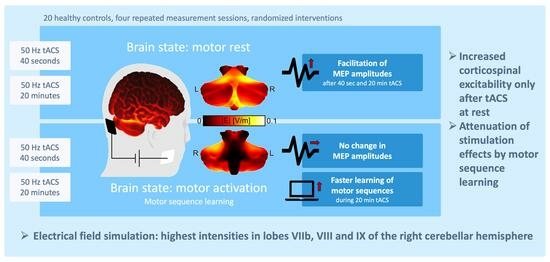
Neuronavigated Cerebellar 50 Hz tACS: Attenuation of Stimulation Effects by Motor Sequence Learning
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Neuronavigation and MRI Data Acquisition
2.3. Transcranial Magnetic Stimulation Measurements
2.4. Transcranial Alternating Current Stimulation
2.5. Motor Sequence Learning
2.6. Data Analysis and Statistical Analysis
2.6.1. Motor Evoked Potentials
2.6.2. Motor Sequence Learning
2.6.3. Evaluation of Side Effects
2.6.4. Electric Field Simulations
3. Results
3.1. Cortical Excitability Measures
3.2. Motor Sequence Learning
3.3. Electric Field Simulation
3.4. Correlation Analyses
3.5. Evaluation of Side Effects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Asan, A.S.; Lang, E.J.; Sahin, M. Entrainment of cerebellar purkinje cells with directional AC electric fields in anesthetized rats. Brain Stimul. 2020, 13, 1548–1558. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; Alekseichuk, I.; Krieg, J.; Doyle, A.; Yu, Y.; Vitek, J.; Johnson, M.; Opitz, A. Dose-dependent effects of transcranial alternating current stimulation on spike timing in awake nonhuman primates. Sci. Adv. 2020, 6, eaaz2747. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.R.; Vieira, P.G.; Csorba, B.A.; Pilly, P.K.; Pack, C.C. Transcranial alternating current stimulation entrains single-neuron activity in the primate brain. Proc. Natl. Acad. Sci. USA 2019, 116, 5747–5755. [Google Scholar] [CrossRef] [PubMed]
- Schubert, C.; Dabbagh, A.; Classen, J.; Kramer, U.M.; Tzvi, E. Alpha oscillations modulate premotor-cerebellar connectivity in motor learning: Insights from transcranial alternating current stimulation. Neuroimage 2021, 241, 118410. [Google Scholar] [CrossRef]
- Tesche, C.D.; Houck, J.M. Discordant alpha-band transcranial alternating current stimulation affects cortico-cortical and cortico-cerebellar connectivity. Brain Connect. 2020, 10, 170–182. [Google Scholar] [CrossRef]
- Veniero, D.; Vossen, A.; Gross, J.; Thut, G. Lasting EEG/MEG aftereffects of rhythmic transcranial brain stimulation: Level of control over oscillatory network activity. Front. Cell Neurosci. 2015, 9, 477. [Google Scholar] [CrossRef]
- Korai, S.A.; Ranieri, F.; Di Lazzaro, V.; Papa, M.; Cirillo, G. Neurobiological after-effects of low intensity transcranial electric stimulation of the human nervous system: From basic mechanisms to metaplasticity. Front. Neurol. 2021, 12, 587771. [Google Scholar] [CrossRef]
- Zaehle, T.; Rach, S.; Herrmann, C.S. Transcranial alternating current stimulation enhances individual alpha activity in human EEG. PLoS ONE 2010, 5, e13766. [Google Scholar] [CrossRef]
- Wischnewski, M.; Engelhardt, M.; Salehinejad, M.A.; Schutter, D.; Kuo, M.F.; Nitsche, M.A. NMDA receptor-mediated motor cortex plasticity after 20 Hz transcranial alternating current stimulation. Cereb. Cortex 2019, 29, 2924–2931. [Google Scholar] [CrossRef]
- Alagapan, S.; Schmidt, S.L.; Lefebvre, J.; Hadar, E.; Shin, H.W.; Frhlich, F. Modulation of cortical oscillations by low-frequency direct cortical stimulation is state-dependent. PLoS Biol. 2016, 14, e1002424. [Google Scholar] [CrossRef]
- Neuling, T.; Rach, S.; Herrmann, C.S. Orchestrating neuronal networks: Sustained after-effects of transcranial alternating current stimulation depend upon brain states. Front. Hum. Neurosci. 2013, 7, 161. [Google Scholar] [CrossRef] [PubMed]
- Feurra, M.; Pasqualetti, P.; Bianco, G.; Santarnecchi, E.; Rossi, A.; Rossi, S. State-dependent effects of transcranial oscillatory currents on the motor system: What you think matters. J. Neurosci. 2013, 33, 17483–17489. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, C.S.; Rach, S.; Neuling, T.; Struber, D. Transcranial alternating current stimulation: A review of the underlying mechanisms and modulation of cognitive processes. Front. Hum. Neurosci. 2013, 7, 279. [Google Scholar] [CrossRef]
- Nowak, M.; Zich, C.; Stagg, C.J. Motor cortical gamma oscillations: What have we learnt and where are we headed? Curr. Behav. Neurosci. Rep. 2018, 5, 136–142. [Google Scholar] [CrossRef]
- Swann, N.C.; de Hemptinne, C.; Miocinovic, S.; Qasim, S.; Wang, S.S.; Ziman, N.; Ostrem, J.L.; San Luciano, M.; Galifianakis, N.B.; Starr, P.A. Gamma oscillations in the hyperkinetic state detected with chronic human brain recordings in Parkinson’s disease. J. Neurosci. 2016, 36, 6445–6458. [Google Scholar] [CrossRef] [PubMed]
- Moisa, M.; Polania, R.; Grueschow, M.; Ruff, C.C. Brain network mechanisms underlying motor enhancement by transcranial entrainment of gamma oscillations. J. Neurosci. 2016, 36, 12053–12065. [Google Scholar] [CrossRef]
- Joundi, R.A.; Jenkinson, N.; Brittain, J.S.; Aziz, T.Z.; Brown, P. Driving oscillatory activity in the human cortex enhances motor performance. Curr. Biol. 2012, 22, 403–407. [Google Scholar] [CrossRef]
- Guerra, A.; Bologna, M.; Paparella, G.; Suppa, A.; Colella, D.; Di Lazzaro, V.; Brown, P.; Berardelli, A. Effects of transcranial alternating current stimulation on repetitive finger movements in healthy humans. Neural Plast. 2018, 2018, 4593095. [Google Scholar] [CrossRef]
- Guell, X.; Schmahmann, J. Cerebellar functional anatomy: A didactic summary based on human fMRI evidence. Cerebellum 2020, 19, 1–5. [Google Scholar] [CrossRef]
- Bruggemann, N. Contemporary functional neuroanatomy and pathophysiology of dystonia. J. Neural Transm. 2021, 128, 499–508. [Google Scholar] [CrossRef]
- Ibrahim, M.F.; Beevis, J.C.; Empson, R.M. Essential tremor—A cerebellar driven disorder? Neuroscience 2020, 462, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Weissbach, A.; Werner, E.; Bally, J.F.; Tunc, S.; Lons, S.; Timmann, D.; Zeuner, K.E.; Tadic, V.; Bruggemann, N.; Lang, A.; et al. Alcohol improves cerebellar learning deficit in myoclonus-dystonia: A clinical and electrophysiological investigation. Ann. Neurol. 2017, 82, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Wessel, M.J.; Draaisma, L.R.; Hummel, F.C. Mini-review: Transcranial alternating current stimulation and the cerebellum. Cerebellum 2022, 22, 120–128. [Google Scholar] [CrossRef]
- Raman, I.M.; Bean, B.P. Ionic currents underlying spontaneous action potentials in isolated cerebellar Purkinje neurons. J. Neurosci. 1999, 19, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- Herzog, R.; Berger, T.M.; Pauly, M.G.; Xue, H.; Rueckert, E.; Münchau, A.; Bäumer, T.; Weissbach, A. Cerebellar transcranial current stimulation—An intraindividual comparison of different techniques. Front. Neurosci. 2022, 16, 987472. [Google Scholar] [CrossRef]
- Naro, A.; Leo, A.; Russo, M.; Cannavo, A.; Milardi, D.; Bramanti, P.; Calabro, R.S. Does transcranial alternating current stimulation induce cerebellum plasticity? Feasibility, safety and efficacy of a novel electrophysiological approach. Brain Stimul. 2016, 9, 388–395. [Google Scholar] [CrossRef]
- Spampinato, D.; Avci, E.; Rothwell, J.; Rocchi, L. Frequency-dependent modulation of cerebellar excitability during the application of non-invasive alternating current stimulation. Brain Stimul. 2021, 14, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Giustiniani, A.; Battaglia, G.; Messina, G.; Morello, H.; Guastella, S.; Iovane, A.; Oliveri, M.; Palma, A.; Proia, P. Transcranial alternating current stimulation (tACS) does not affect sports people’s explosive power: A pilot study. Front. Hum. Neurosci. 2021, 15, 640609. [Google Scholar] [CrossRef]
- Cury, R.G.; França, C.; Reis Barbosa, E.; Jacobsen Teixeira, M.; Ciampi de Andrade, D. Little brain, big expectations. Brain Sci. 2020, 10, 944. [Google Scholar] [CrossRef]
- Miterko, L.N.; Baker, K.B.; Beckinghausen, J.; Bradnam, L.V.; Cheng, M.Y.; Cooperrider, J.; DeLong, M.R.; Gornati, S.V.; Hallett, M.; Heck, D.H.; et al. Consensus paper: Experimental neurostimulation of the cerebellum. Cerebellum 2019, 18, 1064–1097. [Google Scholar] [CrossRef]
- Gomez-Tames, J.; Asai, A.; Mikkonen, M.; Laakso, I.; Tanaka, S.; Uehara, S.; Otaka, Y.; Hirata, A. Group-level and functional-region analysis of electric-field shape during cerebellar transcranial direct current stimulation with different electrode montages. J. Neural Eng. 2019, 16, 036001. [Google Scholar] [CrossRef] [PubMed]
- Sadeghihassanabadi, F.; Misselhorn, J.; Gerloff, C.; Zittel, S. Optimizing the montage for cerebellar transcranial alternating current stimulation (tACS): A combined computational and experimental study. J. Neural Eng. 2022, 19, 026060. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, Z.; Dutta, A. Cerebellar lobules optimal stimulation (CLOS): A computational pipeline to optimize cerebellar lobule-specific electric field distribution. Front. Neurosci. 2019, 13, 266. [Google Scholar] [CrossRef]
- Tzvi, E.; Stoldt, A.; Witt, K.; Kramer, U.M. Striatal-cerebellar networks mediate consolidation in a motor sequence learning task: An fMRI study using dynamic causal modelling. Neuroimage 2015, 122, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Dayan, E.; Cohen, L.G. Neuroplasticity subserving motor skill learning. Neuron 2011, 72, 443–454. [Google Scholar] [CrossRef]
- Doyon, J.; Bellec, P.; Amsel, R.; Penhune, V.; Monchi, O.; Carrier, J.; Lehericy, S.; Benali, H. Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav. Brain Res. 2009, 199, 61–75. [Google Scholar] [CrossRef]
- Giustiniani, A.; Tarantino, V.; Bracco, M.; Bonaventura, R.E.; Oliveri, M. Functional role of cerebellar gamma frequency in motor sequences learning: A tACS study. Cerebellum 2021, 20, 913–921. [Google Scholar] [CrossRef]
- Pauly, M.G.; Steinmeier, A.; Bolte, C.; Hamami, F.; Tzvi, E.; Munchau, A.; Baumer, T.; Weissbach, A. Cerebellar rTMS and PAS effectively induce cerebellar plasticity. Sci. Rep. 2021, 11, 3070. [Google Scholar] [CrossRef]
- Stoodley, C.J.; Schmahmann, J.D. Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies. Neuroimage 2009, 44, 489–501. [Google Scholar] [CrossRef]
- Popa, T.; Velayudhan, B.; Hubsch, C.; Pradeep, S.; Roze, E.; Vidailhet, M.; Meunier, S.; Kishore, A. Cerebellar processing of sensory inputs primes motor cortex plasticity. Cereb. Cortex 2013, 23, 305–314. [Google Scholar] [CrossRef]
- Weissbach, A.; Baumer, T.; Pramstaller, P.P.; Bruggemann, N.; Tadic, V.; Chen, R.; Klein, C.; Munchau, A. Abnormal premotor-motor interaction in heterozygous Parkin- and Pink1 mutation carriers. Clin. Neurophysiol. 2017, 128, 275–280. [Google Scholar] [CrossRef]
- Weissbach, A.; Baumer, T.; Bruggemann, N.; Tadic, V.; Zittel, S.; Cheng, B.; Thomalla, G.; Klein, C.; Munchau, A. Premotor-motor excitability is altered in dopa-responsive dystonia. Mov. Disord. 2015, 30, 1705–1709. [Google Scholar] [CrossRef]
- Nissen, M.J.; Bullemer, P. Attentional requirements of learning: Evidence from performance measures. Cogn. Psychol. 1987, 19, 1–32. [Google Scholar] [CrossRef]
- Curran, T. Higher-order associative learning in amnesia: Evidence from the serial reaction time task. J. Cogn. Neurosci. 1997, 9, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Datta, A.; Bikson, M.; Parra, L.C. ROAST: An open-source, fully-automated, realistic volumetric-approach-based simulator for TES. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; Volume 2018, pp. 3072–3075. [Google Scholar] [CrossRef]
- Huang, Y.; Datta, A.; Bikson, M.; Parra, L.C. Realistic volumetric-approach to simulate transcranial electric stimulation-ROAST-a fully automated open-source pipeline. J. Neural Eng. 2019, 16, 056006. [Google Scholar] [CrossRef] [PubMed]
- Tzourio-Mazoyer, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Mazoyer, B.; Joliot, M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002, 15, 273–289. [Google Scholar] [CrossRef]
- Diedrichsen, J. A spatially unbiased atlas template of the human cerebellum. Neuroimage 2006, 33, 127–138. [Google Scholar] [CrossRef]
- Tsuzuki, D.; Watanabe, H.; Dan, I.; Taga, G. MinR 10/20 system: Quantitative and reproducible cranial landmark setting method for MRI based on minimum initial reference points. J. Neurosci. Methods 2016, 264, 86–93. [Google Scholar] [CrossRef]
- Radecke, J.-O.; Khan, A.; Engel, A.K.; Wolters, C.H.; Schneider, T.R. Individual targeting increases control over inter-individual variability in simulated transcranial electric fields. IEEE Access 2020, 8, 182610–182624. [Google Scholar] [CrossRef]
- D’Angelo, E. Neural circuits of the cerebellum: Hypothesis for function. J. Integr. Neurosci. 2011, 10, 317–352. [Google Scholar] [CrossRef]
- Gandolfi, D.; Lombardo, P.; Mapelli, J.; Solinas, S.; D’Angelo, E. Theta-Frequency resonance at the cerebellum input stage improves spike timing on the millisecond time-scale. Front. Neural Circuits 2013, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Penhune, V.B.; Steele, C.J. Parallel contributions of cerebellar, striatal and M1 mechanisms to motor sequence learning. Behav. Brain Res. 2012, 226, 579–591. [Google Scholar] [CrossRef]
- Tanaka, H.; Ishikawa, T.; Lee, J.; Kakei, S. The cerebro-cerebellum as a locus of forward model: A review. Front. Syst. Neurosci. 2020, 14, 19. [Google Scholar] [CrossRef]
- Tzvi, E.; Munte, T.F.; Kramer, U.M. Delineating the cortico-striatal-cerebellar network in implicit motor sequence learning. Neuroimage 2014, 94, 222–230. [Google Scholar] [CrossRef]
- Kandel, E.R.; Koester, J.D.; Mack, S.H.; Siegelbaum, S.A. The Cerebellum. In Principles of Neural Science, 6th ed.; McGraw Hill: New York, NY, USA, 2021. [Google Scholar]
- Wessel, M.J.; Draaisma, L.R.; de Boer, A.F.W.; Park, C.H.; Maceira-Elvira, P.; Durand-Ruel, M.; Koch, P.J.; Morishita, T.; Hummel, F.C. Cerebellar transcranial alternating current stimulation in the gamma range applied during the acquisition of a novel motor skill. Sci. Rep. 2020, 10, 11217. [Google Scholar] [CrossRef]
- Guerra, A.; Suppa, A.; Asci, F.; De Marco, G.; D’Onofrio, V.; Bologna, M.; Di Lazzaro, V.; Berardelli, A. LTD-like plasticity of the human primary motor cortex can be reversed by gamma-tACS. Brain Stimul. 2019, 12, 1490–1499. [Google Scholar] [CrossRef] [PubMed]
- Guerra, A.; Asci, F.; Zampogna, A.; D’Onofrio, V.; Petrucci, S.; Ginevrino, M.; Berardelli, A.; Suppa, A. Gamma-transcranial alternating current stimulation and theta-burst stimulation: Inter-subject variability and the role of BDNF. Clin. Neurophysiol. 2020, 131, 2691–2699. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Roth, A.; Kuo, M.F.; Fischer, A.K.; Liebetanz, D.; Lang, N.; Tergau, F.; Paulus, W. Timing-dependent modulation of associative plasticity by general network excitability in the human motor cortex. J. Neurosci. 2007, 27, 3807–3812. [Google Scholar] [CrossRef]
- Thirugnanasambandam, N.; Sparing, R.; Dafotakis, M.; Meister, I.G.; Paulus, W.; Nitsche, M.A.; Fink, G.R. Isometric contraction interferes with transcranial direct current stimulation (tDCS) induced plasticity: Evidence of state-dependent neuromodulation in human motor cortex. Restor. Neurol. Neurosci. 2011, 29, 311–320. [Google Scholar] [CrossRef]
- Karabanov, A.; Ziemann, U.; Hamada, M.; George, M.S.; Quartarone, A.; Classen, J.; Massimini, M.; Rothwell, J.; Siebner, H.R. Consensus paper: Probing homeostatic plasticity of human cortex with non-invasive transcranial brain stimulation. Brain Stimul. 2015, 8, 993–1006. [Google Scholar] [CrossRef]
- Ziemann, U.; Siebner, H.R. Modifying motor learning through gating and homeostatic metaplasticity. Brain Stimul. 2008, 1, 60–66. [Google Scholar] [CrossRef]
- Gall, D.; Prestori, F.; Sola, E.; D’Errico, A.; Roussel, C.; Forti, L.; Rossi, P.; D’Angelo, E. Intracellular calcium regulation by burst discharge determines bidirectional long-term synaptic plasticity at the cerebellum input stage. J. Neurosci. 2005, 25, 4813–4822. [Google Scholar] [CrossRef]
- Siebner, H.R. A primer on priming the human motor cortex. Clin. Neurophysiol. 2010, 121, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Naro, A.; Milardi, D.; Cacciola, A.; Russo, M.; Sciarrone, F.; La Rosa, G.; Bramanti, A.; Bramanti, P.; Calabro, R.S. What do we know about the influence of the cerebellum on walking ability? Promising findings from transcranial alternating current stimulation. Cerebellum 2017, 16, 859–867. [Google Scholar] [CrossRef]
- Tzvi, E.; Alizadeh, J.; Schubert, C.; Classen, J. Classification of EEG signals reveals a focal aftereffect of 10 Hz motor cortex transcranial alternating current stimulation. Cereb. Cortex Commun. 2022, 3, tgab067. [Google Scholar] [CrossRef] [PubMed]
- Opitz, A.; Paulus, W.; Will, S.; Antunes, A.; Thielscher, A. Determinants of the electric field during transcranial direct current stimulation. Neuroimage 2015, 109, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Rampersad, S.M.; Aydin, U.; Vorwerk, J.; Oostendorp, T.F.; Neuling, T.; Herrmann, C.S.; Stegeman, D.F.; Wolters, C.H. Investigation of tDCS volume conduction effects in a highly realistic head model. J. Neural Eng. 2014, 11, 016002. [Google Scholar] [CrossRef]





| Side Effects | l-tACSMSL | l-tACSRest | s-tACSMSL | s-tACSRest | X2 | p |
|---|---|---|---|---|---|---|
| Headache | 4 | 2 | 4 | 6 | 2.00 | 0.572 |
| Discomfort | 5 | 4 | 5 | 5 | 0.16 | 0.984 |
| Dizziness | 0 | 1 | 1 | 1 | 0.00 | 1.000 |
| Local heating | 8 | 7 | 9 | 13 | 2.24 | 0.523 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herzog, R.; Bolte, C.; Radecke, J.-O.; von Möller, K.; Lencer, R.; Tzvi, E.; Münchau, A.; Bäumer, T.; Weissbach, A. Neuronavigated Cerebellar 50 Hz tACS: Attenuation of Stimulation Effects by Motor Sequence Learning. Biomedicines 2023, 11, 2218. https://doi.org/10.3390/biomedicines11082218
Herzog R, Bolte C, Radecke J-O, von Möller K, Lencer R, Tzvi E, Münchau A, Bäumer T, Weissbach A. Neuronavigated Cerebellar 50 Hz tACS: Attenuation of Stimulation Effects by Motor Sequence Learning. Biomedicines. 2023; 11(8):2218. https://doi.org/10.3390/biomedicines11082218
Chicago/Turabian StyleHerzog, Rebecca, Christina Bolte, Jan-Ole Radecke, Kathinka von Möller, Rebekka Lencer, Elinor Tzvi, Alexander Münchau, Tobias Bäumer, and Anne Weissbach. 2023. "Neuronavigated Cerebellar 50 Hz tACS: Attenuation of Stimulation Effects by Motor Sequence Learning" Biomedicines 11, no. 8: 2218. https://doi.org/10.3390/biomedicines11082218
APA StyleHerzog, R., Bolte, C., Radecke, J.-O., von Möller, K., Lencer, R., Tzvi, E., Münchau, A., Bäumer, T., & Weissbach, A. (2023). Neuronavigated Cerebellar 50 Hz tACS: Attenuation of Stimulation Effects by Motor Sequence Learning. Biomedicines, 11(8), 2218. https://doi.org/10.3390/biomedicines11082218