2. The History of CPP Development
3. Classification of the CPP-Based Macromolecular Drug Delivery System
3.1. Classification of CPPs
3.2. Classification of Cargos
3.3. CPP and Cargo Connection Types
4. Cellular Uptake Mechanisms, Influencing Factors, and Biological Barriers
5. Optimization Strategies of CPP-Based Delivery Systems
5.1. Enhancing the Endosomal Escape
5.2. Extending Half-Life in Blood
5.3. Targeting CPPs
5.3.1. R6LRVG Targeting CPP
5.3.2. tLyP-1 Targeting CPP
5.3.3. iRGD Targeting CPP
5.4. Stimuli-Responsive Strategies
5.4.1. pH-Responsive Strategy
5.4.2. Enzyme-Responsive Strategy
5.4.3. Light-Responsive Strategy
5.4.4. ROS-Responsive Strategy
5.4.5. Other Responsive Strategies
5.5. Multiple Stimuli-Responsive Strategies
| Responding Strategy | Activatable/Specific Moiety | Loaded Drugs | CPPs | Disease Model | Refs |
|---|---|---|---|---|---|
| pH-responsive | pH-sensitive linker | Irinotecan- and miR-200-loaded liposomes and lipid nanoparticles | RF CPP: LKARFH. NG2 targeting the H peptide. Mitochondria targeting the K peptide. (PEG-lipid derivative with an imine bond confers pH-responsive release, internalization, and intracellular distribution in acidic microenvironment) | Colon cancer | [153] |
| pH-sensitive linker | PLK-1 siRNA-loaded liposome | ehGehGehGehG-(hydrazone)-RRRRRRRR. (Low pH triggers hydrazones to hydrolyse, resulting in loss of the inhibitory domain) | N/A | [154] | |
| pH-sensitive conformational change | DOX-loaded micelle | PLA-PEG -polyHis-GCGGGYGRKKRRQRRR. (Imidazole confers histidines that act as a pH trigger. Low pH protonates histidine, causing it to lose its hydrophobic interactions and the exposed Tat) | Ovarian cancer, breast cancer, and lung cancer | [155] | |
| pH-sensitive conformational change | PTX-loaded liposome | AGYLLGHINLHHLAHL(Aib)HHILC. (the H side-chain charges. Endowed pH responsiveness after complete replacement of all lysine in the sequence with histidine) | Colon cancer | [156] | |
| pH-sensitive conformational change | PET- and SPECT-probes, gold nanoparticles, and magnetic nanoparticles | pHLIP-var3: ACDDQNPWRAYLDLLFPTDTLLLDLLW. pHLIP-var7: ACEEQNPWARYLEWLFPTETLLLEL. (low pH insertion peptide pHLIP reversibly folds and is inserted across membranes in response to pH changes) | Cervix cancer, lung cancer, pancreatic cancer | [157,158] | |
| pH-sensitive conformational change | PTX | (LHHLCHLLHHLCHLAG)2. (Disulfide oxidation forms LH2 dimeric peptide. Lysine is substituted for histidine for endosomal escape, and the dimeric form of amphipathic CPPs shows enhanced CPP activities) | Breast cancer | [159] | |
| pH-sensitive charge conversion | ART-loaded liposome | HEHEHEHEHEHEHEHEHEHEGGGGGRRRRRR. (the histidine-glutamic acid-based masking peptide is modified to R6 via a spacer of 5-mer glycine) | Breast cancer | [134] | |
| pH-sensitive charge conversion and structure shift | TRAIL- and PTX-co-delivered liposomes | C(RGDfK)-AGYLLGHINLHHLAHL(Aib)HHIL-Lys-C18. (a histidine-rich peptide for pH responsiveness, c(RGDfK) peptide for αvβ3 binding, and stearyl chain C18 for membrane anchoring) | Melanoma | [160] | |
| pH-sensitive side-chain modification | DOX | CRRRRRRRRGGGPKKKKKK. (Conjugated DMA to lysine induces intramolecular electrostatic interactions with arginine, thereby inactivating ACPP. Low pH triggers labile amides that are hydrolyzed) | Liver cancer | [161] | |
| pH-sensitive side-chain modification | DOX-loaded PEG-PCL micelle | YGRaKaKRRQRRRC. (Amidized CPPs. Conjugated succinyl moieties to the glutamine and both lysine residues of Tat) | Ovarian cancer | [162] | |
| Enzyme-responsive | MMP-9-sensitive linker | DNase I- and PTX prodrug-loaded NET-regulated nanoparticle | GRKKRRQRRRPQPLGLAGGC. (MMP-9 substrate peptide linked to Tat) | Breast cancer, lung cancer | [163] |
| MMP9-sensitive linker | CsA-loaded, MMP-9-sensitive CPP-decorated reconstituted lipoprotein nanoparticles | ACFAEKFKEAVKDYFAKFWDGSGRRRRRRRRRPVGLIGEGGEGGEGG. (MMP-9 substrate peptide conjugating with APOA-I mimics α-helix peptide through a GSG linker) | Traumatic brain injury | [138] | |
| MMP9-sensitive linker | PGAM1-siRNA- and DTX-loaded nanovesicles | RRRRRRRRRPVGLIGEGGEGGEGG. | Lung cancer | [137] | |
| MMP-2 & -9-sensitive linker | Cy5, Gadolinium chelates | EEEEEEEE-PLGLAG-RRRRRRRRR. EEEEEE-PLGLAG-RRRRRRRRR. (polycationic CPP is coupled via a cleavable linker to a neutralizing peptide) | Image-guided surgery of different kinds of tumors | [164,165] | |
| HAase-sensitive linker | HA-coated, LOX-1-siRNA-loaded nanocomplexes | RQIKIWFQNRRMKWKK. | Atherosclerosis | [141] | |
| Cathepsin-sensitive linker | Dox-loaded SiO2 nanoparticles | EEEEEEPGFKYGRKKRRQRRR. | Lung cancer, ovarian cancer | [166] | |
| Elastase-sensitive linker | Cy5 | EEEEEEEEE-RLQLK(Ac)L-RRRRRRRRR. | Breast cancer | [167] | |
| PSA-sensitive linker | PLK-1 siRNA-loaded liposomes | DGGDGGDGGDGG-HSSKYQ-RRRRRRRR. (PSA is serine protease) | Prostate cancer | [168] | |
| ATG4B-sensitive linker | DTX and CQ–loaded nanoparticles | GTFGFRRRRRRRRR. (Autophagy-specific enzyme ATG4B substrate linked to R9) | Melanoma | [169] | |
| APN-DPP4-sensitive side-chain modification | FITC | GRKKRRQRRRAhxC (Side chain modifications. Aminopeptidase N dipeptidyl peptidase IV) | N/A | [170] | |
| Hypoxia-responsive | Oxygen-sensitive degradation of fusion protein | ODD-beta-Gal | YGRKKRRQRRR-ODD-Casp3(wt) (Tat-oxygen-dependent degradation domain-Caspase 3 fusion protein is selectively stabilized in hypoxic tumors) | Pancreatic cancer | [171] |
| Azoreductase-sensitive modification | Peptide nucleic acid (PNA) | MVTVLFRRLRIRRACGPPRVRV-azo-PEG (Activatable CPP-PEG conjugates. Azoreductase-triggered CPP-inactivation through functionalization with a self-immolative azobenzene moiety) | Colon mucosa | [172] | |
| ROS-responsive | ROS-sensitive polymer | FGL1-siRNA, PD-L1-siRNA | c(CRGDKGPDC) (Proteolysis of iRGD peptide exposes a new motif that can bind to NRP-1 and activate neuropilin, allowing drugs or nanoparticles to leak out from tumor blood vessels and penetrate the tumor tissue.) | Liver cancer | [146] |
| ROS-sensitive linker | FITC, Cy5 | EEEEEEEEE-cleavable linker-RRRRRRRRR. (H2O2-activated CPP. A boronic acid-containing cleavable linker between polycationic CPP and polyanionic fragments) | Lung inflammation | [173] | |
| ATP-responsive | ATP-sensitive release of guest molecules | Photosensitizers | Ac-QYFMpTEpYVA (ATP-triggered release of phosphopeptides from the pegylated GC5A-12C nanocarrier (12C-NC) system. Host-guest ATP-responsive system) | N/A | [174] |
| ATP-sensitive disintegration | Atovaquone (AVO), hemin | c(CRGDKGPDC)-ZIF-90 (iRGD peptide-modified ZI-90/protein nanoparticles disintegrate in the presence of ATP to release protein as a result of the competitive coordination between Zn2+ and ATP) | Breast cancer | [148] | |
| Ultrasound-responsive | Ultrasound-assisted phase-Transformation | Hydroxycamptothecin | CGNKRTR. (Tumor homing-penetrating peptide-functionalized drug-loaded phase-transformation nanoparticles tLyP-1-10-HCPT-PFP) | Breast cancer | [147] |
| Ultrasound-activated cavitation effect | Pefluoropentane, 10-Hydroxycamptothecin-loaded liposome nanoparticle | CGNKRTR. (Truncated form of LyP-1 CPP (CGNKRTRGC)) | Breast cancer | [175] | |
| Ultrasound-dependent endosomal escape | shRNA | Tat-U1A-rose bengal conjugate (Tat cell-penetrating peptide, U1A RNA-binding protein, and rose bengal as a sonosensitizer) | N/A | [176] | |
| GSH-responsive | GSH-sensitive disulfide linker | Podophyllotoxin (PPT), Doxorubicin | PRASHANT. (anti-mitotic PRA octapeptide-linked PPT conjugate that can self-assemble into a vesicle via water and targeted synergistic drug delivery) | N/A | [177] |
| Light-responsive | UV light-sensitive self-immolative linker | Doxorubicin | ARTKQTARKSTGGKAPRKQLATKAARKSAPATGGC35KKPHRYRPGTVALREIRRYQKSTELLIRKLPFQRLVREIAQDFKTDLRFQSSAVMALQEASEAYLVALFEDTNLAAIHAKRVTIMPKDIQLARRIRGERA. (H3-35PC4AP. PC4AP (a photo-caged C4’-oxidized abasic site) as a light-responsive, self-immolative linker to conjugate drugs to a CPP) | N/A | [178] |
| UV light-sensitive linker | Quantum dots, polystyrene particles, Au nanostars, and liposomes | RRRRRRR-o-nitrobenzyl-GGGEEEEEEE. (a photo-caged peptide that undergoes a structural transition from an antifouling ligand to CPP upon photo-irradiation) | N/A | [179] | |
| NIR-sensitive side-chain modification | VB-loaded liposome | CGRRMKPGWKPGKPG. NGR peptide: CYGGRGNG; Synergistic effect (light-released photolabile-protective group PG (4,5-dimethoxy-2-nitrobenzene chloroformate)) | Fibrosarcoma | [180] | |
| UV-sensitive side-chain modification | Proapoptotic peptide (KLAKLAK)2 | Ac-KRRMKNvovWKNvocKnvoc. (Nvoc=6-nitroveratrylcarbonyl; light-activated caged Pen CPP; photo-cleavable groups) | N/A | [181] | |
| UV-sensitive conformational change | Tamra | cis-Ab-LK. Trans-Ab-LK azobenzene (Ab) linker | N/A | [182] | |
| UV/Vis-sensitive conformational change | RhoB | RRRRRRRRR-AB-EEEEEEEEE. (cis-to-trans isomerization of azobenzene (AB) moiety; photoswitchable) | N/A | [183] | |
| UV light-sensitive linker inhibitory domain | Atto655-loaded liposome | YGAKKARQRRAGC-PEG-loop. (modified on both termini of Tat with an alkyl chain; UV-cleavable linker) | N/A | [184] | |
| Multiple-responsive | NIR- and pH-dual sensitive linker | EGFR siRNA | CGRRMKWKK-DMNB-EEEERRRR. (CPP is quenched by a pH-sensitive inhibitory peptide, which is linked via a photo-cleavable group DMNB) | Breast cancer | [185] |
6. Clinical Challenges of CPP-Based Macromolecular Drug Delivery
7. Challenges and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stewart, M.P.; Langer, R.; Jensen, K.F. Intracellular Delivery by Membrane Disruption: Mechanisms, Strategies, and Concepts. Chem. Rev. 2018, 118, 7409–7531. [Google Scholar] [CrossRef] [PubMed]
- Hapala, I. Breaking the barrier: Methods for reversible permeabilization of cellular membranes. Crit. Rev. Biotechnol. 1997, 17, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Stephens, D.J.; Pepperkok, R. The many ways to cross the plasma membrane. Proc. Natl. Acad. Sci. USA 2001, 98, 4295–4298. [Google Scholar] [CrossRef] [PubMed]
- Sinha, B.; Köster, D.; Ruez, R.; Gonnord, P.; Bastiani, M.; Abankwa, D.; Stan, R.V.; Butler-Browne, G.; Vedie, B.; Johannes, L.; et al. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell 2011, 144, 402–413. [Google Scholar] [CrossRef]
- Peraro, M.D.; van der Goot, G. Pore-forming toxins: Ancient, but never really out of fashion. Nat. Rev. Genet. 2015, 14, 77–92. [Google Scholar] [CrossRef]
- Shi, H.; Xue, T.; Yang, Y.; Jiang, C.; Huang, S.; Yang, Q.; Lei, D.; You, Z.; Jin, T.; Wu, F.; et al. Microneedle-mediated gene delivery for the treatment of ischemic myocardial disease. Sci. Adv. 2020, 6, eaaz3621. [Google Scholar] [CrossRef]
- Chen, S.; Haam, J.; Walker, M.; Scappini, E.; Naughton, J.; Martin, N.P. Recombinant Viral Vectors as Neuroscience Tools. Curr. Protoc. Neurosci. 2019, 87, e67. [Google Scholar] [CrossRef]
- Naso, M.F.; Tomkowicz, B.; Perry, W.L., 3rd; Strohl, W.R. Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs 2017, 31, 317–334. [Google Scholar] [CrossRef]
- Davidson, B.L.; Breakefield, X.O. Viral vectors for gene delivery to the nervous system. Nat. Rev. Neurosci. 2003, 4, 353–364. [Google Scholar] [CrossRef]
- Chen, X.; Gonçalves, M.A.F.V. Engineered Viruses as Genome Editing Devices. Mol. Ther. 2016, 24, 447–457. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, H.; Colosi, P. Effect of genome size on AAV vector packaging. Mol. Ther. 2010, 18, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Farrell, P.J. Epstein-Barr Virus and Cancer. Annu. Rev. Pathol. Mech. Dis. 2019, 14, 29–53. [Google Scholar] [CrossRef] [PubMed]
- Sacks, D.; Baxter, B.; Campbell, B.C.V.; Carpenter, J.S.; Cognard, C.; Dippel, D.; Eesa, M.; Fischer, U.; Hausegger, K.; Hirsch, J.A.; et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int. J. Stroke 2018, 13, 612–632. [Google Scholar] [CrossRef] [PubMed]
- Bitzer, M.; Armeanu, S.; Lauer, U.M.; Neubert, W.J. Sendai virus vectors as an emerging negative-strand RNA viral vector system. J. Gene Med. 2003, 5, 543–553. [Google Scholar] [CrossRef]
- Ono, C.; Okamoto, T.; Abe, T.; Matsuura, Y. Baculovirus as a Tool for Gene Delivery and Gene Therapy. Viruses 2018, 10, 510. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, X.; Zhu, D.; Wang, Y.; Zhang, Z.; Zhou, X.; Qiu, N.; Chen, X.; Shen, Y. Nonviral cancer gene therapy: Delivery cascade and vector nanoproperty integration. Adv. Drug Deliv. Rev. 2017, 115, 115–154. [Google Scholar] [CrossRef]
- Chalbatani, G.M.; Dana, H.; Gharagouzloo, E.; Grijalvo, S.; Eritja, R.; Logsdon, C.D.; Memari, F.; Miri, S.R.; Rad, M.R.; Marmari, V. Small interfering RNAs (siRNAs) in cancer therapy: A nano-based approach. Int. J. Nanomed. 2019, 14, 3111–3128. [Google Scholar] [CrossRef]
- Picanço-Castro, V.; Pereira, C.G.; Covas, D.T.; Porto, G.S.; Athanassiadou, A.; Figueiredo, M.L. Emerging patent landscape for non-viral vectors used for gene therapy. Nat. Biotechnol. 2020, 38, 151–157. [Google Scholar] [CrossRef]
- Mintzer, M.A.; Simanek, E.E. Nonviral vectors for gene delivery. Chem. Rev. 2008, 109, 259–302. [Google Scholar] [CrossRef]
- Wang, H.-X.; Li, M.; Lee, C.M.; Chakraborty, S.; Kim, H.-W.; Bao, G.; Leong, K.W. CRISPR/Cas9-Based Genome Editing for Disease Modeling and Therapy: Challenges and Opportunities for Nonviral Delivery. Chem. Rev. 2017, 117, 9874–9906. [Google Scholar] [CrossRef]
- Gessner, I.; Neundorf, I. Nanoparticles Modified with Cell-Penetrating Peptides: Conjugation Mechanisms, Physicochemical Properties, and Application in Cancer Diagnosis and Therapy. Int. J. Mol. Sci. 2020, 21, 2536. [Google Scholar] [CrossRef] [PubMed]
- De Laporte, L.; Rea, J.C.; Shea, L.D. Design of modular non-viral gene therapy vectors. Biomaterials 2006, 27, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G., Jr.; Zigon, E.; Rogers, G.; Davodian, D.; Lu, S.; Jovanovic-Talisman, T.; Jones, J.; Tigges, J.; Tyagi, S.; Ghiran, I.C. Detection of Extracellular Vesicle RNA Using Molecular Beacons. iScience 2019, 23, 100782. [Google Scholar] [CrossRef]
- Habault, J.; Poyet, J.-L. Recent Advances in Cell Penetrating Peptide-Based Anticancer Therapies. Molecules 2019, 24, 927. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Almeida, A.J.; Vale, N. Combination of Cell-Penetrating Peptides with Nanoparticles for Therapeutic Application: A Review. Biomolecules 2019, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Evans, B.C.; Fletcher, B.; Kilchrist, K.V.; Dailing, E.A.; Mukalel, A.J.; Colazo, J.M.; Oliver, M.; Cheung-Flynn, J.; Brophy, C.M.; Tierney, J.W.; et al. An anionic, endosome-escaping polymer to potentiate intracellular delivery of cationic peptides, biomacromolecules, and nanoparticles. Nat. Commun. 2019, 10, 5012–5019. [Google Scholar] [CrossRef]
- Trabulo, S.; Cardoso, A.; Morais, C.; Jurado, A.S.; de Lima, M.P. Cell-penetrating peptides as nucleic acid delivery systems: From biophysics to biological applications. Curr. Pharm. Des. 2013, 19, 2895–2923. [Google Scholar] [CrossRef]
- Frankel, A.D.; Pabo, C.O. Cellular uptake of the tat protein from human immunodeficiency virus. Cell 1988, 55, 1189–1193. [Google Scholar] [CrossRef]
- Green, M.; Loewenstein, P.M. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell 1988, 55, 1179–1188. [Google Scholar] [CrossRef]
- Joliot, A.; Pernelle, C.; Deagostini-Bazin, H.; Prochiantz, A. Antennapedia homeobox peptide regulates neural morphogenesis. Proc. Natl. Acad. Sci. USA 1991, 88, 1864–1868. [Google Scholar] [CrossRef]
- Morris, M.C.; Vidal, P.; Chaloin, L.; Heitz, F.; Divita, G. A new peptide vector for efficient delivery of oligonucleotides into mammalian cells. Nucleic Acids Res. 1997, 25, 2730–2736. [Google Scholar] [CrossRef] [PubMed]
- Vivès, E.; Brodin, P.; Lebleu, B. A Truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J. Biol. Chem. 1997, 272, 16010–16017. [Google Scholar] [CrossRef] [PubMed]
- Elliott, G.; O’Hare, P. Intercellular Trafficking and Protein Delivery by a Herpesvirus Structural Protein. Cell 1997, 88, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Pooga, M.; Hällbrink, M.; Zorko, M.; Langel, U. Cell penetration by transportan. FASEB J. 1998, 12, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Kardani, K.; Milani, A.; Shabani, S.H.; Bolhassani, A. Cell penetrating peptides: The potent multi-cargo intracellular carriers. Expert Opin. Drug Deliv. 2019, 16, 1227–1258. [Google Scholar] [CrossRef]
- Morris, M.C.; Depollier, J.; Mery, J.; Heitz, F.; Divita, G. A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nat. Biotechnol. 2001, 19, 1173–1176. [Google Scholar] [CrossRef]
- Zhao, K.; Zhao, G.-M.; Wu, D.; Soong, Y.; Birk, A.V.; Schiller, P.W.; Szeto, H.H. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J. Biol. Chem. 2004, 279, 34682–34690. [Google Scholar] [CrossRef]
- Yandek, L.E.; Pokorny, A.; Florén, A.; Knoelke, K.; Langel, U.; Almeida, P.F. Mechanism of the cell-penetrating peptide transportan 10 permeation of lipid bilayers. Biophys. J. 2007, 92, 2434–2444. [Google Scholar] [CrossRef]
- Sugahara, K.N.; Teesalu, T.; Karmali, P.P.; Kotamraju, V.R.; Agemy, L.; Girard, O.M.; Hanahan, D.; Mattrey, R.F.; Ruoslahti, E. Tissue-penetrating delivery of compounds and nanoparticles into tumors. Cancer Cell 2009, 16, 510–520. [Google Scholar] [CrossRef]
- Ezzat, K.; EL Andaloussi, S.; Zaghloul, E.M.; Lehto, T.; Lindberg, S.; Moreno, P.M.D.; Viola, J.R.; Magdy, T.; Abdo, R.; Guterstam, P.; et al. PepFect 14, a novel cell-penetrating peptide for oligonucleotide delivery in solution and as solid formulation. Nucleic Acids Res. 2011, 39, 5284–5298. [Google Scholar] [CrossRef]
- Lindberg, S.; Muñoz-Alarcón, A.; Helmfors, H.; Mosqueira, D.; Gyllborg, D.; Tudoran, O.; Langel, U. PepFect15, a novel endosomolytic cell-penetrating peptide for oligonucleotide delivery via scavenger receptors. Int. J. Pharm. 2013, 441, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.J.; Sung, B.H.; Shin, J.R.; Lee, Y.W.; Kim, D.J.; Yang, K.S.; Kim, S.C. A cancer specific cell-penetrating peptide, BR2, for the efficient delivery of an scFv into cancer cells. PLoS ONE 2013, 8, e66084. [Google Scholar] [CrossRef]
- Freire, J.M.; Veiga, A.S.; de Figueiredo, I.R.; de la Torre, B.G.; Santos, N.; Andreu, D.; Da Poian, A.; Castanho, M.A.R.B. Nucleic acid delivery by cell penetrating peptides derived from dengue virus capsid protein: Design and mechanism of action. FEBS J. 2013, 281, 191–215. [Google Scholar] [CrossRef]
- Cerrato, C.P.; Pirisinu, M.; Vlachos, E.N.; Langel, U. Novel cell-penetrating peptide targeting mitochondria. FASEB J. 2015, 29, 4589–4599. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.; Nanda, J.S.; Samuel, J.S.; Kumari, M.; Priyanka, P.; Bedi, G.; Nath, S.K.; Mittal, G.; Khatri, N.; Raghava, G.P.S. Topical Delivery of Protein and Peptide Using Novel Cell Penetrating Peptide IMT-P8. Sci. Rep. 2016, 6, 26278. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Lee, D.; Gujrati, V.; Rejinold, N.S.; Lekshmi, K.M.; Uthaman, S.; Jeong, C.; Park, I.-K.; Jon, S.; Kim, Y.-C. Bioreducible branched poly(modified nona-arginine) cell-penetrating peptide as a novel gene delivery platform. J. Control. Release 2017, 246, 142–154. [Google Scholar] [CrossRef]
- Li, X.; Zheng, L.; Xia, Q.; Liu, L.; Mao, M.; Zhou, H.; Zhao, Y.; Shi, J. A novel cell-penetrating peptide protects against neuron apoptosis after cerebral ischemia by inhibiting the nuclear translocation of annexin A1. Cell Death Differ. 2018, 26, 260–275. [Google Scholar] [CrossRef]
- Hu, G.; Miao, Y.; Luo, X.; Chu, W.; Fu, Y. Identification of a novel cell-penetrating peptide derived from the capsid protein of chicken anemia virus and its application in gene delivery. Appl. Microbiol. Biotechnol. 2020, 104, 10503–10513. [Google Scholar] [CrossRef]
- Min, S.; Kim, K.; Ku, S.; Park, J.; Seo, J.; Roh, S. Newly synthesized peptide, Ara-27, exhibits significant improvement in cell-penetrating ability compared to conventional peptides. Biotechnol. Prog. 2020, 36, e3014. [Google Scholar] [CrossRef]
- Gandhi, N.S.; Wang, E.; Sorolla, A.; Kan, Y.J.; Malik, A.; Batra, J.; Young, K.A.; Tie, W.J.; Blancafort, P.; Mancera, R.L. Design and Characterization of a Cell-Penetrating Peptide Derived from the SOX2 Transcription Factor. Int. J. Mol. Sci. 2021, 22, 9354. [Google Scholar] [CrossRef]
- Cerrato, C.P.; Langel, U. An update on cell-penetrating peptides with intracellular organelle targeting. Expert Opin. Drug Deliv. 2022, 19, 133–146. [Google Scholar] [CrossRef]
- Mandal, D.; Shirazi, A.N.; Parang, K. Cell-penetrating homochiral cyclic peptides as nuclear-targeting molecular transporters. Angew. Chem. Int. Ed. 2011, 50, 9633–9637. [Google Scholar] [CrossRef] [PubMed]
- Tietz, O.; Cortezon-Tamarit, F.; Chalk, R.; Able, S.; Vallis, K.A. Tricyclic cell-penetrating peptides for efficient delivery of functional antibodies into cancer cells. Nat. Chem. 2022, 14, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.; Bhalla, S.; Usmani, S.S.; Singh, S.; Chaudhary, K.; Raghava, G.P.S.; Gautam, A. CPPsite 2.0: A repository of experimentally validated cell-penetrating peptides. Nucleic Acids Res. 2015, 44, D1098–D1103. [Google Scholar] [CrossRef] [PubMed]
- Kardani, K.; Bolhassani, A. Cppsite 2.0: An Available Database of Experimentally Validated Cell-Penetrating Peptides Predicting their Secondary and Tertiary Structures. J. Mol. Biol. 2020, 433, 166703. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Mann, G.; Satish, G.; Brik, A. Enhanced Live-Cell Delivery of Synthetic Proteins Assisted by Cell-Penetrating Peptides Fused to DABCYL. Angew. Chem. Int. Ed. 2021, 60, 7333–7343. [Google Scholar] [CrossRef]
- Sajid, M.I.; Mandal, D.; El-Sayed, N.S.; Lohan, S.; Moreno, J.; Tiwari, R.K. Oleyl Conjugated Histidine-Arginine Cell-Penetrating Peptides as Promising Agents for siRNA Delivery. Pharmaceutics 2022, 14, 881. [Google Scholar] [CrossRef]
- Gentilucci, L.; De Marco, R.; Cerisoli, L. Chemical modifications designed to improve peptide stability: Incorporation of non-natural amino acids, pseudo-peptide bonds, and cyclization. Curr. Pharm. Des. 2010, 16, 3185–3203. [Google Scholar] [CrossRef]
- Qian, Z.; LaRochelle, J.R.; Jiang, B.; Lian, W.; Hard, R.L.; Selner, N.G.; Luechapanichkul, R.; Barrios, A.M.; Pei, D. Early endosomal escape of a cyclic cell-penetrating peptide allows effective cytosolic cargo delivery. Biochemistry 2014, 53, 4034–4046. [Google Scholar] [CrossRef]
- Qian, Z.; Martyna, A.; Hard, R.L.; Wang, J.; Appiah-Kubi, G.; Coss, C.; Phelps, M.A.; Rossman, J.S.; Pei, D. Discovery and Mechanism of Highly Efficient Cyclic Cell-Penetrating Peptides. Biochemistry 2016, 55, 2601–2612. [Google Scholar] [CrossRef]
- Dougherty, P.G.; Sahni, A.; Pei, D. Understanding Cell Penetration of Cyclic Peptides. Chem. Rev. 2019, 119, 10241–10287. [Google Scholar] [CrossRef]
- Leader, B.; Baca, Q.J.; Golan, D.E. Protein therapeutics: A summary and pharmacological classification. Nat. Rev. Drug Discov. 2008, 7, 21–39. [Google Scholar] [CrossRef]
- Bolhassani, A.; Jafarzade, B.S.; Mardani, G. In vitro and in vivo delivery of therapeutic proteins using cell penetrating peptides. Peptides 2017, 87, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, J.; Xu, D. Cell-penetrating peptides as noninvasive transmembrane vectors for the development of novel multifunctional drug-delivery systems. J. Control. Release 2016, 229, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Tat peptide-mediated intracellular delivery of pharmaceutical nanocarriers. Adv. Drug Deliv. Rev. 2008, 60, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, F.; Muhamad, I.I.; Pa’e, N.; Hashim, Z. Strategies in Improving Properties of Cellulose-Based Hydrogels for Smart Applications. In Cellulose-Based Superabsorbent Hydrogels; Mondal, M.I.H., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–22. [Google Scholar] [CrossRef]
- Nam, S.H.; Park, J.; Koo, H. Recent advances in selective and targeted drug/gene delivery systems using cell-penetrating peptides. Arch. Pharmacal Res. 2023, 46, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.C.; Cheon, D.H.; Lee, Y. Challenge to overcome current limitations of cell-penetrating peptides. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2021, 1869, 140604. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Aaron, J.; Sokolov, K. Directional conjugation of antibodies to nanoparticles for synthesis of multiplexed optical contrast agents with both delivery and targeting moieties. Nat. Protoc. 2008, 3, 314–320. [Google Scholar] [CrossRef]
- Bode, S.A.; Timmermans, S.B.P.E.; Eising, S.; van Gemert, S.P.W.; Bonger, K.M.; Löwik, D.W.P.M. Click to enter: Activation of oligo-arginine cell-penetrating peptides by bioorthogonal tetrazine ligations. Chem. Sci. 2018, 10, 701–705. [Google Scholar] [CrossRef]
- Chen, X.; Zaro, J.L.; Shen, W.-C. Fusion protein linkers: Property, design and functionality. Adv. Drug Deliv. Rev. 2012, 65, 1357–1369. [Google Scholar] [CrossRef]
- Turner, J.J.; Arzumanov, A.A.; Gait, M.J. Synthesis, cellular uptake and HIV-1 Tat-dependent trans-activation inhibition activity of oligonucleotide analogues disulphide-conjugated to cell-penetrating peptides. Nucleic Acids Res. 2005, 33, 27–42. [Google Scholar] [CrossRef]
- Gayraud, F.; Klußmann, M.; Neundorf, I. Recent Advances and Trends in Chemical CPP–Drug Conjugation Techniques. Molecules 2021, 26, 1591. [Google Scholar] [CrossRef]
- Shabanpoor, F.; McClorey, G.; Saleh, A.F.; Järver, P.; Wood, M.J.; Gait, M.J. Bi-specific splice-switching PMO oligonucleotides conjugated via a single peptide active in a mouse model of Duchenne muscular dystrophy. Nucleic Acids Res. 2014, 43, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Balogh, B.; Ivánczi, M.; Nizami, B.; Beke-Somfai, T.; Mándity, I.M. ConjuPepDB: A database of peptide-drug conjugates. Nucleic Acids Res. 2020, 49, D1102–D1112. [Google Scholar] [CrossRef] [PubMed]
- Shai, Y. Mode of action of membrane active antimicrobial peptides. Biopolymers 2002, 66, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Gordon, V.D.; Yang, L.; Coridan, R.; Wong, G.C.L. HIV TAT forms pores in membranes by inducing saddle-splay curvature: Potential role of bidentate hydrogen bonding. Angew. Chem. Int. Ed. 2008, 47, 2986–2989. [Google Scholar] [CrossRef] [PubMed]
- Derossi, D.; Calvet, S.; Trembleau, A.; Brunissen, A.; Chassaing, G.; Prochiantz, A. Cell internalization of the third helix of the antennapedia homeodomain is receptor-independent. J. Biol. Chem. 1996, 271, 18188–18193. [Google Scholar] [CrossRef] [PubMed]
- Derossi, D.; Joliot, A.H.; Chassaing, G.; Prochiantz, A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J. Biol. Chem. 1994, 269, 10444–10450. [Google Scholar] [CrossRef]
- Sun, T.-L.; Sun, Y.; Lee, C.-C.; Huang, H.W. Membrane permeability of hydrocarbon-cross-linked peptides. Biophys. J. 2013, 104, 1923–1932. [Google Scholar] [CrossRef]
- Zhang, F.; Guo, H.; Zhang, J.; Chen, Q.; Fang, Q. Identification of the caveolae/raft-mediated endocytosis as the primary entry pathway for aquareovirus. Virology 2018, 513, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Staring, J.; Raaben, M.; Brummelkamp, T.R. Viral escape from endosomes and host detection at a glance. J. Cell Sci. 2018, 131, jcs216259. [Google Scholar] [CrossRef]
- Makvandi, P.; Chen, M.; Sartorius, R.; Zarrabi, A.; Ashrafizadeh, M.; Moghaddam, F.D.; Ma, J.; Mattoli, V.; Tay, F.R. Endocytosis of abiotic nanomaterials and nanobiovectors: Inhibition of membrane trafficking. Nano Today 2021, 40, 101279. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, M.; Boll, W.; van Oijen, A.; Hariharan, R.; Chandran, K.; Nibert, M.L.; Kirchhausen, T. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell 2004, 118, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Akishiba, M.; Takeuchi, T.; Kawaguchi, Y.; Sakamoto, K.; Yu, H.-H.; Nakase, I.; Takatani-Nakase, T.; Madani, F.; Gräslund, A.; Futaki, S. Cytosolic antibody delivery by lipid-sensitive endosomolytic peptide. Nat. Chem. 2017, 9, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Rennick, J.J.; Johnston, A.P.R.; Parton, R.G. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat. Nanotechnol. 2021, 16, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Desale, K.; Kuche, K.; Jain, S. Cell-penetrating peptides (CPPs): An overview of applications for improving the potential of nanotherapeutics. Biomater. Sci. 2020, 9, 1153–1188. [Google Scholar] [CrossRef]
- Mandl, H.K.; Quijano, E.; Suh, H.W.; Sparago, E.; Oeck, S.; Grun, M.; Glazer, P.M.; Saltzman, W.M. Optimizing biodegradable nanoparticle size for tissue-specific delivery. J. Control. Release 2019, 314, 92–101. [Google Scholar] [CrossRef]
- Hickey, J.W.; Santos, J.L.; Williford, J.-M.; Mao, H.-Q. Control of polymeric nanoparticle size to improve therapeutic delivery. J. Control. Release 2015, 219, 536–547. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, Q.; Li, N.; Dai, L.; Liu, Q.; Song, L.; Wang, J.; Li, Y.; Tian, J.; Ding, B.; et al. DNA origami as an in vivo drug delivery vehicle for cancer therapy. ACS Nano 2014, 8, 6633–6643. [Google Scholar] [CrossRef]
- Li, Y.; Zhai, Y.; Liu, W.; Zhang, K.; Liu, J.; Shi, J.; Zhang, Z. Ultrasmall nanostructured drug based pH-sensitive liposome for effective treatment of drug-resistant tumor. J. Nanobiotechnol. 2019, 17, 117. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.P.; Popović, Z.; Chen, O.; Cui, J.; Fukumura, D.; Bawendi, M.G.; Jain, R.K. Fluorescent nanorods and nanospheres for real-time in vivo probing of nanoparticle shape-dependent tumor penetration. Angew. Chem. Int. Ed. 2011, 50, 11417–11420. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Boehmer, T.; Jeudy, S.; Berke, I.C.; Schwartz, T.U. Structural and functional studies of Nup107/Nup133 interaction and its implications for the architecture of the nuclear pore complex. Mol. Cell 2008, 30, 721–731. [Google Scholar] [CrossRef]
- Erazo-Oliveras, A.; Muthukrishnan, N.; Baker, R.; Wang, T.-Y.; Pellois, J.-P. Improving the endosomal escape of cell-penetrating peptides and their cargos: Strategies and challenges. Pharmaceuticals 2012, 5, 1177–1209. [Google Scholar] [CrossRef]
- Allen, J.K.; Brock, D.J.; Kondow-McConaghy, H.M.; Pellois, J.-P. Efficient Delivery of Macromolecules into Human Cells by Improving the Endosomal Escape Activity of Cell-Penetrating Peptides: Lessons Learned from dfTAT and its Analogs. Biomolecules 2018, 8, 50. [Google Scholar] [CrossRef]
- Yamano, S.; Dai, J.; Hanatani, S.; Haku, K.; Yamanaka, T.; Ishioka, M.; Takayama, T.; Yuvienco, C.; Khapli, S.; Moursi, A.M.; et al. Long-term efficient gene delivery using polyethylenimine with modified Tat peptide. Biomaterials 2014, 35, 1705–1715. [Google Scholar] [CrossRef]
- Varkouhi, A.K.; Scholte, M.; Storm, G.; Haisma, H.J. Endosomal escape pathways for delivery of biologicals. J. Control. Release 2011, 151, 220–228. [Google Scholar] [CrossRef]
- Li, W.; Nicol, F.; Szoka, F.C., Jr. GALA: A designed synthetic pH-responsive amphipathic peptide with applications in drug and gene delivery. Adv. Drug Deliv. Rev. 2004, 56, 967–985. [Google Scholar] [CrossRef]
- Kyriakides, T.R.; Cheung, C.Y.; Murthy, N.; Bornstein, P.; Stayton, P.S.; Hoffman, A.S. pH-Sensitive polymers that enhance intracellular drug delivery in vivo. J. Control. Release 2001, 78, 295–303. [Google Scholar] [CrossRef]
- Kobayashi, S.; Nakase, I.; Kawabata, N.; Yu, H.-H.; Pujals, S.; Imanishi, M.; Giralt, E.; Futaki, S. Cytosolic targeting of macromolecules using a pH-dependent fusogenic peptide in combination with cationic liposomes. Bioconjugate Chem. 2009, 20, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Guo, W.; Stephenson, S.M.; Lee, R.J. Efficient intracellular drug and gene delivery using folate receptor-targeted pH-sensitive liposomes composed of cationic/anionic lipid combinations. J. Control. Release 2002, 80, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Yang, H.; Li, T.; Pan, H.; Ren, S.; Luo, G.; Jiang, J.; Yu, L.; Chen, B.; Zhang, Y.; et al. Efficient intracellular delivery of proteins by a multifunctional chimaeric peptide in vitro and in vivo. Nat. Commun. 2021, 12, 5131. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Meng, F.; Kim, B.-K.; Ke, X.; Yeo, Y. In-vitro and in-vivo difference in gene delivery by lithocholic acid-polyethyleneimine conjugate. Biomaterials 2019, 217, 119296. [Google Scholar] [CrossRef]
- He, Y.; Guo, S.; Wu, L.; Chen, P.; Wang, L.; Liu, Y.; Ju, H. Near-infrared boosted ROS responsive siRNA delivery and cancer therapy with sequentially peeled upconversion nano-onions. Biomaterials 2019, 225, 119501. [Google Scholar] [CrossRef]
- Alipour, M.; Hosseinkhani, S. Design, Preparation, and Characterization of Peptide-Based Nanocarrier for Gene Delivery. Methods Mol. Biol. 2019, 2000, 59–69. [Google Scholar] [CrossRef]
- Xie, L.; Tan, Y.; Wang, Z.; Liu, H.; Zhang, N.; Zou, C.; Liu, X.; Liu, G.; Lu, J.; Zheng, H. ε-Caprolactone-Modified Polyethylenimine as Efficient Nanocarriers for siRNA Delivery in Vivo. ACS Appl. Mater. Interfaces 2016, 8, 29261–29269. [Google Scholar] [CrossRef]
- Degors, I.M.S.; Wang, C.; Rehman, Z.U.; Zuhorn, I.S. Carriers Break Barriers in Drug Delivery: Endocytosis and Endosomal Escape of Gene Delivery Vectors. Accounts Chem. Res. 2019, 52, 1750–1760. [Google Scholar] [CrossRef]
- Jia, G.; Han, Y.; An, Y.; Ding, Y.; He, C.; Wang, X.; Tang, Q. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials 2018, 178, 302–316. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, L.; Zhu, C.; Zheng, Q.; Wang, G.; Tong, J.; Fang, Y.; Xia, Y.; Cheng, G.; He, X.; et al. Aptamer-Conjugated Extracellular Nanovesicles for Targeted Drug Delivery. Cancer Res. 2018, 78, 798–808. [Google Scholar] [CrossRef]
- Nakase, I.; Noguchi, K.; Fujii, I.; Futaki, S. Vectorization of biomacromolecules into cells using extracellular vesicles with enhanced internalization induced by macropinocytosis. Sci. Rep. 2016, 6, 34937. [Google Scholar] [CrossRef]
- Xu, H.; Liao, C.; Liang, S.; Ye, B.-C. A Novel Peptide-Equipped Exosomes Platform for Delivery of Antisense Oligonucleotides. ACS Appl. Mater. Interfaces 2021, 13, 10760–10767. [Google Scholar] [CrossRef]
- Liu, D.; Angelova, A.; Liu, J.; Garamus, V.M.; Angelov, B.; Zhang, X.; Li, Y.; Feger, G.; Li, N.; Zou, A. Self-assembly of mitochondria-specific peptide amphiphiles amplifying lung cancer cell death through targeting the VDAC1–hexokinase-II complex. J. Mater. Chem. B 2019, 7, 4706–4716. [Google Scholar] [CrossRef] [PubMed]
- Cerrato, C.P.; Künnapuu, K.; Langel, U. Cell-penetrating peptides with intracellular organelle targeting. Expert Opin. Drug Deliv. 2016, 14, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Date, A.A.; Hanes, J.; Ensign, L.M. Nanoparticles for oral delivery: Design, evaluation and state-of-the-art. J. Control. Release 2016, 240, 504–526. [Google Scholar] [CrossRef] [PubMed]
- Fievez, V.; Plapied, L.; Plaideau, C.; Legendre, D.; Rieux, A.D.; Pourcelle, V.; Freichels, H.; Jérôme, C.; Marchand, J.; Préat, V.; et al. In vitro identification of targeting ligands of human M cells by phage display. Int. J. Pharm. 2010, 394, 35–42. [Google Scholar] [CrossRef]
- Yao, Z.; Che, X.-C.; Lu, R.; Zheng, M.-N.; Zhu, Z.-F.; Li, J.-P.; Jian, X.; Shi, L.-X.; Liu, J.-Y.; Gao, W.-Y. Inhibition by tyroserleutide (YSL) on the invasion and adhesion of the mouse melanoma cell. Mol. Med. 2007, 13, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wei, T.; Hua, Y.; Wang, Z.; Zhang, L. Effective Antitumor of Orally Intestinal Targeting Penetrating Peptide-Loaded Tyroserleutide/PLGA Nanoparticles in Hepatocellular Carcinoma. Int. J. Nanomed. 2021, 16, 4495–4513. [Google Scholar] [CrossRef]
- Ruoslahti, E. Peptides as targeting elements and tissue penetration devices for nanoparticles. Adv. Mater. 2012, 24, 3747–3756. [Google Scholar] [CrossRef]
- Roth, L.; Agemy, L.; Kotamraju, V.R.; Braun, G.; Teesalu, T.; Sugahara, K.N.; Hamzah, J.; Ruoslahti, E. Transtumoral targeting enabled by a novel neuropilin-binding peptide. Oncogene 2011, 31, 3754–3763. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, X.; Teng, B.; Wang, Z.; Li, F.; Zhao, Y.; Guo, Y.; Zeng, Q. Peptide-Targeted High-Density Lipoprotein Nanoparticles for Combinatorial Treatment against Metastatic Breast Cancer. ACS Appl. Mater. Interfaces 2021, 13, 35248–35265. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Duan, X.; Zhang, F.; Ban, X.; Mao, J.; Cao, M.; Han, S.; Shuai, X.; Shen, J. Theranostic Nanomedicine for Synergistic Chemodynamic Therapy and Chemotherapy of Orthotopic Glioma. Adv. Sci. 2020, 7, 2003036. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Hong, S.; Zheng, D.; Huang, Q.; Liu, F.; Zhong, Z.; Zhang, X. Multifunctionalized Gold Sub-Nanometer Particles for Sensitizing Radiotherapy against Glioblastoma. Small 2020, 17, e2006582. [Google Scholar] [CrossRef]
- Liu, M.; Ma, W.; Zhao, D.; Li, J.; Li, Q.; Liu, Y.; Hao, L.; Lin, Y. Enhanced Penetrability of a Tetrahedral Framework Nucleic Acid by Modification with iRGD for DOX-Targeted Delivery to Triple-Negative Breast Cancer. ACS Appl. Mater. Interfaces 2021, 13, 25825–25835. [Google Scholar] [CrossRef]
- Liang, H.; Wu, X.; Zhao, G.; Feng, K.; Ni, K.; Sun, X. Renal Clearable Ultrasmall Single-Crystal Fe Nanoparticles for Highly Selective and Effective Ferroptosis Therapy and Immunotherapy. J. Am. Chem. Soc. 2021, 143, 15812–15823. [Google Scholar] [CrossRef]
- Cho, H.-J.; Park, S.-J.; Jung, W.H.; Cho, Y.; Ahn, D.J.; Lee, Y.-S.; Kim, S. Injectable Single-Component Peptide Depot: Autonomously Rechargeable Tumor Photosensitization for Repeated Photodynamic Therapy. ACS Nano 2020, 14, 15793–15805. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Li, L.; Hu, P.; Yang, Y.; Wei, W.; Deng, X.; Wang, L.; Tay, F.R.; Ma, J. Recent Advances in Stimulus-Responsive Nanocarriers for Gene Therapy. Adv. Sci. 2021, 8, 2100540. [Google Scholar] [CrossRef]
- De Jong, H.; Bonger, K.M.; Löwik, D.W.P.M. Activatable cell-penetrating peptides: 15 years of research. RSC Chem. Biol. 2020, 1, 192–203. [Google Scholar] [CrossRef]
- Roointan, A.; Farzanfar, J.; Mohammadi-Samani, S.; Behzad-Behbahani, A.; Farjadian, F. Smart pH responsive drug delivery system based on poly(HEMA-co-DMAEMA) nanohydrogel. Int. J. Pharm. 2018, 552, 301–311. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z.; Peng, Y.; Ding, J.; Zhou, W. A Smart pH-Sensitive Delivery System for Enhanced Anticancer Efficacy via Paclitaxel Endosomal Escape. Front. Pharmacol. 2019, 10, 10. [Google Scholar] [CrossRef]
- Karimi, M.; Eslami, M.; Sahandi-Zangabad, P.; Mirab, F.; Farajisafiloo, N.; Shafaei, Z.; Ghosh, D.; Bozorgomid, M.; Dashkhaneh, F.; Hamblin, M.R. pH -Sensitive stimulus-responsive nanocarriers for targeted delivery of therapeutic agents. WIREs Nanomed. Nanobiotechnol. 2016, 8, 696–716. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mo, L.; Lu, C.-H.; Fu, T.; Yang, H.-H.; Tan, W. Functional nucleic acid-based hydrogels for bioanalytical and biomedical applications. Chem. Soc. Rev. 2016, 45, 1410–1431. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zu, C.; He, D.; Li, Y.; Chen, Q.; Chen, Q.; Wang, H.; Wang, R.; Chaurasiya, B.; Zaro, J.L.; et al. pH-dependent reversibly activatable cell-penetrating peptides improve the antitumor effect of artemisinin-loaded liposomes. J. Colloid Interface Sci. 2020, 586, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Spears, M.E.; Carlisle, A.E.; Kim, D. Endogenous toxic metabolites and implications in cancer therapy. Oncogene 2020, 39, 5709–5720. [Google Scholar] [CrossRef]
- Gordon, M.R.; Zhao, B.; Anson, F.; Fernandez, A.; Singh, K.; Homyak, C.; Canakci, M.; Vachet, R.W.; Thayumanavan, S. Matrix Metalloproteinase-9-Responsive Nanogels for Proximal Surface Conversion and Activated Cellular Uptake. Biomacromolecules 2018, 19, 860–871. [Google Scholar] [CrossRef]
- Zhang, W.; Gong, C.; Chen, Z.; Li, M.; Li, Y.; Gao, J. Tumor microenvironment-activated cancer cell membrane-liposome hybrid nanoparticle-mediated synergistic metabolic therapy and chemotherapy for non-small cell lung cancer. J. Nanobiotechnol. 2021, 19, 339. [Google Scholar] [CrossRef]
- Chen, L.; Song, Q.; Chen, Y.; Meng, S.; Zheng, M.; Huang, J.; Zhang, Q.; Jiang, J.; Feng, J.; Chen, H.-Z.; et al. Tailored Reconstituted Lipoprotein for Site-Specific and Mitochondria-Targeted Cyclosporine A Delivery to Treat Traumatic Brain Injury. ACS Nano 2020, 14, 6636–6648. [Google Scholar] [CrossRef]
- Liu, L.; Cao, F.; Liu, X.; Wang, H.; Zhang, C.; Sun, H.; Wang, C.; Leng, X.; Song, C.; Kong, D.; et al. Hyaluronic Acid-Modified Cationic Lipid–PLGA Hybrid Nanoparticles as a Nanovaccine Induce Robust Humoral and Cellular Immune Responses. ACS Appl. Mater. Interfaces 2016, 8, 11969–11979. [Google Scholar] [CrossRef]
- Liang, K.; Bae, K.H.; Lee, F.; Xu, K.; Chung, J.E.; Gao, S.J.; Kurisawa, M. Self-assembled ternary complexes stabilized with hyaluronic acid-green tea catechin conjugates for targeted gene delivery. J. Control. Release 2016, 226, 205–216. [Google Scholar] [CrossRef]
- Zhao, Y.; He, Z.; Gao, H.; Tang, H.; He, J.; Guo, Q.; Zhang, W.; Liu, J. Fine Tuning of Core–Shell Structure of Hyaluronic Acid/Cell-Penetrating Peptides/siRNA Nanoparticles for Enhanced Gene Delivery to Macrophages in Antiatherosclerotic Therapy. Biomacromolecules 2018, 19, 2944–2956. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Liu, J.; Yang, M.; Wang, H.; Chu, X.; Zhou, J.; Huo, M.; Yin, T. Biomineralization-inspired dasatinib nanodrug with sequential infiltration for effective solid tumor treatment. Biomaterials 2020, 267, 120481. [Google Scholar] [CrossRef]
- Jain, R.K. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science 2005, 307, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Ding, C.; Wang, C.; Fu, J. UV-light cross-linked and pH de-cross-linked coumarin-decorated cationic copolymer grafted mesoporous silica nanoparticles for drug and gene co-delivery in vitro. Mater. Sci. Eng. C 2019, 108, 110469. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Zhou, Y.; Liu, X.; Chen, Y.; Duan, S.; Zhu, R.; Liu, Y.; Yin, L. Recent Advances on Reactive Oxygen Species-Responsive Delivery and Diagnosis System. Biomacromolecules 2019, 20, 2441–2463. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.-J.; Huang, G.; Wang, Y.; Tang, Y.; Li, H.; Jia, C.-H.; Liu, Y.; You, B.-G.; Zhang, X.-N. Coadministration of iRGD peptide with ROS-sensitive nanoparticles co-delivering siFGL1 and siPD-L1 enhanced tumor immunotherapy. Acta Biomater. 2021, 136, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhao, H.; Zhou, Z.; Xia, Y.; Wang, Z.; Ran, H.; Li, P.; Ren, J. Peptide-Functionalized Phase-Transformation Nanoparticles for Low Intensity Focused Ultrasound-Assisted Tumor Imaging and Therapy. Nano Lett. 2018, 18, 1831–1841. [Google Scholar] [CrossRef]
- Lu, L.; Liu, G.; Lin, C.; Li, K.; He, T.; Zhang, J.; Luo, Z.; Cai, K. Mitochondrial Metabolism Targeted Nanoplatform for Efficient Triple-Negative Breast Cancer Combination Therapy. Adv. Health Mater. 2021, 10, e2100978. [Google Scholar] [CrossRef]
- Fan, W.; Yung, B.; Huang, P.; Chen, X. Nanotechnology for Multimodal Synergistic Cancer Therapy. Chem. Rev. 2017, 117, 13566–13638. [Google Scholar] [CrossRef]
- Nastiuk, K.L.; Krolewski, J.J. Opportunities and challenges in combination gene cancer therapy. Adv. Drug Deliv. Rev. 2016, 98, 35–40. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, J.; Fu, Y.; Zheng, Y.; Shen, W.; Zhou, J.; Yin, T. Deeply Infiltrating iRGD-Graphene Oxide for the Intensive Treatment of Metastatic Tumors through PTT-Mediated Chemosensitization and Strengthened Integrin Targeting-Based Antimigration. Adv. Health Mater. 2021, 10, e2100536. [Google Scholar] [CrossRef]
- Lin, C.; Tong, F.; Liu, R.; Xie, R.; Lei, T.; Chen, Y.; Yang, Z.; Gao, H.; Yu, X. GSH-responsive SN38 dimer-loaded shape-transformable nanoparticles with iRGD for enhancing chemo-photodynamic therapy. Acta Pharm. Sin. B 2020, 10, 2348–2361. [Google Scholar] [CrossRef] [PubMed]
- Juang, V.; Chang, C.; Wang, C.; Wang, H.; Lo, Y. pH-Responsive PEG-Shedding and Targeting Peptide-Modified Nanoparticles for Dual-Delivery of Irinotecan and microRNA to Enhance Tumor-Specific Therapy. Small 2019, 15, e1903296. [Google Scholar] [CrossRef]
- Xiang, B.; Jia, X.-L.; Qi, J.-L.; Yang, L.-P.; Sun, W.-H.; Yan, X.; Yang, S.-K.; Cao, D.-Y.; Du, Q.; Qi, X.-R. Enhancing siRNA-based cancer therapy using a new pH-responsive activatable cell-penetrating peptide-modified liposomal system. Int. J. Nanomed. 2017, 12, 2385–2405. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Gao, Z.; Kim, D.; Park, K.; Kwon, I.C.; Bae, Y.H. Super pH-sensitive multifunctional polymeric micelle for tumor pHe specific TAT exposure and multidrug resistance. J. Control. Release 2008, 129, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Tang, J.; Fu, L.; Ran, R.; Liu, Y.; Yuan, M.; He, Q. A pH-responsive α-helical cell penetrating peptide-mediated liposomal delivery system. Biomaterials 2013, 34, 7980–7993. [Google Scholar] [CrossRef]
- Weerakkody, D.; Moshnikova, A.; Thakur, M.S.; Moshnikova, V.; Daniels, J.; Engelman, D.M.; Andreev, O.A.; Reshetnyak, Y.K. Family of pH (low) insertion peptides for tumor targeting. Proc. Natl. Acad. Sci. USA 2013, 110, 5834–5839. [Google Scholar] [CrossRef]
- Han, H.; Hou, Y.; Chen, X.; Zhang, P.; Kang, M.; Jin, Q.; Ji, J.; Gao, M. Metformin-Induced Stromal Depletion to Enhance the Penetration of Gemcitabine-Loaded Magnetic Nanoparticles for Pancreatic Cancer Targeted Therapy. J. Am. Chem. Soc. 2020, 142, 4944–4954. [Google Scholar] [CrossRef]
- Nam, S.H.; Jang, J.; Cheon, D.H.; Chong, S.-E.; Ahn, J.H.; Hyun, S.; Yu, J.; Lee, Y. pH-Activatable cell penetrating peptide dimers for potent delivery of anticancer drug to triple-negative breast cancer. J. Control. Release 2020, 330, 898–906. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, Y.; Wang, L.; Liu, W.; Xiao, L.; Lin, Q.; Gong, T.; Sun, X.; He, Q.; Zhang, Z.; et al. Improved melanoma suppression with target-delivered TRAIL and Paclitaxel by a multifunctional nanocarrier. J. Control. Release 2020, 325, 10–24. [Google Scholar] [CrossRef]
- Cheng, H.; Zhu, J.-Y.; Xu, X.-D.; Qiu, W.-X.; Lei, Q.; Han, K.; Cheng, Y.-J.; Zhang, X.-Z. Activable Cell-Penetrating Peptide Conjugated Prodrug for Tumor Targeted Drug Delivery. ACS Appl. Mater. Interfaces 2015, 7, 16061–16069. [Google Scholar] [CrossRef]
- Jin, E.; Zhang, B.; Sun, X.; Zhou, Z.; Ma, X.; Sun, Q.; Tang, J.; Shen, Y.; Van Kirk, E.; Murdoch, W.J.; et al. Acid-active cell-penetrating peptides for in vivo tumor-targeted drug delivery. J. Am. Chem. Soc. 2012, 135, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Lu, H.; Xiong, Y.; Ye, L.; Teng, C.; Cao, X.; Li, S.; Sun, S.; Liu, W.; Lv, W.; et al. Tumor-Associated Neutrophil Extracellular Traps Regulating Nanocarrier-Enhanced Inhibition of Malignant Tumor Growth and Distant Metastasis. ACS Appl. Mater. Interfaces 2021, 13, 59683–59694. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Olson, E.S.; Nguyen, Q.T.; Roy, M.; Jennings, P.A.; Tsien, R.Y. Tumor imaging by means of proteolytic activation of cell-penetrating peptides. Proc. Natl. Acad. Sci. USA 2004, 101, 17867–17872. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.T.; Olson, E.S.; Aguilera, T.A.; Jiang, T.; Scadeng, M.; Ellies, L.G.; Tsien, R.Y. Surgery with molecular fluorescence imaging using activatable cell-penetrating peptides decreases residual cancer and improves survival. Proc. Natl. Acad. Sci. USA 2010, 107, 4317–4322. [Google Scholar] [CrossRef]
- Li, J.; Liu, F.; Shao, Q.; Min, Y.; Costa, M.; Yeow, E.K.L.; Xing, B. Enzyme-responsive cell-penetrating peptide conjugated mesoporous silica quantum dot nanocarriers for controlled release of nucleus-targeted drug molecules and real-time intracellular fluorescence imaging of tumor cells. Adv. Health Mater. 2014, 3, 1230–1239. [Google Scholar] [CrossRef]
- Whitney, M.; Crisp, J.L.; Olson, E.S.; Aguilera, T.A.; Gross, L.A.; Ellies, L.G.; Tsien, R.Y. Parallel in vivo and in vitro selection using phage display identifies protease-dependent tumor-targeting peptides. J. Biol. Chem. 2010, 285, 22532–22541. [Google Scholar] [CrossRef]
- Xiang, B.; Dong, D.-W.; Shi, N.-Q.; Gao, W.; Yang, Z.-Z.; Cui, Y.; Cao, D.-Y.; Qi, X.-R. PSA-responsive and PSMA-mediated multifunctional liposomes for targeted therapy of prostate cancer. Biomaterials 2013, 34, 6976–6991. [Google Scholar] [CrossRef]
- Wang, F.; Xie, D.; Lai, W.; Zhou, M.; Wang, J.; Xu, R.; Huang, J.; Zhang, R.; Li, G. Autophagy responsive intra-intercellular delivery nanoparticles for effective deep solid tumor penetration. J. Nanobiotechnol. 2022, 20, 300. [Google Scholar] [CrossRef]
- Bode, S.A.; Hansen, M.B.; Oerlemans, R.A.J.F.; van Hest, J.C.M.; Löwik, D.W.P.M. Enzyme-Activatable Cell-Penetrating Peptides through a Minimal Side Chain Modification. Bioconjug. Chem. 2015, 26, 850–856. [Google Scholar] [CrossRef]
- Harada, H.; Hiraoka, M.; Kizaka-Kondoh, S. Antitumor effect of TAT-oxygen-dependent degradation-caspase-3 fusion protein specifically stabilized and activated in hypoxic tumor cells. Cancer Res. 2002, 62, 2013–2018. [Google Scholar]
- Lee, S.H.; Moroz, E.; Castagner, B.; Leroux, J.-C. Activatable cell penetrating peptide-peptide nucleic acid conjugate via reduction of azobenzene PEG chains. J. Am. Chem. Soc. 2014, 136, 12868–12871. [Google Scholar] [CrossRef] [PubMed]
- Weinstain, R.; Savariar, E.N.; Felsen, C.N.; Tsien, R.Y. In vivo targeting of hydrogen peroxide by activatable cell-penetrating peptides. J. Am. Chem. Soc. 2013, 136, 874–877. [Google Scholar] [CrossRef] [PubMed]
- Han, B.-B.; Pan, Y.-C.; Li, Y.-M.; Guo, D.-S.; Chen, Y.-X. A host-guest ATP responsive strategy for intracellular delivery of phosphopeptides. Chem. Commun. 2020, 56, 5512–5515. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Wang, J.; Luo, Y.; Qiao, B.; Jiang, W.; Zhu, L.; Ran, H.; Wang, Z.; Zhu, W.; Ren, J.; et al. Tumor-penetrating nanoplatform with ultrasound “unlocking” for cascade synergistic therapy and visual feedback under hypoxia. J. Nanobiotechnol. 2023, 21, 30. [Google Scholar] [CrossRef]
- Sumi, N.; Nagahiro, S.; Nakata, E.; Watanabe, K.; Ohtsuki, T. Ultrasound-dependent RNAi using TatU1A-rose bengal conjugate. Bioorg. Med. Chem. Lett. 2022, 68, 128767. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Liu, F.; Tao, B.; Sun, S. GSH-responsive anti-mitotic cell penetrating peptide-linked podophyllotoxin conjugate for improving water solubility and targeted synergistic drug delivery. Bioorganic Med. Chem. Lett. 2019, 29, 1019–1022. [Google Scholar] [CrossRef]
- Zang, C.; Wang, H.; Li, T.; Zhang, Y.; Li, J.; Shang, M.; Du, J.; Xi, Z.; Zhou, C. A light-responsive, self-immolative linker for controlled drug delivery via peptide- and protein-drug conjugates. Chem. Sci. 2019, 10, 8973–8980. [Google Scholar] [CrossRef]
- Lin, Y.; Mazo, M.M.; Skaalure, S.C.; Thomas, M.R.; Schultz, S.R.; Stevens, M.M. Activatable cell-biomaterial interfacing with photo-caged peptides. Chem. Sci. 2018, 10, 1158–1167. [Google Scholar] [CrossRef]
- Xie, X.; Yang, Y.; Yang, Y.; Zhang, H.; Li, Y.; Mei, X. A photo-responsive peptide- and asparagine-glycine-arginine (NGR) peptide-mediated liposomal delivery system. Drug Deliv. 2015, 23, 2445–2456. [Google Scholar] [CrossRef]
- Shamay, Y.; Adar, L.; Ashkenasy, G.; David, A. Light induced drug delivery into cancer cells. Biomaterials 2011, 32, 1377–1386. [Google Scholar] [CrossRef]
- Kim, G.C.; Ahn, J.H.; Oh, J.H.; Nam, S.; Hyun, S.; Yu, J.; Lee, Y. Photoswitching of Cell Penetration of Amphipathic Peptides by Control of α-Helical Conformation. Biomacromolecules 2018, 19, 2863–2869. [Google Scholar] [CrossRef] [PubMed]
- Prestel, A.; Möller, H.M. Spatio-temporal control of cellular uptake achieved by photoswitchable cell-penetrating peptides. Chem. Commun. 2015, 52, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.B.; van Gaal, E.; Minten, I.; Storm, G.; van Hest, J.C.; Löwik, D.W. Constrained and UV-activatable cell-penetrating peptides for intracellular delivery of liposomes. J. Control. Release 2012, 164, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xie, X.; Yang, Y.; Li, Z.; Yu, F.; Gong, W.; Li, Y.; Zhang, H.; Wang, Z.; Mei, X. Polymer Nanoparticles Modified with Photo- and pH-Dual-Responsive Polypeptides for Enhanced and Targeted Cancer Therapy. Mol. Pharm. 2016, 13, 1508–1519. [Google Scholar] [CrossRef] [PubMed]
- Staecker, H.; Jokovic, G.; Karpishchenko, S.; Kienle-Gogolok, A.; Krzyzaniak, A.; Lin, C.-D.; Navratil, P.; Tzvetkov, V.; Wright, N.; Meyer, T. Efficacy and Safety of AM-111 in the Treatment of Acute Unilateral Sudden Deafness—A Double-blind, Randomized, Placebo-controlled Phase 3 Study. Otol. Neurotol. 2019, 40, 584–594. [Google Scholar] [CrossRef]
- Chiquet, C.; Aptel, F.; Creuzot-Garcher, C.; Berrod, J.-P.; Kodjikian, L.; Massin, P.; Deloche, C.; Perino, J.; Kirwan, B.-A.; de Brouwer, S.; et al. Postoperative Ocular Inflammation: A Single Subconjunctival Injection of XG-102 Compared to Dexamethasone Drops in a Randomized Trial. Am. J. Ophthalmol. 2016, 174, 76–84. [Google Scholar] [CrossRef]
- Jankovic, J.; Truong, D.; Patel, A.T.; Brashear, A.; Evatt, M.; Rubio, R.G.; Oh, C.K.; Snyder, D.; Shears, G.; Comella, C. Injectable DaxibotulinumtoxinA in Cervical Dystonia: A Phase 2 Dose-Escalation Multicenter Study. Mov. Disord. Clin. Pract. 2018, 5, 273–282. [Google Scholar] [CrossRef]
- Garcia-Murray, E.; Villasenor, M.L.V.; Acevedo, B.; Luna, S.; Lee, J.; Waugh, J.M.; Hornfeldt, C.S. Safety and efficacy of RT002, an injectable botulinum toxin type A, for treating glabellar lines. Dermatol. Surg. 2015, 41 (Suppl. 1), S47–S55. [Google Scholar] [CrossRef]
- Patel, A.T.; Lew, M.F.; Dashtipour, K.; Isaacson, S.; Hauser, R.A.; Ondo, W.; Maisonobe, P.; Wietek, S.; Rubin, B.; Brashear, A. Sustained functional benefits after a single set of injections with abobotulinumtoxinA using a 2-mL injection volume in adults with cervical dystonia: 12-week results from a randomized, double-blind, placebo-controlled phase 3b study. PLoS ONE 2021, 16, e0245827. [Google Scholar] [CrossRef]
- Hill, M.D.; Goyal, M.; Menon, B.K.; Nogueira, R.G.; McTaggart, R.A.; Demchuk, A.M.; Poppe, A.Y.; Buck, B.H.; Field, T.S.; Dowlatshahi, D.; et al. Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-NA1): A multicentre, double-blind, randomised controlled trial. Lancet 2020, 395, 878–887. [Google Scholar] [CrossRef]
- Cousins, M.J.; Pickthorn, K.; Huang, S.; Critchley, L.; Bell, G. The safety and efficacy of KAI-1678—An inhibitor of epsilon protein kinase C (εPKC)—Versus lidocaine and placebo for the treatment of postherpetic neuralgia: A crossover study design. Pain Med. 2013, 14, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Lincoff, A.M.; Roe, M.; Aylward, P.; Galla, J.; Rynkiewicz, A.; Guetta, V.; Zelizko, M.; Kleiman, N.; White, H.; McErlean, E.; et al. Inhibition of delta-protein kinase C by delcasertib as an adjunct to primary percutaneous coronary intervention for acute anterior ST-segment elevation myocardial infarction: Results of the PROTECTION AMI Randomized Controlled Trial. Eur. Heart J. 2014, 35, 2516–2523. [Google Scholar] [CrossRef] [PubMed]
- Direct Inhibition of δ-Protein Kinase C Enzyme to Limit Total Infarct Size in Acute Myocardial Infarction (DELTA MI) Investigators; Bates, E.; Bode, C.; Costa, M.; Gibson, C.M.; Granger, C.; Green, C.; Grimes, K.; Harrington, R.; Huber, K.; et al. Intracoronary KAI-9803 as an adjunct to primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction. Circulation 2008, 117, 886–896. [Google Scholar] [CrossRef]
- Deloche, C.; Lopez-Lazaro, L.; Mouz, S.; Perino, J.; Abadie, C.; Combette, J. XG-102 administered to healthy male volunteers as a single intravenous infusion: A randomized, double-blind, placebo-controlled, dose-escalating study. Pharmacol. Res. Perspect. 2014, 2, e00020. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Ahn, J.; Kim, J.; Choi, M.; Jeon, H.; Choe, K.; Lee, D.Y.; Kim, P.; Jon, S. Nanoparticle-Assisted Transcutaneous Delivery of a Signal Transducer and Activator of Transcription 3-Inhibiting Peptide Ameliorates Psoriasis-like Skin Inflammation. ACS Nano 2018, 12, 6904–6916. [Google Scholar] [CrossRef]
- Guo, J.; Chen, Y.; Yuan, B.; Liu, S.; Rao, P. Effects of intracellular superoxide removal at acupoints with TAT-SOD on obesity. Free. Radic. Biol. Med. 2011, 51, 2185–2189. [Google Scholar] [CrossRef]
- Lemech, C.R.; Kichenadasse, G.; Marschner, J.-P.; Alevizopoulos, K.; Otterlei, M.; Millward, M. ATX-101, a cell-penetrating protein targeting PCNA, can be safely administered as intravenous infusion in patients and shows clinical activity in a Phase 1 study. Oncogene 2022, 42, 541–544. [Google Scholar] [CrossRef]
- Suckfuell, M.; Lisowska, G.; Domka, W.; Kabacinska, A.; Morawski, K.; Bodlaj, R.; Klimak, P.; Kostrica, R.; Meyer, T. Efficacy and safety of AM-111 in the treatment of acute sensorineural hearing loss. Otol. Neurotol. 2014, 35, 1317–1326. [Google Scholar] [CrossRef]
- Foligné, B.; Plé, C.; Titécat, M.; Dendooven, A.; Pagny, A.; Daniel, C.; Singer, E.; Pottier, M.; Bertin, B.; Neut, C.; et al. Contribution of the Gut Microbiota in P28GST-Mediated Anti-Inflammatory Effects: Experimental and Clinical Insights. Cells 2019, 8, 577. [Google Scholar] [CrossRef]
- Lulla, R.R.; Goldman, S.; Yamada, T.; Beattie, C.W.; Bressler, L.; Pacini, M.; Pollack, I.F.; Fisher, P.G.; Packer, R.J.; Dunkel, I.J.; et al. Phase I trial of p28 (NSC745104), a non-HDM2-mediated peptide inhibitor of p53 ubiquitination in pediatric patients with recurrent or progressive central nervous system tumors: A Pediatric Brain Tumor Consortium Study. Neuro-Oncology 2016, 18, 1319–1325. [Google Scholar] [CrossRef]
- Warso, M.A.; Richards, J.M.; Mehta, D.; Christov, K.; Schaeffer, C.; Bressler, L.R.; Yamada, T.; Majumdar, D.; Kennedy, S.A.; Beattie, C.W.; et al. A first-in-class, first-in-human, phase I trial of p28, a non-HDM2-mediated peptide inhibitor of p53 ubiquitination in patients with advanced solid tumours. Br. J. Cancer 2013, 108, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Singh, M.; Santos, G.S.; Guerlavais, V.; Carvajal, L.A.; Aivado, M.; Zhan, Y.; Oliveira, M.M.; Westerberg, L.S.; Annis, D.A.; et al. Pharmacologic Activation of p53 Triggers Viral Mimicry Response Thereby Abolishing Tumor Immune Evasion and Promoting Antitumor Immunity. Cancer Discov. 2021, 11, 3090–3105. [Google Scholar] [CrossRef] [PubMed]
- Rothbard, J.B.; Garlington, S.; Lin, Q.; Kirschberg, T.; Kreider, E.; McGrane, P.L.; Wender, P.A.; Khavari, P.A. Conjugation of arginine oligomers to cyclosporin A facilitates topical delivery and inhibition of inflammation. Nat. Med. 2000, 6, 1253–1257. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Lattanzio, F.A., Jr.; Samudre, S.S.; DiSandro, G.; Sheppard, J.D., Jr.; Williams, P.B.; Shi, N.-Q.; Qi, X.-R.; Xiang, B.; Zhang, Y.; et al. Efficacy of a phosphorodiamidate morpholino oligomer antisense compound in the inhibition of corneal transplant rejection in a rat cornea transplant model. J. Ocul. Pharmacol. Ther. 2012, 28, 194–201. [Google Scholar] [CrossRef]
- Abes, R.; Arzumanov, A.; Moulton, H.; Abes, S.; Ivanova, G.; Iversen, P.; Gait, M.; Lebleu, B. Cell-penetrating-peptide-based delivery of oligonucleotides: An overview. Biochem. Soc. Trans. 2007, 35, 775–779. [Google Scholar] [CrossRef]
- Brandt, F.; O’Connell, C.; Cazzaniga, A.; Waugh, J.M. Efficacy and safety evaluation of a novel botulinum toxin topical gel for the treatment of moderate to severe lateral canthal lines. Dermatol. Surg. 2010, 36 (Suppl. 4), 2111–2118. [Google Scholar] [CrossRef]
- Glogau, R.; Blitzer, A.; Brandt, F.; Kane, M.; Monheit, G.D.; Waugh, J.M. Results of a randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of a botulinum toxin type A topical gel for the treatment of moderate-to-severe lateral canthal lines. J. Drugs Dermatol. 2012, 11, 38–45. [Google Scholar]
- Miampamba, M.; Liu, J.; Harootunian, A.; Gale, A.J.; Baird, S.; Chen, S.L.; Nguyen, Q.T.; Tsien, R.Y.; González, J.E. Sensitive in vivo Visualization of Breast Cancer Using Ratiometric Protease-activatable Fluorescent Imaging Agent, AVB-620. Theranostics 2017, 7, 3369–3386. [Google Scholar] [CrossRef]
- Gurbel, P.A.; Bliden, K.P.; Turner, S.E.; Tantry, U.S.; Gesheff, M.G.; Barr, T.P.; Covic, L.; Kuliopulos, A. Cell-Penetrating Pepducin Therapy Targeting PAR1 in Subjects with Coronary Artery Disease. Arter. Thromb. Vasc. Biol. 2016, 36, 189–197. [Google Scholar] [CrossRef]
- Unkart, J.T.; Chen, S.L.; Wapnir, I.L.; González, J.E.; Harootunian, A.; Wallace, A.M. Intraoperative Tumor Detection Using a Ratiometric Activatable Fluorescent Peptide: A First-in-Human Phase 1 Study. Ann. Surg. Oncol. 2017, 24, 3167–3173. [Google Scholar] [CrossRef]
- Gowland, C.; Berry, P.; Errington, J.; Jeffrey, P.; Bennett, G.; Godfrey, L.; Pittman, M.; Niewiarowski, A.; Symeonides, S.N.; Veal, G.J. Development of a LC–MS/MS method for the quantification of toxic payload DM1 cleaved from BT1718 in a Phase I study. Bioanalysis 2021, 13, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.B.; Furnish, E.J.; Komalavilas, P.; Flynn, C.R.; Ashby, P.; Hansen, A.; Ly, D.P.; Yang, G.P.; Longaker, M.T.; Panitch, A.; et al. Cell permeant peptide analogues of the small heat shock protein, HSP20, reduce TGF-β1-induced CTGF expression in keloid fibroblasts. J. Investig. Dermatol. 2009, 129, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Coriat, R.; Faivre, S.J.; Mir, O.; Dreyer, C.; Ropert, S.; Bouattour, M.; Desjardins, R.; Goldwasser, F.; Raymond, E. Pharmacokinetics and safety of DTS-108, a human oligopeptide bound to SN-38 with an esterase-sensitive cross-linker in patients with advanced malignancies: A Phase I study. Int. J. Nanomed. 2016, 11, 6207–6216. [Google Scholar] [CrossRef] [PubMed]







| CPPs | Cargos | Recruitment Status | Application | Gov ID | Year | Refs |
|---|---|---|---|---|---|---|
| TAT | Botulinum toxin A | Phase IIIb (completed) | Cervical dystonia | NCT01753310 | 2012 | [190] |
| TAT | JNKI-1 | Phase III (completed) | Postoperative ocular inflammation | NCT02508337, 02235272 | 2015 2017 | [187] |
| TAT | PSD-95 protein inhibitor | Phase III (completed) | Ischemic stroke | NCT02930018 | 2016 | [191] |
| TAT | D-JNKI-1 gel | Phase III (completed) | Hearing loss, idiopathic sudden sensorineural | NCT02809118, 02561091 | 2016 2015 | [186] |
| TAT | Botulinum toxin A | Phase II (completed) | Cervical dystonia | NCT02706795 | 2016 | [188] |
| TAT | δ-PKC inhibitor | Phase II (completed) | Myocardial infarction | NCT00785954 | 2008 | N/A |
| TAT | ε-PKC inhibitor | Phase II (completed) | Pain: postherpetic neuralgia, spinal cord injury, postoperative | NCT01106716, 01135108, 01015235 | 2010 2011 2013 | [192,193] |
| TAT | PKC inhibitor | Phase II (completed) | Acute myocardial infarction | NCT00093197 | 2004 | [194] |
| TAT | Botulinum toxin A | Phase I/II | Glabellar lines | N/A | 2015 | [189] |
| TAT | Dextrogyre peptide | Phase I (completed) | Intraocular inflammation and pain | NCT01570205 | 2012 | [195] |
| TAT | MAGE-A3,HPV-16 | Phase I (completed) | Head and neck carcinoma | NCT00257738 | 2005 | [196] |
| TAT | Cu, Zn-Superoxide dismutase | Phase I | Obesity | N/A | 2011 | [197] |
| ATX-101 | N/A | Phase Ib/Iia (recruiting) | Several cancers | NCT04814875 | 2021 | [198] |
| AM-111 | D-JNKI-1 gel | Phase II (completed) | Acute sensorineural hearing loss | NCT00802425 | 2008 | [199] |
| P28 | P28GST | Phase II (completed) | Intestinal inflammation | NCT02281916 | 2014 | [200] |
| P28 | P28, Non-HDM2-mediated peptide inhibitor of p53 | Phase I | Central nervous system tumors | NCT01975116 | 2016 | [201] |
| P28 | P28, Non-HDM2-mediated peptide inhibitor of p53 | Phase I (completed) | P53 ubiquitination in patients with advanced solid tumors | NCT00914914 | 2013 | [202] |
| ALRN-6924 | Palbociclib | Phase Iia (completed) | Solid tumor, Lymphoma, Peripheral T-cell lymphoma | NCT02264613 | 2014 | [203] |
| ALRN-6924 | Cytarabine | Phase I (completed) | Acute myeloid leukemia, Myelodysplastic syndromes | NCT02909972 | 2016 | |
| ALRN-6924 | Paclitaxel | Phase 1 (active) | Advanced, metastatic or unresectable solid tumors | NCT03725436 | 2018 | |
| ALRN-6924 | Cytarabine | Phase 1 (active) | leukemia, brain tumor, pediatric lymphoma | NCT03654716 | 2018 | |
| ALRN-6924 | Topotecan | Phase 1a (terminated) | Small cell lung cancer | NCT04022876 | 2019 | |
| R7 | Cyclosporin A | Phase II | Psoriasis | N/A | 2003 | [204] |
| (R-Ahx-R)4 | PMO | Phase III (terminated) | Cardiovascular disease, coronary artery bypass | NCT00451256 | 2007 | [205] |
| (R-Ahx-R)4 | PMO targeted to human c-Myc | Phase II | Obstruction of vein graft after cardiovascular bypass surgery | N/A | 2009 | [206] |
| TransMTS | Botulinumtoxin A | Phase III (completed) | Cervical dystonia | NCT03608397 | 2018 | [188] |
| MTS | Botulinumtoxin A | Phase III, Phase II, Phase II (completed) | Skin aging, hyperhidrosis, lateral canthal lines, crow’s feet, and facial wrinkles | NCT02580370, 02565732 | 2015 | [207,208] |
| AVB-620 (ACPP) | Cy5, Cy7 | Phase II (completed) | Breast cancer | NCT03113825 | 2017 | [209] |
| Pepducin | PZ-128 | Phase II | Coronary artery disease | N/A | 2015 | [210] |
| AVB-620 (ACPP) | Cy5, Cy7 | Phase I (completed) | Interpretative tumor detection using a ratiometric activatable fluorescent peptide | NCT02391194 | 2015 | [211] |
| BT1718 | Toxic DM1 | Phase I/Iia (active) | Targeting MT1-MMP for treatment of solid tumors | NCT03486730 | 2018 | [212] |
| PEP-010 | Paclitaxel | Phase 1 (recruiting) | Metastatic solid tumor | NCT04733027 | 2021 | |
| ATP128 | BI 754091 | Phase 1b (recruiting) | Stage IV colorectal cancer | NCT04046445 | 2019 2022 | |
| PTD4 | HSP20 phosphopeptide | Phase II (recruiting) | Scar prevention, reduction | NCT00825916 | 2009 | [213] |
| Charged Oligo peptide | SN38 | Phase I | Tumor | N/A | 2016 | [214] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Huang, J.; Fishelson, Z.; Wang, C.; Zhang, S. Cell-Penetrating Peptide-Based Delivery of Macromolecular Drugs: Development, Strategies, and Progress. Biomedicines 2023, 11, 1971. https://doi.org/10.3390/biomedicines11071971
Sun Z, Huang J, Fishelson Z, Wang C, Zhang S. Cell-Penetrating Peptide-Based Delivery of Macromolecular Drugs: Development, Strategies, and Progress. Biomedicines. 2023; 11(7):1971. https://doi.org/10.3390/biomedicines11071971
Chicago/Turabian StyleSun, Zhe, Jinhai Huang, Zvi Fishelson, Chenhui Wang, and Sihe Zhang. 2023. "Cell-Penetrating Peptide-Based Delivery of Macromolecular Drugs: Development, Strategies, and Progress" Biomedicines 11, no. 7: 1971. https://doi.org/10.3390/biomedicines11071971
APA StyleSun, Z., Huang, J., Fishelson, Z., Wang, C., & Zhang, S. (2023). Cell-Penetrating Peptide-Based Delivery of Macromolecular Drugs: Development, Strategies, and Progress. Biomedicines, 11(7), 1971. https://doi.org/10.3390/biomedicines11071971