The Modulation of NMDA and AMPA/Kainate Receptors by Tocotrienol-Rich Fraction and Α-Tocopherol in Glutamate-Induced Injury of Primary Astrocytes
Abstract
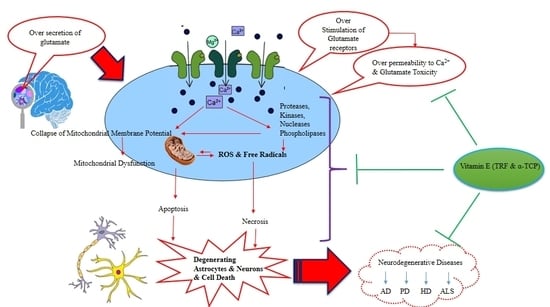
:1. Introduction
2. Results
2.1. Isolation and Purification of Primary Astrocytes
2.2. Cell Viability of Glutamate-Injured Astrocytes against Vitamin E Treatment
2.3. Effects of Vitamin E in Preserving Mitochondrial Membrane Potential of Primary Astrocytes after Glutamate Excitotoxicity
2.4. Effect of Vitamin E on the Expression of Ionotropic Glutamate Receptors Genes
2.5. Calcium (Ca2+) Influx in Primary Astrocytes upon Glutamate Toxicity
3. Discussion
4. Material and Methods
4.1. Preparation of Mixed Glia Cultures
4.2. Isolation of Microglia from Mixed Glia Cultures by Magnetic Sorting
4.3. Purification of Astrocytes from Mixed Glia Culture
4.4. Determination of Primary Astrocytes Purity by Flow Cytometry
4.5. MTT Assay
4.6. Mitochondria Membrane Potential Assay (MMP Assay)
4.7. End-Point RT-PCR
4.8. RT-PCR
4.9. Extracellular Ca2+ Ion Measuring
4.10. Data Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tabrizi, S. Neurodegenerative Diseases Neurobiology Pathogenesis and Therapeutics; BMJ Publishing Group Ltd.: London, UK, 2006. [Google Scholar]
- Selvaraju, T.R.; Khaza’ai, H.; Vidyadaran, S.; Sokhini Abd Mutalib, M.; Ramachandran, V.; Hamdan, Y. Cytoprotective effect of tocotrienol-rich fraction and α-tocopherol vitamin E isoforms against glutamate-induced cell death in neuronal cells. Int. J. Vitam. Nutr. Res. 2014, 84, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Selvaraju, T.R.; Khaza’ai, H.; Vidyadaran, S.; Abd Mutalib, M.S.; Vasudevan, R. The neuroprotective effects of tocotrienol rich fraction and alpha tocopherol against glutamate injury in astrocytes. Bosn. J. Basic Med. Sci. 2014, 14, 195. [Google Scholar] [CrossRef] [PubMed]
- Shukla, V.; Mishra, S.K.; Pant, H.C. Oxidative stress in neurodegeneration. Adv. Pharmacol. Sci. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Coyle, J.T.; Puttfarcken, P. Oxidative stress, glutamate, and neurodegenerative disorders. Science 1993, 262, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.; Tymianski, M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflügers Arch. Eur. J. Physiol. 2010, 460, 525–542. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.Y.; Crews, F.T. TNFα potentiates glutamate neurotoxicity by inhibiting glutamate uptake in organotypic brain slice cultures: Neuroprotection by NFκB inhibition. Brain Res. 2005, 1034, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Nishio, K.; Numakawa, Y.; Ogawa, Y.; Yoshida, Y.; Noguchi, N.; Niki, E. Protective effects of 15-deoxy-Δ12, 14-prostaglandin J2 against glutamate-induced cell death in primary cortical neuron cultures: Induction of adaptive response and enhancement of cell tolerance primarily through up-regulation of cellular glutathione. J. Neurochem. 2007, 102, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Platt, S.R. The role of glutamate in central nervous system health and disease—A review. Vet. J. 2007, 173, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, B.S. Glutamate as a neurotransmitter in the brain: Review of physiology and pathology. J. Nutr. 2000, 130, 1007S–1015S. [Google Scholar] [PubMed]
- Watkins, J.C.; Jane, D.E. The glutamate story. Br. J. Pharmacol. 2006, 147 (Suppl. 1), S100–S108. [Google Scholar] [CrossRef]
- Choi, D.W. Excitotoxic cell death. J. Neurobiol. 1992, 23, 1261–1276. [Google Scholar] [CrossRef] [PubMed]
- Lipton, S.A. Failures and successes of NMDA receptor antagonists: Molecular basis for the use of open-channel blockers like memantine in the treatment of acute and chronic neurologic insults. NeuroRx 2004, 1, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.N.; Yap, W.N.; Lee, D.T.; Ling, M.T.; Wong, Y.C.; Yap, Y.L. Evidence of γ-tocotrienol as an apoptosis-inducing, invasion-suppressing, and chemotherapy drug-sensitizing agent in human melanoma cells. Nutr. Cancer 2009, 61, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Smith, C.L.; Barone, F.C.; Ellison, J.A.; Lysko, P.G.; Li, K.; Simpson, I.A. Astrocytic demise precedes delayed neuronal death in focal ischemic rat brain. Mol. Brain Res. 1999, 68, 29–41. [Google Scholar] [CrossRef]
- Landis, D.M. The early reactions of non-neuronal cells to brain injury. Ann. Rev. Neurosci. 1994, 17, 133–151. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Liao, S.L.; Kuo, J.S. Gliotoxic action of glutamate on cultured astrocytes. J. Neurochem. 2000, 75, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Sundaram, C.; Prasad, S.; Kannappan, R. Tocotrienols, the vitamin E of the 21st century: Its potential against cancer and other chronic diseases. Biochem. Pharmacol. 2010, 80, 1613–1631. [Google Scholar] [CrossRef] [PubMed]
- Theriault, A.; Chao, J.T.; Wang, Q.; Gapor, A.; Adeli, K. Tocotrienol: A review of its therapeutic potential. Clin. Biochem. 1999, 32, 309–319. [Google Scholar] [CrossRef]
- Fang, Y.-Z.; Yang, S.; Wu, G. Free radicals, antioxidants, and nutrition. Nutrition 2002, 18, 872–879. [Google Scholar] [CrossRef]
- Minhajuddin, M.; Beg, Z.H.; Iqbal, J. Hypolipidemic and antioxidant properties of tocotrienol rich fraction isolated from rice bran oil in experimentally induced hyperlipidemic rats. Food Chem. Toxicol. 2005, 43, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Lee, S.M.; Kim, Y.; Yoon, J.; Jeong, H.S.; Lee, J. A tocotrienol-rich fraction from grape seeds inhibits oxidative stress induced by tert-butyl hydroperoxide in HepG2 cells. J. Med. Food 2010, 13, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.J.; Liu, P.L.; Ng, L.T. Tocotrienol-rich fraction of palm oil exhibits anti-inflammatory property by suppressing the expression of inflammatory mediators in human monocytic cells. Mol. Nutr. Food Res. 2008, 52, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Duhem, N.; Danhier, F.; Pourcelle, V.; Schumers, J.M.; Bertrand, O.; Leduff, C.S.; Hoeppener, S.; Schubert, U.S.; Gohy, J.F.; Marchand-Brynaert, J.; et al. Self-assembling doxorubicin–tocopherol succinate prodrug as a new drug delivery system: Synthesis, characterization, and in vitro and in vivo anticancer activity. Bioconjug. Chem. 2013, 25, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Alayoubi, A.; Alqahtani, S.; Kaddoumi, A.; Nazzal, S. Effect of PEG surface conformation on anticancer activity and blood circulation of nanoemulsions loaded with tocotrienol-rich fraction of palm oil. AAPS J. 2013, 15, 1168–1179. [Google Scholar] [CrossRef] [PubMed]
- Fleige, S.; Walf, V.; Huch, S.; Prgomet, C.; Sehm, J.; Pfaffl, M.W. Comparison of relative mRNA quantification models and the impact of RNA integrity in quantitative real-time RT-PCR. Biotechnol. Lett. 2006, 28, 1601–1613. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Miyashita, T.; Fu, T.F.; Lin, W.Y.; Wu, C.L.; Pyzocha, L.; Lin, I.R.; Saitoe, M.; Tully, T.; Chiang, A.S. NMDA receptors mediate olfactory learning and memory in Drosophila. Curr. Biol. 2005, 15, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Aw, S.S.; Lim, I.K.H.; Tang, M.X.M.; Cohen, S.M. A Glio-Protective Role of mir-263a by Tuning Sensitivity to Glutamate. Cell Rep. 2017, 19, 1783–1793. [Google Scholar] [CrossRef] [PubMed]
- Nikodemova, M.; Watters, J.J. Efficient isolation of live microglia with preserved phenotypes from adult mouse brain. J. Neuroinflamm. 2012, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.A.; Cardona, S.M.; Cardona, A.E. Isolation and analysis of mouse microglial cells. Curr. Protoc. Immunol. 2014. [Google Scholar] [CrossRef]
- Gordon, R.; Hogan, C.E.; Neal, M.L.; Anantharam, V.; Kanthasamy, A.G.; Kanthasamy, A. A simple magnetic separation method for high-yield isolation of pure primary microglia. J. Neurosci. Methods 2011, 194, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Tamashiro, T.T.; Dalgard, C.L.; Byrnes, K.R. Primary microglia isolation from mixed glial cell cultures of neonatal rat brain tissue. JoVE J. Vis. Exp. 2012, 15, e3814. [Google Scholar] [CrossRef] [PubMed]
- Daniele, S.G.; Edwards, A.A.; Maguire-Zeiss, K.A. Isolation of cortical microglia with preserved immunophenotype and functionality from murine neonates. JoVE J. Vis. Exp. 2014, 30, e51005. [Google Scholar] [CrossRef] [PubMed]
- Hamby, M.E.; Uliasz, T.F.; Hewett, S.J.; Hewett, J.A. Characterization of an improved procedure for the removal of microglia from confluent monolayers of primary astrocytes. J. Neurosci. Methods 2006, 150, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.-W.; Zhang, L.; Zhu, S.J.; Chen, W.C.; Mei, B. Excitotoxicity effects of glutamate on human neuroblastoma SH-SY5Y cells via oxidative damage. Neurosci. Bull. 2010, 26, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S.; Roy, S.; Parinandi, N.L.; Maurer, M.; Sen, C.K. Characterization of the potent neuroprotective properties of the natural vitamin E α-tocotrienol. J. Neurochem. 2006, 98, 1474–1486. [Google Scholar] [CrossRef] [PubMed]
- Osakada, F.; Hashino, A.; Kume, T.; Katsuki, H.; Kaneko, S.; Akaike, A. Neuroprotective effects of α-tocopherol on oxidative stress in rat striatal cultures. Eur. J. Pharmacol. 2003, 465, 15–22. [Google Scholar] [CrossRef]
- Packer, L.; Weber, S.U.; Rimbach, G. Molecular aspects of α-tocotrienol antioxidant action and cell signalling. J. Nutr. 2001, 131, 369S–373S. [Google Scholar] [PubMed]
- Muller, D.P. Vitamin E and neurological function. Mol. Nutr. Food Res. 2010, 54, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Nishio, K.; Akazawa, Y.O.; Yamanaka, K.; Miyama, A.; Yoshida, Y.; Noguchi, N.; Niki, E. Cytoprotective effects of vitamin E homologues against glutamate-induced cell death in immature primary cortical neuron cultures: Tocopherols and tocotrienols exert similar effects by antioxidant function. Free Radic. Biol. Med. 2010, 49, 1542–1549. [Google Scholar] [CrossRef] [PubMed]
- Kamat, J.; Devasagayam, T. Tocotrienols from palm oil as potent inhibitors of lipid peroxidation and protein oxidation in rat brain mitochondria. Neurosci. Lett. 1995, 195, 179–182. [Google Scholar] [CrossRef]
- Sen, C.K.; Khanna, S.; Roy, S.; Packer, L. Molecular Basis of Vitamin E Action Tocotrienol potently inhibits glutamate-induced pp60c-Src Kinase activation and death of ht4 neuronal cells. J. Biol.Chem. 2000, 275, 13049–13055. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohe, R.; Traber, M.G. Vitamin E: Function and metabolism. FASEB J. 1999, 13, 1145–1155. [Google Scholar] [PubMed]
- Upadhyay, J.; Misra, K. Towards the interaction mechanism of tocopherols and tocotrienols (vitamin E) with selected metabolizing enzymes. Bioinformation 2009, 3, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Grosche, J.; Matyash, V.; Möller, T.; Verkhratsky, A.; Reichenbach, A.; Kettenmann, H. Microdomains for neuron–glia interaction: Parallel fiber signaling to Bergmann glial cells. Nat. Neurosci. 1999, 2, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Seifert, G.; Steinhäuser, C. Ionotropic glutamate receptors in astrocytes. Prog. Brain Res. 2001, 132, 287–299. [Google Scholar] [PubMed]
- Pasti, L.; Volterra, A.; Pozzan, T.; Carmignoto, G. Intracellular calcium oscillations in astrocytes: A highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J. Neurosci. 1997, 17, 7817–7830. [Google Scholar] [PubMed]
- Bezzi, P.; Carmignoto, G.; Pasti, L.; Vesce, S.; Rossi, D.; Rizzini, B.L.; Pozzan, T.; Volterra, A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature 1998, 391, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Araque, A.; Parpura, V.; Sanzgiri, R.P.; Haydon, P.G. Tripartite synapses: Glia, the unacknowledged partner. Trends Neurosci. 1999, 22, 208–215. [Google Scholar] [CrossRef]
- Lee, J.-M.; Zipfel, G.J.; Choi, D.W. The changing landscape of ischaemic brain injury mechanisms. Nature 1999, 399, A7–A14. [Google Scholar] [CrossRef] [PubMed]
- Alberdi, E.; Sánchez-Gómez, M.V.; Marino, A.; Matute, C. Ca2+ influx through AMPA or kainate receptors alone is sufficient to initiate excitotoxicity in cultured oligodendrocytes. Neurobiol. Dis. 2002, 9, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Seifert, G.; Steinhauser, C. Ionotropic glutamate receptors in astrocytes. In Glial Cell. Function; Elsevier Science BV: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Song, I.; Huganir, R.L. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci. 2002, 25, 578–588. [Google Scholar] [CrossRef]
- Sen, C.K.; Khanna, S.; Roy, S. Tocotrienols: Vitamin E beyond tocopherols. Life Sci. 2006, 78, 2088–2098. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Pourcho, R.G. Immunocytochemical localization of kainate-selective glutamate receptor subunits GluR5, GluR6, and GluR7 in the cat retina. Brain Res. 2001, 890, 211–221. [Google Scholar] [CrossRef]
- Lau, J.; Kroes, R.A.; Moskal, J.R.; Linsenmeier, R.A. Diabetes changes expression of genes related to glutamate neurotransmission and transport in the Long-Evans rat retina. Mol. Vis. 2013, 19, 1538–1553. [Google Scholar] [PubMed]
- Jakobs, T.C.; Ben, Y.; Masland, R.H. Expression of mRNA for glutamate receptor subunits distinguishes the major classes of retinal neurons, but is less specific for individual cell types. Mol. Vis. 2007, 13, 933–948. [Google Scholar] [PubMed]
- Brandstätter, J.; Hartveit, E.; Sassoè-Pognetto, M.; Wässle, H. Expression of NMDA and high-affinity kainate receptor subunit mRNAs in the adult rat retina. Eur. J. Neurosci. 1994, 6, 1100–1112. [Google Scholar] [CrossRef] [PubMed]








| Sample | Treatment |
|---|---|
| 1 | Negative control/untreated sample (cells with medium and absolute ethanol only) |
| 2 | Positive control (astrocyte cell, 100 mM of glutamate/astrocytes) |
| 3 | 100 ng/mL TRF + glutamate |
| 4 | 200 ng/mL TRF + glutamate |
| 5 | 300 ng/mL TRF + glutamate |
| 6 | 100 ng/mL α-TCP + glutamate |
| 7 | 200 ng/mL α-TCP + glutamate |
| 8 | 300 ng/mL α-TCP + glutamate |
| Target Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| Gria2 | 5′-TAGACTCTGGCTCCACTAAAGA-3′ | 5′-GTAGTCCTCACAAACACAGAGG-3′ |
| GRIK1 | 5′-GTCAGTGCTGTGCAGTCTATT-3′ | 5′-AGCTGCATAATCTGGGTAAAGG-3′ |
| Grin2A | 5′-CACGGTCATGCTGAAGAT-3′ | 5′-TCTTGACGAAGCTGATGAA-3′ |
| ACTB | 5′-GCTCCGGCATGTGCAARG-3′ | 5′-CATCACACCCTGGTGCCT-3′ |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abedi, Z.; Khaza’ai, H.; Vidyadaran, S.; Mutalib, M.S.A. The Modulation of NMDA and AMPA/Kainate Receptors by Tocotrienol-Rich Fraction and Α-Tocopherol in Glutamate-Induced Injury of Primary Astrocytes. Biomedicines 2017, 5, 68. https://doi.org/10.3390/biomedicines5040068
Abedi Z, Khaza’ai H, Vidyadaran S, Mutalib MSA. The Modulation of NMDA and AMPA/Kainate Receptors by Tocotrienol-Rich Fraction and Α-Tocopherol in Glutamate-Induced Injury of Primary Astrocytes. Biomedicines. 2017; 5(4):68. https://doi.org/10.3390/biomedicines5040068
Chicago/Turabian StyleAbedi, Zahra, Huzwah Khaza’ai, Sharmili Vidyadaran, and Mohd Sokhini Abd Mutalib. 2017. "The Modulation of NMDA and AMPA/Kainate Receptors by Tocotrienol-Rich Fraction and Α-Tocopherol in Glutamate-Induced Injury of Primary Astrocytes" Biomedicines 5, no. 4: 68. https://doi.org/10.3390/biomedicines5040068
APA StyleAbedi, Z., Khaza’ai, H., Vidyadaran, S., & Mutalib, M. S. A. (2017). The Modulation of NMDA and AMPA/Kainate Receptors by Tocotrienol-Rich Fraction and Α-Tocopherol in Glutamate-Induced Injury of Primary Astrocytes. Biomedicines, 5(4), 68. https://doi.org/10.3390/biomedicines5040068