Tumor-Associated Macrophages in Oncolytic Virotherapy: Friend or Foe?
Abstract
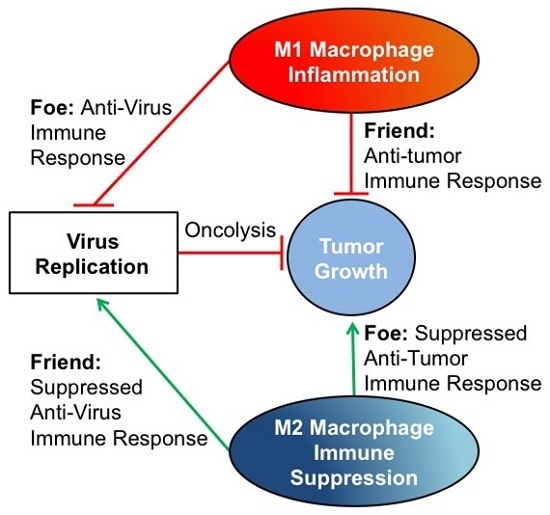
:1. Introduction
2. Macrophages, Prognosis, and Immunotherapy
3. The Tumor Macrophage Influence on Oncolytic Virus Efficacy
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- American Cancer Society. Cancer Facts and Figures 2013; American Cancer Society: Atlanta, GA, USA, 2013. [Google Scholar]
- Armenian, S.H.; Kremer, L.C.; Sklar, C. Approaches to reduce the long-term burden of treatment-related complications in survivors of childhood cancer. Am. Soc. Clin. Oncol. Educ. Book 2015, 196–204. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. SEER Cancer Statistics Review 1975–2004; National Cancer Institute: Bethesda, MD, USA, 2005. [Google Scholar]
- Jakeman, P.G.; Hills, T.E.; Fischer, K.D.; Seymour, L.W. Macrophages and their interactions with oncolytic viruses. Curr. Opin. Pharmacol. 2015, 24, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Cassady, K.A.; Haworth, K.B.; Jackson, J.; Markert, J.M.; Cripe, T.P. To Infection and beyond: The Multi-Pronged Anti-Cancer Mechanisms of Oncolytic Viruses. Viruses 2016, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- LeddonLeddon, J.L.; Chun, C.Y.; Currier, M.A.; Wang, P.Y.; Jung, F.A.; Denton, N.L.; Cripe, K.M.; Haworth, K.B.; Arnold, M.A.; Gross, A.C.; et al. Oncolytic HSV virotherapy in murine sarcomas differentially triggers an antitumor T-cell response in the absence of virus permissivity. Mol. Ther. Oncolytics 2015. [Google Scholar] [CrossRef]
- Varghese, S.; Rabkin, S. Oncolytic herpes simplex virus vectors for cancer virotherapy. Cancer Gene Ther. 2002, 9, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Gujar, S.; Dielschneider, R.; Clements, D.; Helson, E.; Shmulevitz, M.; Marcato, P.; Pan, D.; Pan, L.-Z.; Ahn, D.-G.; Alawadhi, A.; et al. Multifaceted therapeutic targeting of ovarian peritoneal carcinomatosis through virus-induced immunomodulation. Mol. Ther. 2013, 21, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Marchini, A.; Scott, E.M.; Rommelaere, J. Overcoming Barriers in Oncolytic Virotherapy with HDAC Inhibitors and Immune Checkpoint Blockade. Viruses 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Tung, L.Y.; Kaneda, Y. Systemic Administration of Platelets Incorporating Inactivated Sendai Virus Eradicates Melanoma in Mice. Mol. Ther. 2014, 22, 2046–2055. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, C. Amgen announces oncolytic virus shrinks tumors. Nat. Biotechnol. 2013, 31, 471–472. [Google Scholar] [CrossRef] [PubMed]
- Andtbacka, R.H.I.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S.; et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 2015, 33, 2780–2788. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.L.; Amatruda, T.; Reid, T.; Gonzalez, R.; Glaspy, J.; Whitman, E.; Harrington, K.; Nemunaitis, J.; Zloza, A.; Wolf, M.; et al. Systemic versus local responses in melanoma patients treated with talimogene laherparepvec from a multi-institutional phase II study. J. Immunother. Cancer 2016, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Fan, J.; Guo, M.; Huang, B. Oncolytic and immunologic cancer therapy with GM-CSF-armed vaccinia virus of Tian Tan strain Guang9. Cancer Lett. 2016, 372, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Solinas, G.; Germano, G.; Mantovani, A.; Allavena, P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J. Leukoc. Biol. 2009, 86, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sica, A. Macrophages, innate immunity and cancer: Balance, tolerance, and diversity. Curr. Opin. Immunol. 2010, 22, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Takeda, N.; O’Dea, E.L.; Doedens, A.; Kim, J.W.; Weidemann, A.; Stockmann, C.; Asagiri, M.; Simon, M.C.; Hoffmann, A.; Johnson, R.S. Differential activation and antagonistic function of HIF-α isoforms in macrophages are essential for NO homeostasis. Genes Dev. 2010, 24, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.A.; Liao, W.; Sarkar, A.; Kim, M.V.; Bivona, M.R.; Liu, K.; Pamer, E.G.; Li, M.O. The Cellular and Molecular Origin of Tumor-Associated Macrophages. Science 2014, 344, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Zheng, M.; Kim, B.; Rouse, B.T. Role of matrix metalloproteinase-9 in angiogenesis caused by ocular infection with herpes simplex virus. J. Clin. Investig. 2002, 110, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Asano, K.; Nabeyama, A.; Miyake, Y.; Qiu, C.H.; Kurita, A.; Tomura, M.; Kanagawa, O.; Fujii, S.; Tanaka, M. CD169-Positive Macrophages Dominate Anti-tumor Immunity by Crosspresenting Dead Cell-Associated Antigens. Immunity 2011, 34, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Ugel, S.; Sanctis, D.S.; Mandruzzato, S.; Bronte, V. Tumor-induced myeloid deviation: When myeloid-derived suppressor cells meet tumor-associated macrophages. J. Clin. Investig. 2015, 125, 3365–3376. [Google Scholar] [CrossRef] [PubMed]
- Sousa, S.; Brion, R.; Lintunen, M.; Kronqvist, P.; Sandholm, J.; Mönkkönen, J.; Kellokumpu-Lehtinen, P.-L.; Lauttia, S.; Tynninen, O.; Joensuu, H.; et al. Human breast cancer cells educate macrophages toward the M2 activation status. Breast Cancer Res. 2015, 17, 101. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Fukushi, J.; Yamamoto, S.; Matsumoto, Y.; Setsu, N.; Oda, Y.; Yamada, H.; Okada, S.; Watari, K.; Ono, M.; et al. Macrophage infiltration predicts a poor prognosis for human ewing sarcoma. Am. J. Pathol. 2011, 179, 1157–1170. [Google Scholar] [CrossRef] [PubMed]
- Melchjorsen, J.; Siren, J.; Julkunen, I.; Paludan, S.R.; Matikainen, S. Induction of cytokine expression by herpes simplex virus in human monocyte-derived macrophages and dendritic cells is dependent on virus replication and is counteracted by ICP27 targeting NF-κB and IRF-3. J. Gen. Virol. 2006, 87, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Y.; Huang, L.; Xiao, Q.; Chen, X.; Zhong, J.; Chen, Y.; Yang, D.; Han, Z.; Shu, Y.; et al. Let-7a suppresses macrophage infiltrations and malignant phenotype of Ewing sarcoma via STAT3/NF-κB positive regulatory circuit. Cancer Lett. 2016, 374, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Biswas, B.; Sharma, M.C.; Mridha, A.R.; Bakhshi, S. Expression of Cathepsin L in tumor cells and tumor-associated macrophages in patients with Ewing sarcoma family of tumors: A pilot study. Indian J. Pathol. Microbiol. 2015, 58, 170–174. [Google Scholar] [PubMed]
- Hesketh, A.J.; Maloney, C.; Behr, C.A.; Edelman, M.C.; Glick, R.D.; Al-Abed, Y.; Symons, M.; Soffer, S.Z.; Steinberg, B.M. The Macrophage Inhibitor CNI-1493 Blocks Metastasis in a Mouse Model of Ewing Sarcoma through Inhibition of Extravasation. PLoS ONE 2015, 10, e0145197. [Google Scholar] [CrossRef] [PubMed]
- Currier, M.A.; Eshun, F.K.; Sholl, A.; Chernoguz, A.; Crawford, K.; Divanovic, S.; Boon, L.; Goins, W.F.; Frischer, J.S.; Collins, M.H.; et al. VEGF Blockade Enables Oncolytic Cancer Virotherapy in Part by Modulating Intratumoral Myeloid Cells. Mol. Ther. 2013, 21, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Teicher, B.A.; Bagley, R.G.; Rouleau, C.; Kruger, A.; Ren, Y.; Kurtzberg, L. Characteristics of human Ewing/PNET sarcoma models. Ann. Saudi Med. 2011, 31, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.; Knowles, H.J.; Athanasou, N.A. Ewing sarcoma cells express RANKL and support osteoclastogenesis. J. Pathol. 2011, 225, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Zhou, Z.; Cao, Y.; Duan, X.; Kleinerman, E.S. VEGF165 promotes the osteolytic bone destruction of ewing’s sarcoma tumors by upregulating RANKL. Oncol. Res. 2009, 18, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Hume, D.A.; Sasmono, T.; Himes, S.R.; Sharma, S.M.; Bronisz, A.; Constantin, M.; Ostrowski, M.C.; Ross, I.L. The Ewing Sarcoma Protein (EWS) Binds Directly to the Proximal Elements of the Macrophage-Specific Promoter of the CSF-1 Receptor (csf1r) Gene. J. Immunol. 2008, 180, 6733–6742. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.S.; Adamopoulos, I.E.; Sabokbar, A.; Giele, H.; Gibbons, C.L.; Athanasou, N.A. Cellular and humoral mechanisms of osteoclast formation in Ewing’s sarcoma. Br. J. Cancer 2007, 96, 1716–1722. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Guan, H.; Duan, X.; Kleinerman, E.S. Zoledronic acid inhibits primary bone tumor growth in Ewing sarcoma. Cancer 2005, 104, 1713–1720. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Eissler, N.; Blanc, K.L.; Johnsen, J.I.; Kogner, P.; Kiessling, R. Targeting suppressive myeloid cells potentiates checkpoint inhibitors to control spontaneous neuroblastoma. Clin. Cancer Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Xu, Z.; Hao, D. MicroRNA-451 inhibits neuroblastoma proliferation, invasion and migration by targeting macrophage migration inhibitory factor. Mol. Med. Rep. 2016, 13, 2253–2260. [Google Scholar] [CrossRef] [PubMed]
- Larsson, K.; Kock, A.; Idborg, H.; Arsenian Henriksson, M.; Martinsson, T.; Johnsen, J.I.; Korotkova, M.; Kogner, P.; Jakobsson, P.J. COX/mPGES-1/PGE2 pathway depicts an inflammatory-dependent high-risk neuroblastoma subset. Proc. Natl. Acad. Sci. USA 2015, 112, 8070–8075. [Google Scholar] [CrossRef] [PubMed]
- Francis, L.; Guo, Z.S.; Liu, Z.; Ravindranathan, R.; Urban, J.A.; Sathaiah, M.; Magge, D.; Kalinski, P.; Bartlett, D.L. Modulation of chemokines in the tumor microenvironment enhances oncolytic virotherapy for colorectal cancer. Oncotarget 2016, 7907. [Google Scholar] [CrossRef]
- Ehrig, K.; Kilinc, M.O.; Chen, N.G.; Stritzker, J.; Buckel, L.; Zhang, Q.; Szalay, A.A. Growth inhibition of different human colorectal cancer xenografts after a single intravenous injection of oncolytic vaccinia virus GLV-1h68. J. Transl. Med. 2013, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Lavilla-Alonso, S.; Bauer, M.M.; Abo-Ramadan, U.; Ristimäki, A.; Halavaara, J.; Desmond, R.A.; Wang, D.; Escutenaire, S.; Ahtiainen, L.; Saksela, K.; et al. Macrophage metalloelastase (MME) as adjuvant for intra-tumoral injection of oncolytic adenovirus and its influence on metastases development. Cancer Gene Ther. 2012, 19, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Chen, W.Y.; Lin, S.F.; Wong, R.J. Epithelial-Mesenchymal Transition Enhances Response to Oncolytic Herpesviral Therapy Through Nectin-1. Hum. Gene Ther. 2014, 25, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Meisen, W.H.; Wohleb, E.S.; Jaime-Ramirez, A.C.; Bolyard, C.; Yoo, J.Y.; Russell, L.; Hardcastle, J.; Dubin, S.; Muili, K.; Yu, J.; et al. The Impact of Macrophage- and Microglia-Secreted TNFα on Oncolytic HSV-1 Therapy in the Glioblastoma Tumor Microenvironment. Clin. Cancer Res. 2015, 21, 3274–3285. [Google Scholar] [CrossRef] [PubMed]
- Thorne, A.H.; Meisen, W.H.; Russell, L.; Yoo, J.Y.; Bolyard, C.M.; Lathia, J.D.; Rich, J.; Puduvalli, V.K.; Mao, H.; Yu, J.; et al. Role of Cysteine-rich 61 Protein (CCN1) in Macrophage-mediated Oncolytic Herpes Simplex Virus Clearance. Mol. Ther. 2014, 22, 1678–1687. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, K.; Russell, L.; Kaur, B.; Friedman, A. Effects of CCN1 and Macrophage Content on Glioma Virotherapy: A Mathematical Model. Bull. Math. Biol. 2015, 77, 984–1012. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Chen, X.; Chu, J.; Xu, B.; Meisen, W.H.; Chen, L.; Zhang, L.; Zhang, J.; He, X.; Wang, Q.E.; et al. TGFβ Treatment Enhances Glioblastoma Virotherapy by Inhibiting the Innate Immune Response. Cancer Res. 2015, 75, 5273–5282. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.Q.; Zhang, L.; Ohba, K.; Ichiyama, K.; Yamamoto, N. Macrophage response to oncolytic paramyxoviruses potentiates virus-mediated tumor cell killing. Eur. J. Immunol. 2016, 46, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Gerseny, H.; Zhang, Z.; Chen, Y.J.; Berg, A.; Zhang, Z.; Stock, S.; Seth, P. Oncolytic adenovirus expressing soluble TGFβ receptor II-Fc-mediated inhibition of established bone metastases: A safe and effective systemic therapeutic approach for breast cancer. Mol. Ther. 2011, 19, 1609–1618. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Suksanpaisan, L.; Steele, M.B.; Russell, S.J.; Peng, K.W. Induction of antiviral genes by the tumor microenvironment confers resistance to virotherapy. Sci. Rep. 2013, 3, 2375. [Google Scholar] [CrossRef] [PubMed]
- Lucas, T.; Benihoud, K.; Vigant, F.; Schmidt, C.Q.; Wortmann, A.; Bachem, M.G.; Simmet, T.; Kochanek, S. Hexon modification to improve the activity of oncolytic adenovirus vectors against neoplastic and stromal cells in pancreatic cancer. PLoS ONE 2015, 10, e0117254. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Knolhoff, B.L.; Meyer, M.A.; Nywening, T.M.; West, B.L.; Luo, J.; Wang-Gillam, A.; Goedegebuure, S.P.; Linehan, D.C.; DeNardo, D.G. CSF1/CSF1R blockade reprogram tumor-infiltrating macrophages and improves response to T cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014, 74, 5057–5069. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yuan, S.-J.; Chen, Y.-T.; Xie, Y.-B.; Cui, L.; Yang, W.-Z.; Yang, D.-X.; Tian, Y.-Z. Preclinical evaluation of herpes simplex virus armed with granulocyte-macrophage colony-stimulating factor in pancreatic carcinoma. World J. Gastroenterol. 2013, 19, 5138–5143. [Google Scholar] [CrossRef] [PubMed]
- Muthana, M.; Rodrigues, S.; Chen, Y.Y.; Welford, A.; Hughes, R.; Tazzyman, S.; Essand, M.; Morrow, F.; Lewis, C.E. Macrophage Delivery of an Oncolytic Virus Abolishes Tumor Regrowth and Metastasis after chemotherapy or irradiation. Cancer Res. 2013, 73, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Bollino, D.; Colunga, A.; Li, B.; Aurelian, L. ΔPK oncolytic activity includes modulation of the tumour cell milieu. J. Gen. Virol. 2015, 97, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Weiskopf, K.; Weismann, I.L. Macrophages are critical effectors of antibody therapies for cancer. MAbs 2015, 7, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Eisenstein, S.; Chen, S.H.; Pan, P.Y. Immune cells: More than simple carriers for systemic delivery of oncolytic viruses. Oncol. Virother. 2014, 3, 83–91. [Google Scholar]
- Han, Q.; Shi, H.; Liu, F. CD163+ M2-type tumor-associated macrophage support the suppression of tumor-infiltrating T cells in osteosarcoma. Int. Immunopharmacol. 2016, 34, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Theoleyre, S.; Mori, K.; Cherrier, B.; Passuti, N.; Gouin, F.; Rédini, F.; Heymann, D. Phenotypic and functional analysis of lymphocytes infiltrating osteolytic tumors: Use as a possible therapeutic approach of osteosarcoma. PMC Cancer 2005, 5, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Zhang, P.; Li, Q.; Zhou, D.; Liu, P. Expression of high mobility group box 1 protein predicts a poorer prognosis for patients with osteosarcoma. Oncol. Lett. 2016, 11, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Sumiyoshi, M.; Taniquchi, M.; Baba, K.; Kimura, Y. Anti-tumor and antimetastatic actions of xanthoangelol and 4-hydroxyderricin isolated from Angelica keiskei roots through the inhibited activation and differentiation of M2 macrophages. Phytomedicine 2015, 22, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Van Valen, F.; Winkelmann, W.; Burdach, S.; Gobel, U.; Jurgens, H. Interferon gamma and tumour necrosis factor alpha induce a synergistic antiproliferative response in human Ewing’s sarcoma cells in vitro. J. Cancer Res. Clin. Oncol. 1993, 119, 615–621. [Google Scholar] [CrossRef] [PubMed]

| Tumor Type | Macrophage Polarization | Friend or Foe? | Oncolytic Viruses Tested | Signaling Pathways Involved | Reference Number |
|---|---|---|---|---|---|
| Osteosarcoma | M2 | Foe | none | RAGE, CD24, NfκB, VEGF, MCP-1, HMGB1, IL-10, pSTAT3 | [14,20,21,22,25,26,27,28] |
| Ewing Sarcoma | M2 | Foe | none | EWS/FLI1, STAT3, MMP-2, CCND-2, VEGF, MCP-1, M-CSF, RANKL, TNFα, IL-1, VEGF | [22,23,25,26,27,28,29,30,31,32,33,34] |
| Neuroblastoma | M1 | Friend | none | MYCN | [24,35] |
| - | M2 | Foe | none | MIF, Cyclo-oxygenase–prostaglandin E2 pathway, M-CSF | [22,35,36,37] |
| Colorectal Cancer | M1 | Friend | vaccinia virus | GCP-2, KC/GROα, IFNγ, CXCL10, IL-3, IL-6, Lymphotactin, M-CSF1, MIP-1 beta, MCP-1, MCP-3, MCP-5, RANTES, macrophage metallelastase | [38,39,40] |
| Glioblastoma | M1 | Foe | herpes simplex virus | TNFα, CCN1, IL-1β, IFNγ, CXCL10, MCP-1, MCP-3 | [41,42,43,44] |
| - | M2 | Friend | herpes simplex virus | TGFβ | [45] |
| Breast Cancer | M1 | Friend | paramyxovirus | Human Monocyte-Derived | [46] |
| - | M2 | Foe | adenovirus | TGFβ | [47] |
| - | M1 | Foe | vesicular stomatitis virus | JAK/STAT, IFNα, IFNβ | [48] |
| Pancreatic Cancer | M2 | Friend | adenovirus | TGFβR, TGFβ | [49] |
| - | - | - | herpes simplex virus | Nectin-1, TGFβ | [41] |
| - | M2 | Foe | herpes simplex virus | CSF1R | [50] |
| - | M1 | Friend | herpes simplex virus | GM-CSF | [51] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denton, N.L.; Chen, C.-Y.; Scott, T.R.; Cripe, T.P. Tumor-Associated Macrophages in Oncolytic Virotherapy: Friend or Foe? Biomedicines 2016, 4, 13. https://doi.org/10.3390/biomedicines4030013
Denton NL, Chen C-Y, Scott TR, Cripe TP. Tumor-Associated Macrophages in Oncolytic Virotherapy: Friend or Foe? Biomedicines. 2016; 4(3):13. https://doi.org/10.3390/biomedicines4030013
Chicago/Turabian StyleDenton, Nicholas L., Chun-Yu Chen, Thomas R. Scott, and Timothy P. Cripe. 2016. "Tumor-Associated Macrophages in Oncolytic Virotherapy: Friend or Foe?" Biomedicines 4, no. 3: 13. https://doi.org/10.3390/biomedicines4030013
APA StyleDenton, N. L., Chen, C. -Y., Scott, T. R., & Cripe, T. P. (2016). Tumor-Associated Macrophages in Oncolytic Virotherapy: Friend or Foe? Biomedicines, 4(3), 13. https://doi.org/10.3390/biomedicines4030013