An Abraded Surface of Doxorubicin-Loaded Surfactant-Containing Drug Delivery Systems Effectively Reduces the Survival of Carcinoma Cells
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Carrier Preparation
2.3. Particle Analysis
2.4. Tissue Culture
2.5. In Vitro Tests
2.6. Small-Angle X-ray Scattering (SAXS)
3. Results
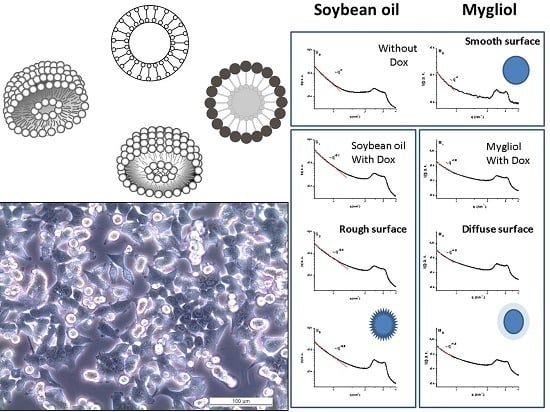
3.1. Synthesis and Characterization of Carrier Systems
3.2. In Vitro Performance of Synthesized Carrier Systems on HeLa and HCT116 Cells
3.3. SAXS Characterization
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics 2015. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Storsberg, J.; Schmidt, C. Nanomaterials—Tools, Technology and Methodology of Nanotechnology Based Biomedical Systems for Diagnostics and Therapy. Biomedicines 2015, 3, 203–223. [Google Scholar]
- Weissig, V.; Pettinger, T.K.; Murdock, N. Nanopharmaceuticals (part 1): Products on the market. Int. J. Nanomed. 2014, 9, 4357–4373. [Google Scholar] [CrossRef] [PubMed]
- Weissig, V.; Guzman-Villanueva, D. Nanopharmaceuticals (part 2): Products in the pipeline. Int. J. Nanomed. 2015, 10, 1245–1257. [Google Scholar] [CrossRef] [PubMed]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, C.; Misra, V.; Januszewski, A.; Yosef, H.; Ashford, R.; Keary, I.; Davidson, N. Multicenter experience of nonpegylated liposomal doxorubicin use in the management of metastatic breast cancer. Clin. Breast Cancer 2014, 14, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Fiegl, M.; Mlineritsch, B.; Hubalek, M.; Bartsch, R.; Pluschnig, U.; Steger, G.G. Single-agent pegylated liposomal doxorubicin (PLD) in the treatment of metastatic breast cancer: Results of an Austrian observational trial. BMC Cancer 2011, 11, 373. [Google Scholar] [CrossRef] [PubMed]
- Rau, K.M.; Lin, Y.C.; Chen, Y.Y.; Chen, J.S.; Lee, K.D.; Wang, C.H.; Chang, H.K. Pegylated liposomal doxorubicin (Lipo-Dox®) combined with cyclophosphamide and 5-fluorouracil is effective and safe as salvage chemotherapy in taxane-treated metastatic breast cancer, an open-label, multi-center, non-comparative phase II study. BMC Cancer 2015, 15, 423. [Google Scholar] [CrossRef] [PubMed]
- Denard, B.; Lee, C.; Ye, J. Doxorubicin blocks proliferation of cancer cells through proteolytic activation of CREB3L1. Elife 2012, 1, e00090. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, Y.; Ghanefar, M.; Bayeva, M.; Wu, R.; Khechaduri, A.; Naga Prasad, S.V.; Mutharasan, R.K.; Naik, T.J.; Ardehali, H. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J. Clin. Investig. 2014, 124, 617–630. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.E.; Wigler, N.; Inbar, M.; Rosso, R.; Grischke, E.; Santoro, A.; Catane, R.; Kieback, D.G.; Tomczak, P.; Ackland, S.P.; et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann. Oncol. 2004, 15, 440–449. [Google Scholar] [CrossRef] [PubMed]
- FDA Approval of Generic Version of Cancer Drug Doxil Is Expected to Help Resolve Shortage. Available online: http://www.webcitation.org/6Yz2BdWO5 (accessed on 2 June 2015).
- Lorusso, D.; Di Stefano, A.; Carone, V.; Fagotti, A.; Pisconti, S.; Scambia, G. Pegylated liposomal doxorubicin-related palmar-plantar erythrodysesthesia (‘hand-foot’ syndrome). Ann. Oncol. 2007, 18, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Farr, K.P.; Safwat, A. Palmar-plantar erythrodysesthesia associated with chemotherapy and its treatment. Case Rep. Oncol. 2011, 4, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.; Choo, S.P.; Tan, E.H. Travel warning with capecitabine. Ann. Oncol. 2009, 20, 1281. [Google Scholar] [CrossRef] [PubMed]
- Ţălu, Ş.; Stach, S.; Sueiras, V.; Ziebarth, N.M. Fractal analysis of AFM images of the surface of Bowman’s membrane of the human cornea. Ann. Biomed. Eng. 2015, 43, 906–916. [Google Scholar] [CrossRef] [PubMed]
- Davies, N.A.; Harrison, N.K.; Morris, R.H.K.; Noble, S.; Lawrence, M.J.; D’Silva, L.A.; Broome, L.; Brown, M.R.; Hawkins, K.M.; Williams, P.R.; et al. Fractal dimension (df) as a new structural biomarker of clot microstructure in different stages of lung cancer. Thromb. Haemost. 2015, 114, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Dokukin, M.E.; Guz, N.V.; Woodworth, C.D.; Sokolov, I. Emergence of fractal geometry on the surface of human cervical epithelial cells during progression towards cancer. New J. Phys. 2015, 17. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Fabinyi, S.; Rehfeldt, S.; Klöpzig, S.; Jentzen, V.; Bohrisch, J.; Messner, A.; Storsberg, J. Non-antagonistic influence of Krumeich’s intrastromal corneal ring in an experimental tissue culture system. Ophthalmologe 2016. [Google Scholar] [CrossRef]
- Cavalcanti, L.P.; Torriani, I.L.; Plivelic, T.S.; Oliveira, C.L.P.; Kellermann, G.; Neuenschwander, R. Two new sealed sample cells for small angle X-ray scattering from macromolecules in solution and complex fluids using synchrotron radiation. Rev. Sci. Instrum. 2004, 75, 4541–4546. [Google Scholar] [CrossRef]
- Chinsriwongkul, A.; Chareanputtakhun, P.; Ngawhirunpat, T.; Rojanarata, T.; Sila-on, W.; Ruktanonchai, U.; Opanasopit, P. Nanostructured lipid carriers (NLC) for parenteral delivery of an anticancer drug. AAPS PharmSciTech 2012, 13, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.L.; Gonzalez, M.V.; Leppard, S.W.; Brown, J.E.; Stratford, P.W.; Phillips, G.J.; Lloyd, A.W. Doxorubicin eluting beads—1: Effects of drug loading on bead characteristics and drug distribution. J. Mater. Sci. Mater. Med. 2007, 18, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zheng, C.; Xin, J.; Chen, F.; Ling, H.; Sun, L.; Webster, T.J.; Ming, X.; Liu, J. Enhanced tumor delivery and antitumor response of doxorubicin-loaded albumin nanoparticles formulated based on a Schiff base. Int. J. Nanomed. 2016, 11, 3875–3890. [Google Scholar]
- Purdie, L.; Alexander, C.; Spain, S.G.; Magnusson, J.P. Influence of Polymer Size on Uptake and Cytotoxicity of Doxorubicin-Loaded DNA-PEG Conjugates. Bioconjug. Chem. 2016, 27, 1244–1152. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Guo, D.; Xiao, K.; Wang, X.; Wang, L.; Luo, J. A drug-specific nanocarrier design for efficient anticancer therapy. Nat. Commun. 2015, 6, 7449. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Brazel, C.S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Control. Release 2001, 73, 121–136. [Google Scholar] [CrossRef]
- Jain, T.K.; Richey, J.; Strand, M.; Leslie-Pelecky, D.L.; Flask, C.A.; Labhasetwar, V. Magnetic nanoparticles with dual functional properties: Drug delivery and magnetic resonance imaging. Biomaterials 2008, 29, 4012–4021. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi-Aliabadi, H.; Minaiyan, M.; Dabestan, A. Cytotoxic evaluation of doxorubicin in combination with simvastatin against human cancer cells. Res. Pharm. Sci. 2010, 5, 127–133. [Google Scholar] [PubMed]
- Kochetkov, D.V.; Il’inskaia, G.V.; Komarov, P.G.; Strom, E.; Agapova, L.S.; Ivanov, A.V.; Budanov, A.V.; Frolova, E.I.; Chumakov, P.M. Transcriptional inhibition of human papilloma virus in cervical carcinoma cells reactivates functions of the tumor suppressor p53. Mol. Biol. 2007, 41, 515–523. [Google Scholar] [CrossRef]
- Angelova, A.; Angelov, B.; Garamus, V.M.; Couvreur, P.; Lesieur, S. Small-angle X-ray scattering investigations of biomolecular confinement, loading, and release from liquid-crystalline nanochannel assemblies. J. Phys. Chem. Lett. 2012, 3, 445–457. [Google Scholar] [CrossRef] [PubMed]






| Fatty Acids Composition for Soybean Oil | (%) |
|---|---|
| C 16:0 Palmitic acid | 10.62 |
| C 18:0 Stearic acid | 2.81 |
| C 18:1 Oleic acid | 25.25 |
| C 18:2 Linoleic acid | 53.29 |
| C 18:3 Linoleic acid | 5.95 |
| C 20:0 Arachidic acid | 0.35 |
| C 22:0 Behecid acid | 0.70 |
| Trans-isomer fatty acids | 1.08 |
| Fatty Acids Composition for Mygliol 812 | (%) |
|---|---|
| Caproic acid | 0.10 |
| Caprylic acid | 56.3 |
| Capric acid | 43.1 |
| Lauric acid | 0.30 |
| Myristic acid | 0.10 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, C.; Yokaichiya, F.; Doğangüzel, N.; Dias Franco, M.K.K.; Cavalcanti, L.P.; Brown, M.A.; Alkschbirs, M.I.; De Araujo, D.R.; Kumpugdee-Vollrath, M.; Storsberg, J. An Abraded Surface of Doxorubicin-Loaded Surfactant-Containing Drug Delivery Systems Effectively Reduces the Survival of Carcinoma Cells. Biomedicines 2016, 4, 22. https://doi.org/10.3390/biomedicines4030022
Schmidt C, Yokaichiya F, Doğangüzel N, Dias Franco MKK, Cavalcanti LP, Brown MA, Alkschbirs MI, De Araujo DR, Kumpugdee-Vollrath M, Storsberg J. An Abraded Surface of Doxorubicin-Loaded Surfactant-Containing Drug Delivery Systems Effectively Reduces the Survival of Carcinoma Cells. Biomedicines. 2016; 4(3):22. https://doi.org/10.3390/biomedicines4030022
Chicago/Turabian StyleSchmidt, Christian, Fabiano Yokaichiya, Nurdan Doğangüzel, Margareth K. K. Dias Franco, Leide P. Cavalcanti, Mark A. Brown, Melissa I. Alkschbirs, Daniele R. De Araujo, Mont Kumpugdee-Vollrath, and Joachim Storsberg. 2016. "An Abraded Surface of Doxorubicin-Loaded Surfactant-Containing Drug Delivery Systems Effectively Reduces the Survival of Carcinoma Cells" Biomedicines 4, no. 3: 22. https://doi.org/10.3390/biomedicines4030022
APA StyleSchmidt, C., Yokaichiya, F., Doğangüzel, N., Dias Franco, M. K. K., Cavalcanti, L. P., Brown, M. A., Alkschbirs, M. I., De Araujo, D. R., Kumpugdee-Vollrath, M., & Storsberg, J. (2016). An Abraded Surface of Doxorubicin-Loaded Surfactant-Containing Drug Delivery Systems Effectively Reduces the Survival of Carcinoma Cells. Biomedicines, 4(3), 22. https://doi.org/10.3390/biomedicines4030022