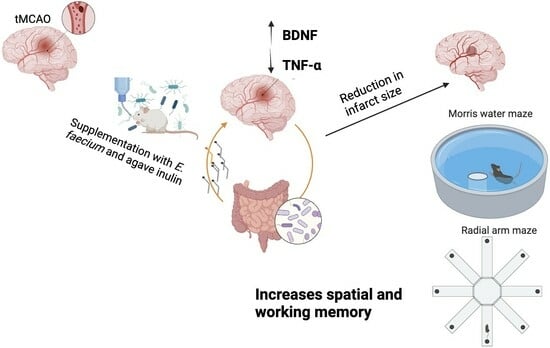
Supplementation with a Symbiotic Induced Neuroprotection and Improved Memory in Rats with Ischemic Stroke
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Cerebral Ischemia Model
2.3. Administration of the Vehicle and Symbiotic
2.4. Zea Longa Scale
2.5. Morris Water Maze
2.6. Eight-Arm Radial Maze
2.7. Brain Infarct Volume
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Statistical Analysis
3. Results
3.1. Symbiotic Supplementation Reduced the Neurological Deficit Observed after tMCAO
3.2. Symbiotic Supplementation Reduced the Infarct Volume after tMCAO
3.3. Supplementation with the Symbiotic Enhances Spatial Memory after tMCAO
3.4. Work Memory Was Also Enhanced by Symbiotic Supplementation
3.5. Hippocampus of Symbiotic-Supplemented Rats Presented an Increase in BDNF and a Reduction in TNF-α
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.; Sacco, R.L.; Hacke, W.; Fisher, M.; Pandian, J.; Lindsay, P. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int. J. Stroke 2022, 17, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Jia, M.; Wang, Y.; Wang, Q.; Wu, J. Cell Death Mechanisms in Cerebral Ischemia-Reperfusion Injury. Neurochem. Res. 2022, 47, 3525–3542. [Google Scholar] [CrossRef] [PubMed]
- Jurcau, A.; Simion, A. Neuroinflammation in Cerebral Ischemia and Ischemia/Reperfusion Injuries: From Pathophysiology to Therapeutic Strategies. Int. J. Mol. Sci. 2021, 23, 14. [Google Scholar] [CrossRef] [PubMed]
- Radenovic, L.; Nenadic, M.; Ułamek-Kozioł, M.; Januszewski, S.; Czuczwar, S.J.; Andjus, P.R.; Pluta, R. Heterogeneity in brain distribution of activated microglia and astrocytes in a rat ischemic model of Alzheimer’s disease after 2 years of survival. Aging 2020, 12, 12251–12267. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, T.; Bennett, S.; Koh, C.L.; McKenna, K.T. Occupational therapy for cognitive impairment in stroke patients. Cochrane Database Syst. Rev. 2010, 2010, CD006430. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Biesbroek, J.M.; Shi, L.; Liu, W.; Kuijf, H.J.; Chu, W.W.; Abrigo, J.M.; Lee, R.K.; Leung, T.W.; Lau, A.Y.; et al. Strategic infarct location for post-stroke cognitive impairment: A multivariate lesion-symptom mapping study. J. Cereb. Blood Flow Metab. 2018, 38, 1299–1311. [Google Scholar] [CrossRef]
- Lugtmeijer, S.; Lammers, N.A.; de Haan, E.H.F.; de Leeuw, F.E.; Kessels, R.P.C. Post-Stroke Working Memory Dysfunction: A Meta-Analysis and Systematic Review. Neuropsychol. Rev. 2021, 31, 202–219. [Google Scholar] [CrossRef]
- Morrison, H.W.; White, M.M.; Rothers, J.L.; Taylor-Piliae, R.E. Examining the Associations between Post-Stroke Cognitive Function and Common Comorbid Conditions among Stroke Survivors. Int. J. Environ. Res. Public Health 2022, 19, 13445. [Google Scholar] [CrossRef]
- Li, N.; Wang, X.; Sun, C.; Wu, X.; Lu, M.; Si, Y.; Ye, X.; Wang, T.; Yu, X.; Zhao, X.; et al. Change of intestinal microbiota in cerebral ischemic stroke patients. BMC Microbiol. 2019, 19, 191. [Google Scholar] [CrossRef]
- Yamashiro, K.; Kurita, N.; Urabe, T.; Hattori, N. Role of the Gut Microbiota in Stroke Pathogenesis and Potential Therapeutic Implications. Ann. Nutr. Metab. 2021, 77, 36–44. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Farabegoli, F.; Santaclara, F.J.; Costas, D.; Alonso, M.; Abril, A.G.; Espiñeira, M.; Ortea, I.; Costas, C. Exploring the Anti-Inflammatory Effect of Inulin by Integrating Transcriptomic and Proteomic Analyses in a Murine Macrophage Cell Model. Nutrients 2023, 15, 859. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, X.; Zhu, L.; Yang, X.; He, F.; Wang, T.; Bao, T.; Lu, H.; Wang, H.; Yang, S. Inulin alleviates inflammation of alcoholic liver disease via SCFAs-inducing suppression of M1 and facilitation of M2 macrophages in mice. Int. Immunopharmacol. 2020, 78, 106062. [Google Scholar] [CrossRef] [PubMed]
- Ayala Monter, M.A.; Pinto Ruiz, R.; González Muñoz, S.S.; Bárcena Gama, J.R.; Hernández Mendo, O.; Torres Salado, N. Efecto prebiótico de dos fuentes de inulina en el crecimiento in vitro de Lactobacillus salivarius y Enterococcus faecium. Rev. Mex. Cienc. Pecu. 2018, 9, 346–361. [Google Scholar] [CrossRef]
- Yang, Q.; He, Y.; Tian, L.L.; Zhang, Z.; Qiu, L.; Tao, X.Y.; Wei, H. Anti-tumor effect of infant-derived Enterococcus via the inhibition of proliferation and inflammation as well as the promotion of apoptosis. Food Funct. 2023, 14, 2223–2238. [Google Scholar] [CrossRef] [PubMed]
- Romo-Araiza, A.; Gutiérrez-Salmeán, G.; Galván, E.J.; Hernández-Frausto, M.; Herrera-López, G.; Romo-Parra, H.; García-Contreras, V.; Fernández-Presas, A.M.; Jasso-Chávez, R.; Borlongan, C.V.; et al. Probiotics and Prebiotics as a Therapeutic Strategy to Improve Memory in a Model of Middle-Aged Rats. Front. Aging Neurosci. 2018, 10, 416. [Google Scholar] [CrossRef] [PubMed]
- Servín-Casas, G.A.; Romo-Araiza, A.; Gutierrez-Salmean, G.; Martinez-Solis, E.; Ibarra-García, A.P.; Cruz-Martinez, Y.; Rodriguez-Barrera, R.; García, E.; Incontri-Abraham, D.; Ibarra, A. Memory improvement in senile rats after prebiotic and probiotic supplementation is not induced by GLP-1. CNS Neurosci. Ther. 2022, 28, 1986–1992. [Google Scholar] [CrossRef]
- Romo-Araiza, A.; Picazo-Aguilar, R.I.; Griego, E.; Márquez, L.A.; Galván, E.J.; Cruz, Y.; Fernández-Presas, A.M.; Chávez-Guerra, A.; Rodríguez-Barrera, R.; Azpiri-Cardós, A.P.; et al. Symbiotic Supplementation (E. faecium and Agave Inulin) Improves Spatial Memory and Increases Plasticity in the Hippocampus of Obese Rats: A Proof-of-Concept Study. Cell Transplant. 2023, 32, 9636897231177357. [Google Scholar] [CrossRef]
- Longa, E.Z.; Weinstein, P.R.; Carlson, S.; Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989, 20, 84–91. [Google Scholar] [CrossRef]
- Penley, S.C.; Gaudet, C.M.; Threlkeld, S.W. Use of an eight-arm radial water maze to assess working and reference memory following neonatal brain injury. J. Vis. Exp. 2013, 82, 50940. [Google Scholar]
- Nouraee, C.; Fisher, M.; Di Napoli, M.; Salazar, P.; Farr, T.D.; Jafarli, A.; Divani, A.A. A Brief Review of Edema-Adjusted Infarct Volume Measurement Techniques for Rodent Focal Cerebral Ischemia Models with Practical Recommendations. J. Vasc. Interv. Neurol. 2019, 10, 38–45. [Google Scholar]
- Chiu, K.; Lau, W.M.; Lau, H.T.; So, K.F.; Chang, R.C. Micro-dissection of rat brain for RNA or protein extraction from specific brain region. J. Vis. Exp. 2007, 7, 269. [Google Scholar]
- Dominguez-Lara, S. Magnitud del efecto, una guía rápida. Educ. Méd. 2018, 19, 251–254. [Google Scholar] [CrossRef]
- Honarpisheh, P.; Bryan, R.M.; McCullough, L.D. Aging Microbiota-Gut-Brain Axis in Stroke Risk and Outcome. Circ. Res. 2022, 130, 1112–1144. [Google Scholar] [CrossRef] [PubMed]
- Pluta, R.; Januszewski, S.; Czuczwar, S.J. The Role of Gut Microbiota in an Ischemic Stroke. Int. J. Mol. Sci. 2021, 22, 915. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhu, Q.; Bi, C.; Yuan, J.; Chen, Y.; Hu, X. Bibliometric analysis of tumor necrosis factor in post-stroke neuroinflammation from 2003 to 2021. Front. Immunol. 2022, 13, 1040686. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-Y.; Wang, Y.-Y.; Chang, C.-Y.; Wu, C.-C.; Chen, W.-Y.; Liao, S.-L.; Chen, C.-J. TNF-α Receptor Inhibitor Alleviates Metabolic and Inflammatory Changes in a Rat Model of Ischemic Stroke. Antioxidants 2021, 10, 851. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, H.M.; Swerdlow, R.H. TNF-α in cerebral ischemia: Another stroke against you? J. Neurochem. 2015, 132, 369–372. [Google Scholar] [CrossRef]
- Chen, A.Q.; Fang, Z.; Chen, X.L.; Yang, S.; Zhou, Y.F.; Mao, L.; Xia, Y.P.; Jin, H.J.; Li, Y.N.; You, M.F.; et al. Microglia-derived TNF-α mediates endothelial necroptosis aggravating blood brain–barrier disruption after ischemic stroke. Cell Death Dis. 2019, 10, 487. [Google Scholar] [CrossRef]
- Iosif, R.E.; Ahlenius, H.; Ekdahl, C.T.; Darsalia, V.; Thored, P.; Jovinge, S.; Kokaia, Z.; Lindvall, O. Suppression of stroke-induced progenitor proliferation in adult subventricular zone by tumor necrosis factor receptor 1. J. Cereb. Blood Flow Metab. 2008, 28, 1574–1587. [Google Scholar] [CrossRef]
- Liguz-Lecznar, M.; Zakrzewska, R.; Kossut, M. Inhibition of TNF-α R1 signaling can rescue functional cortical plasticity impaired in early post-stroke period. Neurobiol. Aging 2015, 36, 2877–2884. [Google Scholar] [CrossRef] [PubMed]
- Sayyah, M.; Seydyousefi, M.; Moghanlou, A.E.; Metz, G.A.S.; Shamsaei, N.; Faghfoori, M.H.; Faghfoori, Z. Activation of BDNF- and VEGF-mediated Neuroprotection by Treadmill Exercise Training in Experimental Stroke. Metab. Brain Dis. 2022, 37, 1843–1853. [Google Scholar] [CrossRef] [PubMed]
- Eyileten, C.; Sharif, L.; Wicik, Z.; Jakubik, D.; Jarosz-Popek, J.; Soplinska, A.; Postula, M.; Czlonkowska, A.; Kaplon-Cieslicka, A.; Mirowska-Guzel, D. The Relation of the Brain-Derived Neurotrophic Factor with MicroRNAs in Neurodegenerative Diseases and Ischemic Stroke. Mol. Neurobiol. 2021, 58, 329–347. [Google Scholar] [CrossRef] [PubMed]
- Havlovska, Y.Y.; Lytvynenko, N.V.; Shkodina, A.D. Serum Level of Brain-Derived Neurotrophic Factor and Thrombotic Type Are Predictive of Cognitive Impairment in the Acute Period of Ischemic Strokes Patients. Neurol. Res. Int. 2023, 2023, 5578850. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, M.; Wang, H.; Li, N.; Lu, Z.; Li, L.; Hui, S.; Xu, H. Gut microbiota and short chain fatty acids partially mediate the beneficial effects of inulin on metabolic disorders in obese ob/ob mice. J. Food Biochem. 2022, 46, e14063. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhang, L.; Xue, J.; Yang, X.; Dong, X.; Sha, L.; Lei, H.; Zhang, X.; Zhu, L.; Wang, Z.; et al. Dietary inulin alleviates diverse stages of type 2 diabetes mellitus via anti-inflammation and modulating gut microbiota in db/db mice. Food Funct. 2019, 10, 1915–1927. [Google Scholar] [CrossRef] [PubMed]
- Bao, T.; Wang, Z.; Zhu, L.; Lu, H.; Wang, T.; Zhang, Y.; Zhang, X.; Wang, H.; Yang, S. Inulin increases the proportion of monocytic myeloid-derived suppressor cells in peripheral blood, liver, spleen and regulates the secretion of plasma inflammatory cytokines in mice with non-alcoholic fatty liver disease. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2020, 36, 228–235. [Google Scholar] [PubMed]
- Ondee, T.; Pongpirul, K.; Janchot, K.; Kanacharoen, S.; Lertmongkolaksorn, T.; Wongsaroj, L.; Somboonna, N.; Ngamwongsatit, N.; Leelahavanichkul, A. Lactiplantibacillus plantarum dfa1 Outperforms Enterococcus faecium dfa1 on Anti-Obesity in High Fat-Induced Obesity Mice Possibly through the Differences in Gut Dysbiosis Attenuation, despite the Similar Anti-Inflammatory Properties. Nutrients 2021, 14, 80. [Google Scholar] [CrossRef]
- Divyashri, G.; Krishna, G.; Muralidhara; Prapulla, S.G. Probiotic attributes, antioxidant, anti-inflammatory and neuromodulatory effects of Enterococcus faecium CFR 3003: In vitro and in vivo evidence. J. Med. Microbiol. 2015, 64, 1527–1540. [Google Scholar] [CrossRef]
- Lee, J.; D’aigle, J.; Atadja, L.; Quaicoe, V.; Honarpisheh, P.; Ganesh, B.P.; Hassan, A.; Graf, J.; Petrosino, J.F.; Putluri, N.; et al. Gut Microbiota-Derived Short-Chain Fatty Acids Promote Poststroke Recovery in Aged Mice. Circ. Res. 2020, 127, 453–465. [Google Scholar] [CrossRef]
- National Research Council. Guía Para el Cuidado y Uso de Animales de Laboratorio; Ediciones UC: Santiago, Chile, 2017; ISBN-13: 978-9561421080.
- Diario Oficial de la Federación. Norma Oficial Mexicana NOM-062-ZOO-1999. Especificaciones Técnicas para la Producción, Cuidado y uso de los Animales de Laboratorio. México. 2001. Available online: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.gob.mx/cms/uploads/attachment/file/203498/NOM-062-ZOO-1999_220801.pdf (accessed on 8 November 2023).






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Martínez, Y.; Aguilar-Ponce, L.; Romo-Araiza, A.; Chávez-Guerra, A.; Martiñón, S.; Ibarra-García, A.P.; Arias-Santiago, S.; Gálvez-Susano, V.; Ibarra, A. Supplementation with a Symbiotic Induced Neuroprotection and Improved Memory in Rats with Ischemic Stroke. Biomedicines 2024, 12, 209. https://doi.org/10.3390/biomedicines12010209
Cruz-Martínez Y, Aguilar-Ponce L, Romo-Araiza A, Chávez-Guerra A, Martiñón S, Ibarra-García AP, Arias-Santiago S, Gálvez-Susano V, Ibarra A. Supplementation with a Symbiotic Induced Neuroprotection and Improved Memory in Rats with Ischemic Stroke. Biomedicines. 2024; 12(1):209. https://doi.org/10.3390/biomedicines12010209
Chicago/Turabian StyleCruz-Martínez, Yolanda, Leslie Aguilar-Ponce, Alejandra Romo-Araiza, Almudena Chávez-Guerra, Susana Martiñón, Andrea P. Ibarra-García, Stella Arias-Santiago, Vanessa Gálvez-Susano, and Antonio Ibarra. 2024. "Supplementation with a Symbiotic Induced Neuroprotection and Improved Memory in Rats with Ischemic Stroke" Biomedicines 12, no. 1: 209. https://doi.org/10.3390/biomedicines12010209
APA StyleCruz-Martínez, Y., Aguilar-Ponce, L., Romo-Araiza, A., Chávez-Guerra, A., Martiñón, S., Ibarra-García, A. P., Arias-Santiago, S., Gálvez-Susano, V., & Ibarra, A. (2024). Supplementation with a Symbiotic Induced Neuroprotection and Improved Memory in Rats with Ischemic Stroke. Biomedicines, 12(1), 209. https://doi.org/10.3390/biomedicines12010209