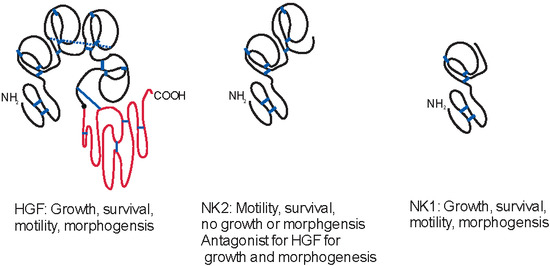
Hepatocyte Growth Factor Isoforms in Tissue Repair, Cancer, and Fibrotic Remodeling
Abstract
:1. Introduction
2. Hepatocyte Growth Factor (HGF) Isoforms


3. Regulation of Expression of HGF and Its Isoforms
4. Cellular Signaling by HGF and Its Truncated Isoforms
5. Biological Functions of HGF and Its Isoforms
5.1. HGF Isoforms during Development
5.2. Role of HGF Isoforms for Tissue Homeostasis and Repair
5.3. HGF Isoforms in Cancer
6. Clinical Applications of HGF Isoforms
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gohda, E.; Tsubouchi, H.; Nakayama, H.; Hirono, S.; Sakiyama, O.; Takahashi, K.; Miyazaki, H.; Hashimoto, S.; Daikuhara, Y. Purification and partial characterization of hepatocyte growth factor from plasma of a patient with fulminant hepatic failure. J. Clin. Investig. 1988, 81, 414–419. [Google Scholar] [PubMed]
- Zarnegar, R.; Michalopoulos, G. Purification and biological characterization of human hepatopoietin A, a polypeptide growth factor for hepatocytes. Cancer Res. 1989, 49, 3314–3320. [Google Scholar]
- Nakamura, T.; Nawa, K.; Ichihara, A.; Kaise, N.; Nishino, T. Purification and subunit structure of hepatocyte growth factor from rat platelets. FEBS Lett. 1987, 224, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Rubin, J.S.; Chan, A.M.; Bottaro, D.P.; Burgess, W.H.; Taylor, W.G.; Cech, A.C.; Hirschfield, D.W.; Wong, J.; Miki, T.; Finch, P.W.; et al. A broad-spectrum human lung fibroblast-derived mitogen is a variant of hepatocyte growth factor. Proc. Natl. Acad. Sci. USA 1991, 88, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Mizuno, S. The discovery of hepatocyte growth factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proc. Jpn. Acad. B 2010, 86, 588–610. [Google Scholar] [CrossRef] [PubMed]
- Benkhoucha, M.; Santiago-Raber, M.L.; Schneiter, G.; Chofflon, M.; Funakoshi, H.; Nakamura, T.; Lalive, P.H. Hepatocyte growth factor inhibits CNS autoimmunity by inducing tolerogenic dendritic cells and CD25+FOXP3+ regulatory T cells. Proc. Natl. Acad. Sci. USA 2010, 107, 6424–6429. [Google Scholar] [CrossRef] [PubMed]
- Hickey, D.G.; Frenkel, S.R.; di Cesare, P.E. Clinical applications of growth factors for articular cartilage repair. Am. J. Orthop. 2003, 32, 70–76. [Google Scholar] [PubMed]
- Stoker, M. Effect of scatter factor on motility of epithelial cells and fibroblasts. J. Cell. Physiol. 1989, 139, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Nakamura, T. Hepatocyte growth factor (HGF) as a tissue organizer for organogenesis and regeneration. Biochem. Biophys. Res. Commun. 1997, 239, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Goke, M.; Kanai, M.; Podolsky, D.K. Intestinal fibroblasts regulate intestinal epithelial cell proliferation via hepatocyte growth factor. Am. J. Physiol. 1998, 274, G809–G818. [Google Scholar] [PubMed]
- Tabata, M.J.; Kim, K.; Liu, J.G.; Yamashita, K.; Matsumura, T.; Kato, J.; Iwamoto, M.; Wakisaka, S.; Matsumoto, K.; Nakamura, T.; et al. Hepatocyte growth factor is involved in the morphogenesis of tooth germ in murine molars. Development 1996, 122, 1243–1251. [Google Scholar] [PubMed]
- Hamanoue, M.; Takemoto, N.; Matsumoto, K.; Nakamura, T.; Nakajima, K.; Kohsaka, S. Neurotrophic effect of hepatocyte growth factor on central nervous system neurons in vitro. J. Neurosci. Res. 1996, 43, 554–564. [Google Scholar]
- Matsumoto, K.; Date, K.; Ohmichi, H.; Nakamura, T. Hepatocyte growth factor in lung morphogenesis and tumor invasion: Role as a mediator in epithelium-mesenchyme and tumor-stroma interactions. Cancer Chemother. Pharmacol. 1996, 38, S42–S47. [Google Scholar] [CrossRef] [PubMed]
- Montesano, R.; Matsumoto, K.; Nakamura, T.; Orci, L. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell 1991, 67, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Bladt, F.; Goedecke, S.; Brinkmann, V.; Zschiesche, W.; Sharpe, M.; Gherardi, E.; Birchmeier, C. Scatter factor/hepatocyte growth factor is essential for liver development. Nature 1995, 373, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Takayama, H.; la Rochelle, W.J.; Anver, M.; Bockman, D.E.; Merlino, G. Scatter factor/hepatocyte growth factor as a regulator of skeletal muscle and neural crest development. Proc. Natl. Acad. Sci. USA 1996, 93, 5866–5871. [Google Scholar] [CrossRef] [PubMed]
- Santos, O.F.; Barros, E.J.; Yang, X.M.; Matsumoto, K.; Nakamura, T.; Park, M.; Nigam, S.K. Involvement of hepatocyte growth factor in kidney development. Dev. Biol. 1994, 163, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Sonnenberg, E.; Meyer, D.; Weidner, K.M.; Birchmeier, C. Scatter factor/hepatocyte growth factor and its receptor, the c-Met tyrosine kinase, can mediate a signal exchange between mesenchyme and epithelia during mouse development. J. Cell Biol. 1993, 123, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Maina, F.; Casagranda, F.; Audero, E.; Simeone, A.; Comoglio, P.M.; Klein, R.; Ponzetto, C. Uncoupling of GRB2 from the Met receptor in vivo reveals complex roles in muscle development. Cell 1996, 87, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Birchmeier, C.; Gherardi, E. Developmental roles of HGF/SF and its receptor, the c-Met tyrosine kinase. Trends Cell Biol. 1998, 8, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Bottaro, D.P.; Rubin, J.S.; Faletto, D.L.; Chan, A.M.; Kmiecik, T.E.; vande Woude, G.F.; Aaronson, S.A. Identification of the hepatocyte growth factor receptor as the c-Met proto-oncogene product. Science 1991, 251, 802–804. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Dean, M.; Kaul, K.; Braun, M.J.; Gonda, M.A.; vande Woude, G. Sequence of Met protooncogene cDNA has features characteristic of the tyrosine kinase family of growth-factor receptors. Proc. Natl. Acad. Sci. USA 1987, 84, 6379–6383. [Google Scholar] [CrossRef] [PubMed]
- Zachow, R.; Uzumcu, M. The hepatocyte growth factor system as a regulator of female and male gonadal function. J. Endocrinol. 2007, 195, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, S.; Matsumoto, K.; Nakamura, T. HGF as a renotrophic and anti-fibrotic regulator in chronic renal disease. Front. Biosci. 2008, 13, 7072–7086. [Google Scholar] [CrossRef] [PubMed]
- Madonna, R.; Rokosh, G.; de Caterina, R.; Bolli, R. Hepatocyte growth factor/Met gene transfer in cardiac stem cells—Potential for cardiac repair. Basic Res. Cardiol. 2010, 105, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D.; Wuenschell, C.; Flores-Delgado, G.; Anderson, K. Commitment and differentiation of lung cell lineages. Biochem. Cell Biol. 1998, 76, 971–995. [Google Scholar] [CrossRef] [PubMed]
- Kasai, S.; Sugimura, K.; Matsumoto, K.; Nishi, N.; Kishimoto, T.; Nakamura, T. Hepatocyte growth factor is a paracrine regulator of rat prostate epithelial growth. Biochem. Biophys. Res. Commun. 1996, 228, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Tajima, H.; Nakamura, T. Hepatocyte growth factor is a potent stimulator of human melanocyte DNA synthesis and growth. Biochem. Biophys. Res. Commun. 1991, 176, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.M.; Park, M. Expression of the hepatocyte growth factor/scatter factor receptor tyrosine kinase is localized to epithelia in the adult mouse. Lab. Investig. 1995, 73, 483–491. [Google Scholar] [PubMed]
- Nakamura, Y.; Morishita, R.; Higaki, J.; Kida, I.; Aoki, M.; Moriguchi, A.; Yamada, K.; Hayashi, S.; Yo, Y.; Nakano, H.; et al. Hepatocyte growth factor is a novel member of the endothelium-specific growth factors: Additive stimulatory effect of hepatocyte growth factor with basic fibroblast growth factor but not with vascular endothelial growth factor. J. Hypertens. 1996, 14, 1067–1072. [Google Scholar] [PubMed]
- Hayashi, S.; Morishita, R.; Higaki, J.; Aoki, M.; Moriguchi, A.; Kida, I.; Yoshiki, S.; Matsumoto, K.; Nakamura, T.; Kaneda, Y.; et al. Autocrine-paracrine effects of over-expression of hepatocyte growth factor gene on growth of endothelial cells. Biochem. Biophys. Res. Commun. 1996, 220, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Nishizawa, T.; Hagiya, M.; Seki, T.; Shimonishi, M.; Sugimura, A.; Tashiro, K.; Shimizu, S. Molecular cloning and expression of human hepatocyte growth factor. Nature 1989, 342, 440–443. [Google Scholar] [PubMed]
- Donate, L.E.; Gherardi, E.; Srinivasan, N.; Sowdhamini, R.; Aparicio, S.; Blundell, T.L. Molecular evolution and domain structure of plasminogen-related growth factors (HGF/SF and HGF1/MSP). Protein Sci. 1994, 3, 2378–2394. [Google Scholar] [PubMed]
- Chan, A.M.; Rubin, J.S.; Bottaro, D.P.; Hirschfield, D.W.; Chedid, M.; Aaronson, S.A. Identification of a competitive HGF antagonist encoded by an alternative transcript. Science 1991, 254, 1382–1385. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, K.; Kitamura, A.; Naka, D.; Kitamura, N. An alternatively processed mRNA generated from human hepatocyte growth factor gene. Eur. J. Biochem. 1991, 197, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Cioce, V.; Csaky, K.G.; Chan, A.M.; Bottaro, D.P.; Taylor, W.G.; Jensen, R.; Aaronson, S.A.; Rubin, J.S. Hepatocyte growth factor (HGF)/NK1 is a naturally occurring HGF/scatter factor variant with partial agonist/antagonist activity. J. Biol. Chem. 1996, 271, 13110–13115. [Google Scholar] [CrossRef] [PubMed]
- Jakubczak, J.L.; LaRochelle, W.J.; Merlino, G. NK1, a natural splice variant of hepatocyte growth factor/scatter factor, is a partial agonist in vivo. Mol. Cell. Biol. 1998, 18, 1275–1283. [Google Scholar] [PubMed]
- Lokker, N.A.; Godowski, P.J. Generation and characterization of a competitive antagonist of human hepatocyte growth factor, HGF/NK1. J. Biol. Chem. 1993, 268, 17145–17150. [Google Scholar] [PubMed]
- Lokker, N.A.; Mark, M.R.; Luis, E.A.; Bennett, G.L.; Robbins, K.A.; Baker, J.B.; Godowski, P.J. Structure-function analysis of hepatocyte growth factor: Identification of variants that lack mitogenic activity yet retain high affinity receptor binding. EMBO J. 1992, 11, 2503–2510. [Google Scholar]
- Skeel, A.; Yoshimura, T.; Showalter, S.D.; Tanaka, S.; Appella, E.; Leonard, E.J. Macrophage stimulating protein: Purification, partial amino acid sequence, and cellular activity. J. Exp. Med. 1991, 173, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information Genbank. Available online: http://www.Ncbi.Nlm.Nih.Gov/Genbank/ (accessed on 31 October 2014).
- National Center for Biotechnology Information Gnomon, Eukaryotic Gene Prediction Tool. Available online: http://www.Ncbi.Nlm.Nih.Gov/Genome/Guide/Gnomon.Shtml (accessed on 31 October 2014).
- Basic Local Alignment Search Tool (Blast). Available online: http://Blast.Ncbi.Nlm.Nih.Gov/Blast.Cgi (accessed on 31 October 2014).
- Mungunsukh, O.; Day, R.M. Transforming growth factor-beta1 selectively inhibits hepatocyte growth factor expression via a micro-RNA-199-dependent posttranscriptional mechanism. Mol. Biol. Cell 2013, 24, 2088–2097. [Google Scholar] [CrossRef] [PubMed]
- European Molecular Biology Laboratory—European Bioinformatics Institute Clustal Omega Program for Multiple Sequence Alignment. Available online: http://www.Ebi.Ac.Uk/Tools/Msa/Clustalo/ (accessed on 31 October 2014).
- Miyazawa, K.; Kitamura, A.; Kitamura, N. Structural organization and the transcription initiation site of the human hepatocyte growth factor gene. Biochemistry 1991, 30, 9170–9176. [Google Scholar] [CrossRef] [PubMed]
- Okajima, A.; Miyazawa, K.; Kitamura, N. Characterization of the promoter region of the rat hepatocyte-growth-factor/scatter-factor gene. Eur. J. Biochem. 1993, 213, 113–119. [Google Scholar] [CrossRef]
- Bell, A.W.; Jiang, J.G.; Chen, Q.; Liu, Y.; Zarnegar, R. The upstream regulatory regions of the hepatocyte growth factor gene promoter are essential for its expression in transgenic mice. J. Biol. Chem. 1998, 273, 6900–6908. [Google Scholar] [CrossRef] [PubMed]
- Gohda, E.; Matsunaga, T.; Kataoka, H.; Takebe, T.; Yamamoto, I. Induction of hepatocyte growth factor in human skin fibroblasts by epidermal growth factor, platelet-derived growth factor and fibroblast growth factor. Cytokine 1994, 6, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Tajima, H.; Okazaki, H.; Nakamura, T. Negative regulation of hepatocyte growth factor gene expression in human lung fibroblasts and leukemic cells by transforming growth factor-beta 1 and glucocorticoids. J. Biol. Chem. 1992, 267, 24917–24920. [Google Scholar] [PubMed]
- Matsumoto, K.; Okazaki, H.; Nakamura, T. Up-regulation of hepatocyte growth factor gene expression by interleukin-1 in human skin fibroblasts. Biochem. Biophys. Res. Commun. 1992, 188, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Tajima, H.; Hamanoue, M.; Kohno, S.; Kinoshita, T.; Nakamura, T. Identification and characterization of “injurin”, an inducer of expression of the gene for hepatocyte growth factor. Proc. Natl. Acad. Sci. USA 1992, 89, 3800–3804. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Teramoto, H.; Uetani, K.; Igawa, K.; Shimizu, E. Stretch induces a growth factor in alveolar cells via protein kinase. Respir. Physiol. 2001, 127, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Michalopoulos, G.K.; Zarnegar, R. Structural and functional characterization of the mouse hepatocyte growth factor gene promoter. J. Biol. Chem. 1994, 269, 4152–4160. [Google Scholar] [PubMed]
- Jiang, J.G.; Johnson, C.; Zarnegar, R. Peroxisome proliferator-activated receptor gamma-mediated transcriptional up-regulation of the hepatocyte growth factor gene promoter via a novel composite cis-acting element. J. Biol. Chem. 2001, 276, 25049–25056. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.G.; Zarnegar, R. A novel transcriptional regulatory region within the core promoter of the hepatocyte growth factor gene is responsible for its inducibility by cytokines via the C/EBP family of transcription factors. Mol. Cell. Biol. 1997, 17, 5758–5770. [Google Scholar] [PubMed]
- Jiang, J.G.; Chen, Q.; Bell, A.; Zarnegar, R. Transcriptional regulation of the hepatocyte growth factor (HGF) gene by the SP family of transcription factors. Oncogene 1997, 14, 3039–3049. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.G.; deFrances, M.C.; Machen, J.; Johnson, C.; Zarnegar, R. The repressive function of AP2 transcription factor on the hepatocyte growth factor gene promoter. Biochem. Biophys. Res. Commun. 2000, 272, 882–886. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; deFrances, M.C.; Zou, C.; Johnson, C.; Ferrell, R.; Zarnegar, R. Somatic mutation and functional polymorphism of a novel regulatory element in the HGF gene promoter causes its aberrant expression in human breast cancer. J. Clin. Investig. 2009, 119, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Flann, K.L.; Rathbone, C.R.; Cole, L.C.; Liu, X.; Allen, R.E.; Rhoads, R.P. Hypoxia simultaneously alters satellite cell-mediated angiogenesis and hepatocyte growth factor expression. J. Cell. Physiol. 2014, 229, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.; Bradley, L.; Bomford, A. Mechanism of regulation of HGF/SF gene expression in fibroblasts by TGF-beta1. Biochem. Biophys. Res. Commun. 2000, 271, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Rubin, J.S.; Bottaro, D.P.; Aaronson, S.A. Hepatocyte growth factor/scatter factor and its receptor, the c-Met proto-oncogene product. Biochim. Biophys. Acta 1993, 1155, 357–371. [Google Scholar] [PubMed]
- Brinkmann, V.; Foroutan, H.; Sachs, M.; Weidner, K.M.; Birchmeier, W. Hepatocyte growth factor/scatter factor induces a variety of tissue-specific morphogenic programs in epithelial cells. J. Cell Biol. 1995, 131, 1573–1586. [Google Scholar] [CrossRef] [PubMed]
- Giordano, S.; Bardelli, A.; Zhen, Z.; Menard, S.; Ponzetto, C.; Comoglio, P.M. A point mutation in the Met oncogene abrogates metastasis without affecting transformation. Proc. Natl. Acad. Sci. USA 1997, 94, 13868–13872. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Naujokas, M.A.; Fixman, E.D.; Torossian, K.; Park, M. Tyrosine 1356 in the carboxyl-terminal tail of the HGF/SF receptor is essential for the transduction of signals for cell motility and morphogenesis. J. Biol. Chem. 1994, 269, 29943–29948. [Google Scholar] [PubMed]
- Ponzetto, C.; Bardelli, A.; Zhen, Z.; Maina, F.; dalla Zonca, P.; Giordano, S.; Graziani, A.; Panayotou, G.; Comoglio, P.M. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell 1994, 77, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Weidner, K.M.; Sachs, M.; Riethmacher, D.; Birchmeier, W. Mutation of juxtamembrane tyrosine residue 1001 suppresses loss-of-function mutations of the Met receptor in epithelial cells. Proc. Natl. Acad. Sci. USA 1995, 92, 2597–2601. [Google Scholar] [CrossRef] [PubMed]
- Furge, K.A.; Zhang, Y.W.; vande Woude, G.F. Met receptor tyrosine kinase: Enhanced signaling through adapter proteins. Oncogene 2000, 19, 5582–5589. [Google Scholar] [CrossRef] [PubMed]
- Graziani, A.; Gramaglia, D.; Cantley, L.C.; Comoglio, P.M. The tyrosine-phosphorylated hepatocyte growth factor/scatter factor receptor associates with phosphatidylinositol 3-kinase. J. Biol. Chem. 1991, 266, 22087–22090. [Google Scholar] [PubMed]
- Sakkab, D.; Lewitzky, M.; Posern, G.; Schaeper, U.; Sachs, M.; Birchmeier, W.; Feller, S.M. Signaling of hepatocyte growth factor/scatter factor (HGF) to the small GTPase Rap1 via the large docking Protein Gab1 and the adapter protein CRKL. J. Biol. Chem. 2000, 275, 10772–10778. [Google Scholar] [CrossRef] [PubMed]
- Riordan, S.M.; Lidder, S.; Williams, R.; Skouteris, G.G. The beta-subunit of the hepatocyte growth factor/scatter factor (HGF/SF) receptor phosphorylates and associates with CrkII: Expression of CrkII enhances HGF/SF-induced mitogenesis. Biochem. J. 2000, 350, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Chen, Z.; Li, Z.Y.; Yeh, N.T.; Bancroft, C.C.; van Waes, C. Hepatocyte growth factor/scatter factor-induced activation of MEK and PI3K signal pathways contributes to expression of proangiogenic cytokines interleukin-8 and vascular endothelial growth factor in head and neck squamous cell carcinoma. Cancer Res. 2001, 61, 5911–5918. [Google Scholar] [PubMed]
- Kodama, A.; Matozaki, T.; Fukuhara, A.; Kikyo, M.; Ichihashi, M.; Takai, Y. Involvement of an SHP-2-Rho small G protein pathway in hepatocyte growth factor/scatter factor-induced cell Scattering. Mol. Biol. Cell 2000, 11, 2565–2575. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, G.; Weidner, K.M.; Schwarz, H.; Birchmeier, W. The motility signal of scatter factor/hepatocyte growth factor mediated through the receptor tyrosine kinase Met requires intracellular action of Ras. J. Biol. Chem. 1994, 269, 21936–21939. [Google Scholar] [PubMed]
- Royal, I.; Lamarche-Vane, N.; Lamorte, L.; Kaibuchi, K.; Park, M. Activation of cdc42, rac, PAK, and rho-kinase in response to hepatocyte growth factor differentially regulates epithelial cell colony spreading and dissociation. Mol. Biol. Cell 2000, 11, 1709–1725. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, N.; Tremblay, E.; Elliott, B. Phosphatidylinositol 3-kinase activity is required for hepatocyte growth factor-induced mitogenic signals in epithelial cells. J. Biol. Chem. 1996, 271, 24850–24855. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, N.; Hung, W.; Tremblay, E.; Saulnier, R.; Elliott, B. c-Src kinase activity is required for hepatocyte growth factor-induced motility and anchorage-independent growth of mammary carcinoma cells. J. Biol. Chem. 1998, 273, 33714–33721. [Google Scholar] [CrossRef] [PubMed]
- Nakagami, H.; Morishita, R.; Yamamoto, K.; Taniyama, Y.; Aoki, M.; Kim, S.; Matsumoto, K.; Nakamura, T.; Higaki, J.; Ogihara, T. Anti-apoptotic action of hepatocyte growth factor through mitogen-activated protein kinase on human aortic endothelial cells. J. Hypertens. 2000, 18, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Royal, I.; Fournier, T.M.; Park, M. Differential requirement of Grb2 and PI3-Kinase in HGF/SF-induced cell motility and tubulogenesis. J. Cell. Physiol. 1997, 173, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Ma, Y.X.; Wang, J.A.; Yuan, R.Q.; Meng, Q.; Cao, Y.; Laterra, J.J.; Goldberg, I.D.; Rosen, E.M. The cytokine hepatocyte growth factor/scatter factor inhibits apoptosis and enhances DNA repair by a common mechanism involving signaling through phosphatidyl inositol 3' kinase. Oncogene 2000, 19, 2212–2223. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, Y.; Kim, H.P.; Song, R.; Zarnegar, R.; Ryter, S.W.; Choi, A.M. Hepatocyte growth factor protects against hypoxia/reoxygenation-induced apoptosis in endothelial cells. J. Biol. Chem. 2004, 279, 5237–5243. [Google Scholar] [CrossRef] [PubMed]
- Mildner, M.; Eckhart, L.; Lengauer, B.; Tschachler, E. Hepatocyte growth factor/scatter factor inhibits UVb-induced apoptosis of human keratinocytes but not of keratinocyte-derived cell lines via the phosphatidylinositol 3-kinase/Akt pathway. J. Biol. Chem. 2002, 277, 14146–14152. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Hayashida, M.; Kawano, H.; Sugimoto, K.; Nakano, T.; Shiraki, K. Hepatocyte growth factor promotes cell survival from Fas-mediated cell death in hepatocellular carcinoma cells via Akt activation and Fas-death-inducing signaling complex suppression. Hepatology 2000, 32, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Marquez, A.P.; Mungunsukh, O.; Day, R.M. Hepatocyte growth factor inhibits apoptosis by the profibrotic factor angiotensin II via extracellular signal-regulated kinase 1/2 in endothelial cells and tissue explants. Mol. Biol. Cell 2010, 21, 4240–4250. [Google Scholar] [PubMed]
- Stahl, S.J.; Wingfield, P.T.; Kaufman, J.D.; Pannell, L.K.; Cioce, V.; Sakata, H.; Taylor, W.G.; Rubin, J.S.; Bottaro, D.P. Functional and biophysical characterization of recombinant human hepatocyte growth factor isoforms produced in Escherichia coli. Biochem. J. 1997, 326, 763–772. [Google Scholar]
- Day, R.M.; Cioce, V.; Breckenridge, D.; Castagnino, P.; Bottaro, D.P. Differential signaling by Alternative HGF isoforms through c-Met: Activation of both MAP kinase and PI 3-kinase pathways is insufficient for mitogenesis. Oncogene 1999, 18, 3399–3406. [Google Scholar] [CrossRef] [PubMed]
- Montesano, R.; Soriano, J.V.; Malinda, K.M.; Ponce, M.L.; Bafico, A.; Kleinman, H.K.; Bottaro, D.P.; Aaronson, S.A. Differential effects of hepatocyte growth factor isoforms on epithelial and endothelial tubulogenesis. Cell Growth Differ. 1998, 9, 355–365. [Google Scholar] [PubMed]
- Jones, D.S., 2nd; Tsai, P.C.; Cochran, J.R. Engineering hepatocyte growth factor fragments with high stability and activity as Met receptor agonists and antagonists. Proc. Natl. Acad. Sci. USA 2011, 108, 13035–13040. [Google Scholar] [CrossRef] [PubMed]
- Rubin, J.S.; Day, R.M.; Breckenridge, D.; Atabey, N.; Taylor, W.G.; Stahl, S.J.; Wingfield, P.T.; Kaufman, J.D.; Schwall, R.; Bottaro, D.P. Dissociation of heparan sulfate and receptor binding eomains of hepatocyte growth factor reveals that heparan sulfate-c-Met interaction facilitates signaling. J. Biol. Chem. 2001, 276, 32977–32983. [Google Scholar] [CrossRef] [PubMed]
- Schwall, R.H.; Chang, L.Y.; Godowski, P.J.; Kahn, D.W.; Hillan, K.J.; Bauer, K.D.; Zioncheck, T.F. Heparin induces dimerization and confers proliferative activity onto the hepatocyte growth factor antagonists NK1 and NK2. J. Cell Biol. 1996, 133, 709–718. [Google Scholar] [PubMed]
- Sakata, H.; Stahl, S.J.; Taylor, W.G.; Rosenberg, J.M.; Sakaguchi, K.; Wingfield, P.T.; Rubin, J.S. Heparin binding and oligomerization of hepatocyte growth factor/scatter factor isoforms. Heparan sulfate glycosaminoglycan requirement for Met binding and signaling. J. Biol. Chem. 1997, 272, 9457–9463. [Google Scholar] [CrossRef] [PubMed]
- Tolbert, W.D.; Daugherty-Holtrop, J.; Gherardi, E.; vande Woude, G.; Xu, H.E. Structural basis for agonism and antagonism of hepatocyte growth factor. Proc. Natl. Acad. Sci. USA 2010, 107, 13264–13269. [Google Scholar] [CrossRef] [PubMed]
- Pavone, L.M.; Cattaneo, F.; Rea, S.; de Pasquale, V.; Spina, A.; Sauchelli, E.; Mastellone, V.; Ammendola, R. Intracellular signaling cascades triggered by the NK1 fragment of hepatocyte growth factor in human prostate epithelial cell line PNT1A. Cell. Signal. 2011, 23, 1961–1971. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, T.; Jakubczak, J.; Vieira, W.; Bottaro, D.P.; Breckenridge, D.; Larochelle, W.J.; Merlino, G. Disassociation of Met-mediated biological responses in vivo: The natural hepatocyte growth factor/scatter factor splice variant NK2 antagonizes growth but facilitates metastasis. Mol. Cell. Biol. 2000, 20, 2055–2065. [Google Scholar] [CrossRef] [PubMed]
- Wolf, H.K.; Zarnegar, R.; Oliver, L.; Michalopoulos, G.K. Hepatocyte growth factor in human placenta and trophoblastic disease. Am. J. Pathol. 1991, 138, 1035–1043. [Google Scholar] [PubMed]
- Woolf, A.S.; Kolatsi-Joannou, M.; Hardman, P.; Andermarcher, E.; Moorby, C.; Fine, L.G.; Jat, P.S.; Noble, M.D.; Gherardi, E. Roles of hepatocyte growth factor/scatter factor and the Met receptor in the early development of the metanephros. J. Cell Biol. 1995, 128, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Maina, F.; Hilton, M.C.; Ponzetto, C.; Davies, A.M.; Klein, R. Met receptor signaling is required for sensory nerve development and HGF promotes axonal growth and survival of sensory neurons. Genes Dev. 1997, 11, 3341–3350. [Google Scholar] [CrossRef] [PubMed]
- Takebayashi, T.; Iwamoto, M.; Jikko, A.; Matsumura, T.; Enomoto-Iwamoto, M.; Myoukai, F.; Koyama, E.; Yamaai, T.; Matsumoto, K.; Nakamura, T.; et al. Hepatocyte growth factor/scatter factor modulates cell motility, proliferation, and proteoglycan synthesis of chondrocytes. J. Cell Biol. 1995, 129, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Bladt, F.; Riethmacher, D.; Isenmann, S.; Aguzzi, A.; Birchmeier, C. Essential role for the c-Met receptor in the migration of myogenic precursor cells into the limb bud. Nature 1995, 376, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Chuva de Sousa Lopes, S.M.; Mummery, C.I. Differentiation in early development. In Essentials of Stem Cell Biology, 2nd ed.; Lanza, R., Ed.; Academic Press: San Diego, CA, USA, 2009; pp. 119–130. [Google Scholar]
- Defrances, M.C.; Wolf, H.K.; Michalopoulos, G.K.; Zarnegar, R. The presence of hepatocyte growth factor in the developing rat. Development 1992, 116, 387–395. [Google Scholar] [PubMed]
- Lindsey, J.S.; Brenner, R.M. Novel hepatocyte growth factor/scatter factor isoform transcripts in the macaque endometrium and placenta. Mol. Hum. Reprod. 2002, 8, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Kitta, K.; Day, R.M.; Ikeda, T.; Suzuki, Y.J. Hepatocyte growth factor protects cardiac myocytes against oxidative stress-induced apoptosis. Free Radic. Biol. Med. 2001, 31, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Sugita, K.; Inukai, T.; Goi, K.; Kagami, K.; Kawasaki, K.; Nakazawa, S. Hepatocyte growth factor protects small airway epithelial cells from apoptosis induced by tumor necrosis factor-alpha or oxidative stress. Pediatr. Res. 2004, 56, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Hepatocyte growth factor promotes renal epithelial cell survival by dual mechanisms. Am. J. Physiol. 1999, 277, F624–F633. [Google Scholar] [PubMed]
- Bussolino, F.; di Renzo, M.F.; Ziche, M.; Bocchietto, E.; Olivero, M.; Naldini, L.; Gaudino, G.; Tamagnone, L.; Coffer, A.; Comoglio, P.M. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J. Cell Biol. 1992, 119, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Van Adelsberg, J.; Sehgal, S.; Kukes, A.; Brady, C.; Barasch, J.; Yang, J.; Huan, Y. Activation of hepatocyte growth factor (HGF) by endogenous HGF activator is required for metanephric kidney morphogenesis in vitro. J. Biol. Chem. 2001, 276, 15099–15106. [Google Scholar] [CrossRef] [PubMed]
- Stolz, D.B.; Michalopoulos, G.K. Comparative effects of hepatocyte growth factor and epidermal growth factor on motility, morphology, mitogenesis, and signal transduction of primary rat hepatocytes. J. Cell. Biochem. 1994, 55, 445–464. [Google Scholar] [CrossRef] [PubMed]
- Singh-Kaw, P.; Zarnegar, R.; Siegfried, J.M. Stimulatory effects of hepatocyte growth factor on normal and neoplastic human bronchial epithelial cells. Am. J. Physiol. 1995, 268, L1012–L1020. [Google Scholar] [PubMed]
- Mason, R.J.; McCormick-Shannon, K.; Rubin, J.S.; Nakamura, T.; Leslie, C.C. Hepatocyte growth factor is a mitogen for alveolar type II cells in rat lavage fluid. Am. J. Physiol. 1996, 271, L46–L53. [Google Scholar] [PubMed]
- Liu, Y.; Sun, A.M.; Dworkin, L.D. Hepatocyte growth factor protects renal epithelial cells from apoptotic cell death. Biochem. Biophys. Res. Commun. 1998, 246, 821–826. [Google Scholar] [CrossRef]
- Yo, Y.; Morishita, R.; Yamamoto, K.; Tomita, N.; Kida, I.; Hayashi, S.; Moriguchi, A.; Kato, S.; Matsumoto, K.; Nakamura, T.; et al. Actions of hepatocyte growth factor as a local modulator in the kidney: Potential role in pathogenesis of renal disease. Kidney Int. 1998, 53, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, S.M.; Allen, R.E. Skeletal muscle satellite cell proliferation in response to members of the fibroblast growth factor family and hepatocyte growth factor. J. Cell. Physiol. 1999, 181, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Q.; Tseng, S.C. Differential regulation of keratinocyte growth factor and hepatocyte growth factor/scatter factor by different cytokines in human corneal and limbal fibroblasts. J. Cell. Physiol. 1997, 172, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Crosby, L.M.; Waters, C.M. Epithelial repair mechanisms in the lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010, 298, L715–L731. [Google Scholar] [CrossRef] [PubMed]
- Skibinski, G.; Elborn, J.S.; Ennis, M. Bronchial epithelial cell growth regulation in fibroblast cocultures: The role of hepatocyte growth factor. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 293, L69–L76. [Google Scholar] [CrossRef] [PubMed]
- Trusolino, L.; Bertotti, A.; Comoglio, P.M. Met dignalling: Principles and functions in development, organ regeneration and cancer. Nat. Rev. Mol. Cell Biol. 2010, 11, 834–848. [Google Scholar] [CrossRef] [PubMed]
- Powell, E.M.; Mars, W.M.; Levitt, P. Hepatocyte growth factor/scatter factor is a motogen for interneurons migrating from the ventral to dorsal telencephalon. Neuron 2001, 30, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Zarnegar, R.; deFrances, M.C.; Kost, D.P.; Lindroos, P.; Michalopoulos, G.K. Expression of hepatocyte growth factor mRNA in regenerating rat liver after partial hepatectomy. Biochem. Biophys. Res. Commun. 1991, 177, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Igawa, T.; Matsumoto, K.; Kanda, S.; Saito, Y.; Nakamura, T. Hepatocyte growth factor may function as a renotropic factor for regeneration in rats with acute renal injury. Am. J. Physiol. 1993, 265, F61–F69. [Google Scholar] [PubMed]
- Ueda, H.; Nakamura, T.; Matsumoto, K.; Sawa, Y.; Matsuda, H. A potential cardioprotective role of hepatocyte growth factor in myocardial infarction in rats. Cardiovasc. Res. 2001, 51, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Adamson, I.Y.; Bakowska, J. KGF and HGF are growth factors for mesothelial cells in pleural lavage fluid after intratracheal asbestos. Exp. Lung Res. 2001, 27, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Yanagita, K.; Matsumoto, K.; Sekiguchi, K.; Ishibashi, H.; Niho, Y.; Nakamura, T. Hepatocyte growth factor may act as a pulmotrophic factor on lung regeneration after acute lung injury. J. Biol. Chem. 1993, 268, 21212–21217. [Google Scholar] [PubMed]
- Douglas, D.; Chen, G.; Khalil, N. Increase in the beta chain of hepatocyte growth factor (HGF beta) precedes c-Met expression after bleomycin-induced lung injury in the rat. Exp. Lung Res. 2002, 28, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Matsumoto, K.; Inoue, T.; Nose, T.; Murayama, S.; Teshima, T.; Ozeki, S.; Koizumi, M.; Nakamura, T. Induction of hepatocyte growth factor in the liver, kidney and lung following total body irradiation in rat. Cytokine 1996, 8, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tolbert, E.M.; Lin, L.; Thursby, M.A.; Sun, A.M.; Nakamura, T.; Dworkin, L.D. Up-regulation of hepatocyte growth factor receptor: An amplification and targeting mechanism for hepatocyte growth factor action in acute renal failure. Kidney Int. 1999, 55, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Verghese, G.M.; McCormick-Shannon, K.; Mason, R.J.; Matthay, M.A. Hepatocyte growth factor and keratinocyte growth factor in the pulmonary edema fluid of patients with acute lung injury. Biologic and clinical significance. Am. J. Respir. Crit. Care Med. 1998, 158, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Yoshinouchi, T.; Sakamoto, T.; Fujieda, H.; Murao, S.; Sato, H.; Kobayashi, H.; Ohe, T. Hepatocyte growth factor (HGF): A new biochemical marker for acute myocardial infarction. Heart Vessels 1997, 12, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, S.; Goto, Y.; Sumida, H.; Noguchi, T.; Baba, T.; Miyazaki, S.; Nonogi, H. Angiotensin-converting enzyme inhibition restores hepatocyte growth factor production in patients with congestive heart failure. Hypertension 1999, 33, 1374–1378. [Google Scholar] [CrossRef] [PubMed]
- Yanagita, K.; Nagaike, M.; Ishibashi, H.; Niho, Y.; Matsumoto, K.; Nakamura, T. Lung may have an endocrine function producing hepatocyte growth factor in response to injury of distal organs. Biochem. Biophys. Res. Commun. 1992, 182, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Kawaida, K.; Matsumoto, K.; Shimazu, H.; Nakamura, T. Hepatocyte growth factor prevents scute renal failure and accelerates renal regeneration in mice. Proc. Natl. Acad. Sci. USA 1994, 91, 4357–4361. [Google Scholar] [CrossRef] [PubMed]
- Adamson, I.Y.; Bakowska, J. Relationship of keratinocyte growth factor and hepatocyte growth factor levels in rat lung lavage fluid to epithelial cell regeneration after bleomycin. Am. J. Pathol. 1999, 155, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Ohmichi, H.; Matsumoto, K.; Nakamura, T. In vivo mitogenic action of HGF on lung epithelial cells: Pulmotrophic role in lung regeneration. Am. J. Physiol. 1996, 270, L1031–L1039. [Google Scholar] [PubMed]
- Panos, R.J.; Patel, R.; Bak, P.M. Intratracheal administration of hepatocyte growth factor/scatter factor stimulates rat alveolar type II cell proliferation in vivo. Am. J. Respir. Cell Mol. Biol. 1996, 15, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Burr, A.W.; Toole, K.; Chapman, C.; Hines, J.E.; Burt, A.D. Anti-hepatocyte growth factor antibody inhibits hepatocyte proliferation during liver regeneration. J. Pathol. 1998, 185, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Rajur, K.; Tolbert, E.; Dworkin, L.D. Endogenous hepatocyte growth factor ameliorates chronic renal injury by activating matrix degradation pathways. Kidney Int. 2000, 58, 2028–2043. [Google Scholar] [CrossRef] [PubMed]
- Phaneuf, D.; Moscioni, A.D.; leClair, C.; Raper, S.E.; Wilson, J.M. Generation of a mouse expressing a conditional knockout of the hepatocyte growth factor hene: Demonstration of impaired liver regeneration. DNA Cell Biol. 2004, 23, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Borowiak, M.; Garratt, A.N.; Wustefeld, T.; Strehle, M.; Trautwein, C.; Birchmeier, C. Met provides essential signals for liver regeneration. Proc. Natl. Acad. Sci. USA 2004, 101, 10608–10613. [Google Scholar] [CrossRef] [PubMed]
- Huh, C.G.; Factor, V.M.; Sanchez, A.; Uchida, K.; Conner, E.A.; Thorgeirsson, S.S. Hepatocyte growth factor/c-Met signaling pathway is required for efficient liver regeneration and repair. Proc. Natl. Acad. Sci. USA 2004, 101, 4477–4482. [Google Scholar] [CrossRef] [PubMed]
- Chmielowiec, J.; Borowiak, M.; Morkel, M.; Stradal, T.; Munz, B.; Werner, S.; Wehland, J.; Birchmeier, C.; Birchmeier, W. c-Met is essential for wound healing in the skin. J. Cell Biol. 2007, 177, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Sato, S.; Dai, W.; Yamanaka, N. The protective effect of hepatocyte growth-promoting factor (PHGF) against hydrogen peroxide-induced acute lung injury in rats. Med. Electron Microsc. 2001, 34, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, S.; Matsumoto, K.; Li, M.Y.; Nakamura, T. HGF reduces advancing lung fibrosis in mice: A potential role for MMP-dependent myofibroblast apoptosis. FASEB J. 2005, 19, 580–582. [Google Scholar] [PubMed]
- Okano, J.; Shiota, G.; Kawasaki, H. Protective action of hepatocyte growth factor for acute liver injury caused by d-galactosamine in transgenic mice. Hepatology 1997, 26, 1241–1249. [Google Scholar] [PubMed]
- Yang, J.; Dai, C.; Liu, Y. Hepatocyte growth factor gene therapy and angiotensin II blockade synergistically attenuate renal interstitial fibrosis in mice. J. Am. Soc. Nephrol. 2002, 13, 2464–2477. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Ebina, M.; Orson, F.M.; Nakamura, A.; Kubota, K.; Koinuma, D.; Akiyama, K.; Maemondo, M.; Okouchi, S.; Tahara, M.; et al. Hepatocyte growth factor gene transfer to alveolar septa for effective suppression of lung fibrosis. Mol. Ther. 2005, 12, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, S.; Huang, L.; Michalopoulos, G.K.; Liu, Y. Sustained expression of naked plasmid DNA encoding hepatocyte growth factor in mice promotes liver and overall body growth. Hepatology 2001, 33, 848–859. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, T.; Takagi, H.; Horiguchi, N.; Toyoda, M.; Sato, K.; Takayama, H.; Mori, M. CCL4-induced acute liver injury in mice is inhibited by hepatocyte growth factor over-expression but stimulated by NK2 over-expression. FEBS Lett. 2002, 532, 391–395. [Google Scholar] [CrossRef]
- Umeda, Y.; Marui, T.; Matsuno, Y.; Shirahashi, K.; Iwata, H.; Takagi, H.; Matsumoto, K.; Nakamura, T.; Kosugi, A.; Mori, Y.; et al. Skeletal muscle targeting in vivo electroporation-mediated HGF gene therapy of bleomycin-induced pulmonary fibrosis in mice. Lab. Investig. 2004, 84, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, M.; Takayama, H.; Horiguchi, N.; Otsuka, T.; Fukusato, T.; Merlino, G.; Takagi, H.; Mori, M. Overexpression of hepatocyte growth factor/scatter factor promotes vascularization and granulation tissue formation in vivo. FEBS Lett. 2001, 509, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Takahara, T.; Yata, Y.; Kuwabara, Y.; Shinno, E.; Nonome, K.; Minemura, M.; Takahara, S.; Li, X.; Yamato, E.; et al. Hepatocyte growth factor gene therapy accelerates regeneration in cirrhotic mouse livers after hepatectomy. Gut 2003, 52, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Bevan, D.; Gherardi, E.; Fan, T.P.; Edwards, D.; Warn, R. Diverse and potent activities of HGF/SF in skin wound repair. J. Pathol. 2004, 203, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Uenoyama, M.; Tomita, N.; Morishita, R.; Kaneda, Y.; Ogihara, T.; Matsumoto, K.; Nakamura, T.; Maruta, A.; Matsuyama, S.; et al. Gene transfer of human hepatocyte growth factor into rat skin wounds mediated by liposomes coated with the Sendai virus (Hemagglutinating Virus of Japan). Am. J. Pathol. 2002, 161, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Ono, I.; Yamashita, T.; Hida, T.; Jin, H.Y.; Ito, Y.; Hamada, H.; Akasaka, Y.; Ishii, T.; Jimbow, K. Local administration of hepatocyte growth factor gene enhances the regeneration of dermis in acute incisional wounds. J. Surg. Res. 2004, 120, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Warzecha, Z.; Dembinski, A.; Ceranowicz, P.; Konturek, S.; Tomaszewska, R.; Stachura, J.; Nakamura, T.; Konturek, P.C. Inhibition of cyclooxygenase-2 reduces the protective effect of hepatocyte growth factor in experimental pancreatitis. Eur. J. Pharmacol. 2004, 486, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Yang, J.; Liu, Y. Single injection of naked plasmid encoding hepatocyte growth factor prevents cell death and ameliorates acute renal failure in mice. J. Am. Soc. Nephrol. 2002, 13, 411–422. [Google Scholar] [PubMed]
- Kitamura, K.; Fujiyoshi, K.; Yamane, J.; Toyota, F.; Hikishima, K.; Nomura, T.; Funakoshi, H.; Nakamura, T.; Aoki, M.; Toyama, Y.; et al. Human hepatocyte growth factor promotes functional recovery in primates after spinal cord injury. PLoS One 2011, 6, e27706. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Sakai, K.; Matsumoto, K. Hepatocyte growth factor twenty years on: Much more than a growth factor. J. Gastroenterol. Hepatol. 2011, 26, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Yaekashiwa, M.; Nakayama, S.; Ohnuma, K.; Sakai, T.; Abe, T.; Satoh, K.; Matsumoto, K.; Nakamura, T.; Takahashi, T.; Nukiwa, T. Simultaneous or delayed Administration of hepatocyte growth factor equally represses the fibrotic changes in murine lung injury induced by bleomycin. A morphologic study. Am. J. Respir. Crit. Care Med. 1997, 156, 1937–1944. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, Y. Blockage of tubular epithelial to myofibroblast transition by hepatocyte growth factor prevents renal interstitial fibrosis. J. Am. Soc. Nephrol. 2002, 13, 96–107. [Google Scholar] [PubMed]
- Dohi, M.; Hasegawa, T.; Yamamoto, K.; Marshall, B.C. Hepatocyte growth factor attenuates collagen accumulation in a murine model of pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2000, 162, 2302–2307. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Kakubari, M.; Kawamura, M.; Sugimoto, J.; Matsumoto, K.; Ishii, T. The decrease in total collagen fibers in the liver by hepatocyte growth factor after formation of cirrhosis induced by thioacetamide. Biochem. Pharmacol. 2000, 59, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Tahara, M.; Matsumoto, K.; Nukiwa, T.; Nakamura, T. Hepatocyte growth factor leads to recovery from alcohol-induced fatty liver in rats. J. Clin. Investig. 1999, 103, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Dai, C.; Liu, Y. Systemic administration of naked plasmid encoding hepatocyte growth factor ameliorates chronic renal fibrosis in mice. Gene Ther. 2001, 8, 1470–1479. [Google Scholar] [PubMed]
- Gazdhar, A.; Fachinger, P.; van Leer, C.; Pierog, J.; Gugger, M.; Friis, R.; Schmid, R.A.; Geiser, T. Gene transfer of hepatocyte growth factor by electroporation reduces bleomycin-induced lung fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 292, L529–L536. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, T.; Imado, T.; Kitano, S.; Sano, H. Hepatocyte growth factor ameliorates dermal sclerosis in the tight-skin mouse model of scleroderma. Arthrit. Res. Ther. 2006, 8, R161. [Google Scholar] [CrossRef]
- Zhou, D.; Tan, R.J.; Lin, L.; Zhou, L.; Liu, Y. Activation of hepatocyte growth factor receptor, c-Met, in renal tubules is required for renoprotection after acute kidney injury. Kidney Int. 2013, 84, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shen, W.; Yang, Y.; Liu, G. Therapeutic implications of mesenchymal stem cells transfected with hepatocyte growth factor transplanted in rat kidney with unilateral ureteral obstruction. J. Pediatr. Surg. 2011, 46, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Henry, T.D.; Hirsch, A.T.; Goldman, J.; Wang, Y.L.; Lips, D.L.; McMillan, W.D.; Duval, S.; Biggs, T.A.; Keo, H.H. Safety of a non-viral plasmid-encoding dual isoforms of hepatocyte growth factor in critical limb ischemia patients: A Phase I study. Gene Ther. 2011, 18, 788–794. [Google Scholar] [PubMed]
- Ajroud-Driss, S.; Christiansen, M.; Allen, J.A.; Kessler, J.A. Phase 1/2 open-label dose-escalation study of plasmid DNA expressing two isoforms of hepatocyte growth factor in patients with painful diabetic peripheral neuropathy. Mol. Ther. 2013, 21, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Guevremont, M.; Martel-Pelletier, J.; Massicotte, F.; Tardif, G.; Pelletier, J.P.; Ranger, P.; Lajeunesse, D.; Reboul, P. Human adult chondrocytes express hepatocyte growth factor (HGF) isoforms but not HGF: Potential implication of osteoblasts on the presence of HGF in cartilage. J. Bone Miner. Res. 2003, 18, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.; Gherardi, E.; Mallorqui-Fernandez, N.; Bocci, M.; Sobkowicz, A.; Rees, M.; Rowe, A.; Ellmerich, S.; Massie, I.; Soeda, J.; et al. Protein rngineered variants of hepatocyte growth factor/scatter factor promote proliferation of primary human hepatocytes and in rodent liver. Gastroenterology 2012, 142, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Gaddy, D.F.; Riedel, M.J.; Pejawar-Gaddy, S.; Kieffer, T.J.; Robbins, P.D. In vivo expression of HGF/NK1 and GLP-1 from dsAAV vectors enhances pancreatic β-cell proliferation and improves pathology in the db/db mouse model of fiabetes. Diabetes 2010, 59, 3108–3116. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, T.; Horiguchi, N.; Kanda, D.; Kosone, T.; Yamazaki, Y.; Yuasa, K.; Sohara, N.; Kakizaki, S.; Sato, K.; Takagi, H.; et al. Overexpression of NK2 inhibits liver regeneration after partial hepatectomy in mice. World J. Gastroenterol. 2005, 11, 7444–7449. [Google Scholar] [PubMed]
- Hagiwara, S.; Otsuka, T.; Yamazaki, Y.; Kosone, T.; Sohara, N.; Ichikawa, T.; Sato, K.; Kakizaki, S.; Takagi, H.; Mori, M. Overexpression of NK2 promotes liver fibrosis in carbon tetrachloride-induced chronic liver injury. Liver Int. 2008, 28, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.G.; Burrows, J.; Salgia, R. c-Met as a target for human cancer and characterization of inhibitors for therapeutic intervention. Cancer Lett. 2005, 225, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Ramanujum, R.; Lin, Y.L.; Liu, J.K.; He, S. Regulatory expression of MMP-8/MMP-9 and inhibition of proliferation, migration and invasion in human lung cancer A549 cells in the presence of HGF variants. Kaohsiung J. Med. Sci. 2013, 29, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Yashiro, M.; Chung, Y.S.; Inoue, T.; Nishimura, S.; Matsuoka, T.; Fujihara, T.; Sowa, M. Hepatocyte growth factor (HGF) produced by peritoneal fibroblasts may affect mesothelial cell morphology and promote peritoneal dissemination. Int. J. Cancer 1996, 67, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Takeuchi, E.; Tang, J.T.; Fukushima, S.; Inoue, T.; Shinkawa, K.; Watanabe, Y.; Tanaka, E.; Teshima, T.; Ozeki, S.; et al. Effect of thoracic irradiation on hepatocyte growth factor in rat lung and in bronchoalveolar lavage fluid of patients with thoracic malignancies. Eur. Respir. J. 1997, 10, 2539–2544. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Dean, M.; Cooper, C.S.; Schmidt, M.; O’Brien, S.J.; Blair, D.G.; vande Woude, G.F. Mechanism of Met oncogene activation. Cell 1986, 45, 895–904. [Google Scholar] [CrossRef]
- Cooper, C.S.; Park, M.; Blair, D.G.; Tainsky, M.A.; Huebner, K.; Croce, C.M.; vande Woude, G.F. Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature 1984, 311, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Faletto, D.L.; Tsarfaty, I.; Kmiecik, T.E.; Gonzatti, M.; Suzuki, T.; vande Woude, G.F. Evidence for non-covalent clusters of the c-Met proto-oncogene product. Oncogene 1992, 7, 1149–1157. [Google Scholar] [PubMed]
- Bardelli, A.; Pugliese, L.; Comoglio, P.M. “Invasive-growth” signaling by the Met/HGF receptor: The hereditary renal carcinoma connection. Biochim. Biophys. Acta 1997, 1333, M41–M51. [Google Scholar] [PubMed]
- Schmidt, L.; Duh, F.M.; Chen, F.; Kishida, T.; Glenn, G.; Choyke, P.; Scherer, S.W.; Zhuang, Z.; Lubensky, I.; Dean, M.; et al. Germline and somatic mutations in the tyrosine kinase domain of the Met proto-oncogene in papillary renal carcinomas. Nat. Genet. 1997, 16, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.S.; Tempest, P.R.; Beckman, M.P.; Heldin, C.H.; Brookes, P. Amplification and over-expression of the Met gene in spontaneously transformed NIH3T3 mouse fibroblasts. EMBO J. 1986, 5, 2623–2628. [Google Scholar] [PubMed]
- Ponzetto, C.; Giordano, S.; Peverali, F.; Della Valle, G.; Abate, M.L.; Vaula, G.; Comoglio, P.M. c-Met is amplified but not mutated in a cell line with an activated Met tyrosine kinase. Oncogene 1991, 6, 553–559. [Google Scholar]
- Rahimi, N.; Tremblay, E.; McAdam, L.; Park, M.; Schwall, R.; Elliott, B. Identification of a hepatocyte growth factor autocrine loop in a murine mammary carcinoma. Cell Growth Differ. 1996, 7, 263–270. [Google Scholar] [PubMed]
- Jeffers, M.; Schmidt, L.; Nakaigawa, N.; Webb, C.P.; Weirich, G.; Kishida, T.; Zbar, B.; vande Woude, G.F. Activating mutations for the Met tyrosine kinase receptor in human cancer. Proc. Natl. Acad. Sci. USA 1997, 94, 11445–11450. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Gao, C.F.; Lee, C.C.; Kim, M.D.; vande Woude, G.F. An alternatively spliced form of Met receptor is tumorigenic. Exp. Mol. Med. 2006, 38, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Rong, S.; Bodescot, M.; Blair, D.; Dunn, J.; Nakamura, T.; Mizuno, K.; Park, M.; Chan, A.; Aaronson, S.; vande Woude, G.F. Tumorigenicity of the Met proto-oncogene and the gene for hepatocyte growth factor. Mol. Cell. Biol. 1992, 12, 5152–5158. [Google Scholar] [PubMed]
- Takayama, H.; LaRochelle, W.J.; Sabnis, S.G.; Otsuka, T.; Merlino, G. Renal tubular hyperplasia, polycystic disease, and glomerulosclerosis in transgenic mice over-expressing hepatocyte growth factor/scatter factor. Lab. Investig. 1997, 77, 131–138. [Google Scholar] [PubMed]
- Takayama, H.; LaRochelle, W.J.; Sharp, R.; Otsuka, T.; Kriebel, P.; Anver, M.; Aaronson, S.A.; Merlino, G. Diverse tumorigenesis associated with aberrant development in mice over-expressing hepatocyte growth factor/scatter factor. Proc. Natl. Acad. Sci. USA 1997, 94, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Hjertner, O.; Torgersen, M.L.; Seidel, C.; Hjorth-Hansen, H.; Waage, A.; Borset, M.; Sundan, A. Hepatocyte growth factor (HGF) induces interleukin-11 secretion from osteoblasts: A possible role for HGF in myeloma-associated osteolytic bone disease. Blood 1999, 94, 3883–3888. [Google Scholar] [PubMed]
- Sakata, H.; Takayama, H.; Sharp, R.; Rubin, J.S.; Merlino, G.; LaRochelle, W.J. Hepatocyte growth factor/scatter factor over-expression induces growth, abnormal development, and tumor formation in transgenic mouse livers. Cell Growth Differ. 1996, 7, 1513–1523. [Google Scholar] [PubMed]
- Horiguchi, N.; Takayama, H.; Toyoda, M.; Otsuka, T.; Fukusato, T.; Merlino, G.; Takagi, H.; Mori, M. Hepatocyte growth factor promotes hepatocarcinogenesis through c-Met autocrine activation and enhanced angiogenesis in transgenic mice treated with diethylnitrosamine. Oncogene 2002, 21, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Teofili, L.; di Febo, A.L.; Pierconti, F.; Maggiano, N.; Bendandi, M.; Rutella, S.; Cingolani, A.; di Renzo, N.; Musto, P.; Pileri, S.; et al. Expression of the c-Met proto-oncogene and its ligand, hepatocyte growth factor, in hodgkin disease. Blood 2001, 97, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Eagles, G.; Warn, A.; Ball, R.Y.; Baillie-Johnson, H.; Arakaki, N.; Daikuhara, Y.; Warn, R.M. Hepatocyte growth factor/scatter factor is present in most pleural effusion fluids from cancer patients. Br. J. Cancer 1996, 73, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Sezer, O.; Jakob, C.; Eucker, J.; Niemoller, K.; Gatz, F.; Wernecke, K.; Possinger, K. Serum levels of the angiogenic cytokines basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF) in multiple myeloma. Eur. J. Haematol. 2001, 66, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Takanami, I.; Tanana, F.; Hashizume, T.; Kikuchi, K.; Yamamoto, Y.; Yamamoto, T.; Kodaira, S. Hepatocyte growth Factor and c-Met/hepatocyte growth factor receptor in pulmonary adenocarcinomas: An evaluation of their expression as prognostic markers. Oncology 1996, 53, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.L.; Chen, H.L.; Kuo, H.M.; He, S.P. NK3 and NK4 of HGF enhance filamin production via STAT pathway, but not NK1 and NK2 in human breast cancer cells. Acta Pharmacol. Sin. 2008, 29, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Youles, M.; Holmes, O.; Petoukhov, M.V.; Nessen, M.A.; Stivala, S.; Svergun, D.I.; Gherardi, E. Engineering the NK1 fragment of hepatocyte growth factor/scatter factor as a Met receptor antagonist. J. Mol. Biol. 2008, 377, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Guerin, C.; Luddy, C.; Abounader, R.; Lal, B.; Laterra, J. Glioma inhibition by HGF/NK2, an antagonist of scatter factor/hepatocyte growth factor. Biochem. Biophys. Res. Commun. 2000, 273, 287–293. [Google Scholar] [CrossRef]
- Jin, H.; Wyss, J.M.; Yang, R.; Schwall, R. The therapeutic potential of hepatocyte growth factor for myocardial infarction and heart failure. Curr. Pharm. Des. 2004, 10, 2525–2533. [Google Scholar] [CrossRef] [PubMed]
- Kagawa, T.; Takemura, G.; Kosai, K.; Murata, I.; Ohno, T.; Takahashi, T.; Esaki, M.; Maruyama, R.; Fujiwara, T.; Ohashi, H.; et al. Hepatocyte growth factor gene therapy slows down the progression of diabetic nephropathy in Db/Db mice. Nephron Physiol. 2006, 102, 92–102. [Google Scholar] [CrossRef]
- Makino, H.; Aoki, M.; Hashiya, N.; Yamasaki, K.; Azuma, J.; Sawa, Y.; Kaneda, Y.; Ogihara, T.; Morishita, R. Long-term follow-up evaluation of results from clinical trial using hepatocyte growth factor gene to treat severe peripheral arterial disease. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2503–2509. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.J.; Zhang, Y.R.; Chen, B.; Zhang, S.L.; Jia, E.Z.; Wang, L.S.; Zhu, T.B.; Li, C.J.; Wang, H.; Huang, J.; et al. Phase I clinical trial on intracoronary administration of Ad-hHGF treating severe coronary artery disease. Mol. Biol. Rep. 2009, 36, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Date, K.; Matsumoto, K.; Shimura, H.; Tanaka, M.; Nakamura, T. Hgf/Nk4 is a specific antagonist for pleiotrophic actions of hepatocyte growth factor. FEBS Lett. 1997, 420, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.S.; Kim, H.J.; Yoon, J.H.; Yoo, S.C.; Jo, H.; Lee, S.Y.; Min, C.K.; Ryu, H.S. Endometrial cancer invasion depends on cancer-derived tumor necrosis factor-alpha and stromal derived hepatocyte growth factor. Int. J. Cancer 2009, 124, 2528–2538. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.P.; Zhang, B.L.; Duan, J.W.; Wu, H.H.; Wang, B.Q.; Yu, Z.P.; Yang, W.J.; Shan, Y.F.; Zhou, M.T.; Zhang, Q.Y. Effect of NK4 transduction in bone marrow-derived mesenchymal stem cells on biological characteristics of pancreatic cancer cells. Int. J. Mol. Sci. 2014, 15, 3729–3745. [Google Scholar] [CrossRef] [PubMed]
- Date, K.; Matsumoto, K.; Kuba, K.; Shimura, H.; Tanaka, M.; Nakamura, T. Inhibition of tumor growth and invasion by a four-kringle antagonist (HGF/NK4) for hepatocyte growth factor. Oncogene 1998, 17, 3045–3054. [Google Scholar] [CrossRef] [PubMed]
- Tomioka, D.; Maehara, N.; Kuba, K.; Mizumoto, K.; Tanaka, M.; Matsumoto, K.; Nakamura, T. Inhibition of growth, invasion, and metastasis of human pancreatic carcinoma cells by NK4 in an orthotopic mouse model. Cancer Res. 2001, 61, 7518–7524. [Google Scholar] [PubMed]
- Hirao, S.; Yamada, Y.; Koyama, F.; Fujimoto, H.; Takahama, Y.; Ueno, M.; Kamada, K.; Mizuno, T.; Maemondo, M.; Nukiwa, T.; et al. Tumor suppression effect using NK4, a molecule acting as an antagonist of HGF, on human gastric carcinomas. Cancer Gene Ther. 2002, 9, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Heideman, D.A.; Overmeer, R.M.; van Beusechem, V.W.; Lamers, W.H.; Hakvoort, T.B.; Snijders, P.J.; Craanen, M.E.; Offerhaus, G.J.; Meijer, C.J.; Gerritsen, W.R. Inhibition of angiogenesis and HGF-cMet-elicited malignant processes in human hepatocellular carcinoma cells using adenoviral vector-mediated NK4 gene therapy. Cancer Gene Ther. 2005, 12, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Wright, T.G.; Singh, V.K.; Li, J.J.; Foley, J.H.; Miller, F.; Jia, Z.; Elliott, B.E. Increased production and secretion of HGF alpha-chain and an antagonistic HGF fragment in a human breast cancer progression model. Int. J. Cancer 2009, 125, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, S.; Nakamura, T. HGF–Met cascade, a key target for inhibiting cancer metastasis: The impact of NK4 discovery on cancer biology and therapeutics. Int. J. Mol. Sci. 2013, 14, 888–919. [Google Scholar] [PubMed]
- Rabquer, B.J.; Koch, A.E. NK4 therapy: A new approach to target angiogenesis and inflammation in rheumatoid arthritis. Arthrit. Res. Ther. 2013, 15, 119. [Google Scholar] [CrossRef]
- Tsunemi, S.; Iwasaki, T.; Kitano, S.; Matsumoto, K.; Takagi-Kimura, M.; Kubo, S.; Tamaoki, T.; Sano, H. Molecular targeting of hepatocyte growth factor by an Antagonist, NK4, in the treatment of rheumatoid arthritis. Arthrit. Res. Ther. 2013, 15, R75. [Google Scholar] [CrossRef]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mungunsukh, O.; McCart, E.A.; Day, R.M. Hepatocyte Growth Factor Isoforms in Tissue Repair, Cancer, and Fibrotic Remodeling. Biomedicines 2014, 2, 301-326. https://doi.org/10.3390/biomedicines2040301
Mungunsukh O, McCart EA, Day RM. Hepatocyte Growth Factor Isoforms in Tissue Repair, Cancer, and Fibrotic Remodeling. Biomedicines. 2014; 2(4):301-326. https://doi.org/10.3390/biomedicines2040301
Chicago/Turabian StyleMungunsukh, Ognoon, Elizabeth A. McCart, and Regina M. Day. 2014. "Hepatocyte Growth Factor Isoforms in Tissue Repair, Cancer, and Fibrotic Remodeling" Biomedicines 2, no. 4: 301-326. https://doi.org/10.3390/biomedicines2040301
APA StyleMungunsukh, O., McCart, E. A., & Day, R. M. (2014). Hepatocyte Growth Factor Isoforms in Tissue Repair, Cancer, and Fibrotic Remodeling. Biomedicines, 2(4), 301-326. https://doi.org/10.3390/biomedicines2040301