Platelet-Rich Plasma (PRP) Rinses for the Treatment of Non-Responding Oral Lichen Planus: A Case Report
Abstract
:1. Introduction
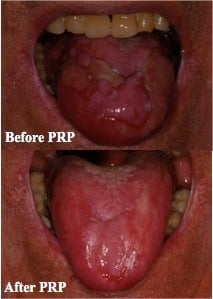
2. Description of the Case
3. Discussion and Conclusions
Author Contributions
Conflicts of Interest
References
- Thongprasom, K.; Prapinjumrune, C.; Carrozzo, M. Novel therapies for oral lichen planus. J. Oral Pathol. Med. 2013, 42, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Ghosh, S.; Gupta, S. Interventions for the management of oral lichen planus: A review of the conventional and novel therapies. Oral Dis. 2017, 23, 1029–1042. [Google Scholar] [CrossRef] [PubMed]
- Fornaini, C.; Raybaud, H.; Augros, C.; Rocca, J.P. New clinical approach for use of Er:YAG laser in the surgical treatment of oral lichen planus: A report of two cases. Photomed. Laser Surg. 2012, 30, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Fornaini, C. LLLT in the Symptomatic Treatment of Oral Lichen Planus. Laser Ther. 2012, 21, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, R.S.; Schwarz, E.M.; Maloney, M.D. Platelet-rich plasma therapy—Future or trend? Arthritis Res. Ther. 2012, 14, 219. [Google Scholar] [CrossRef] [PubMed]
- Gonchar, I.V.; Lipunov, A.R.; Afanasov, I.M.; Larina, V.; Faller, A.P.; Kibardin, A.V. Platelet rich plasma and growth factors cocktails for diabetic foot ulcers treatment: State of art developments and future prospects. Diabetes Metab. Syndr. 2017. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, N.N.; Candido, K.D.; Desai, R.; Kaye, A.D. Is Platelet-Rich Plasma a Future Therapy in Pain Management? Med. Clin. N. Am. 2016, 100, 199–217. [Google Scholar] [CrossRef] [PubMed]
- Raeissadat, S.A.; Babaee, M.; Rayegani, S.M.; Hashemi, Z.; Hamidieh, A.A.; Mojgani, P.; Fouladi Vanda, H. An overview of platelet products (PRP, PRGF, PRF, etc.) in the Iranian studies. Future Sci. OA 2017, 3, FSO231. [Google Scholar] [CrossRef] [PubMed]
- Fornaini, C.; Cella, L.; Oppici, A.; Parlatore, A.; Clini, F.; Fontana, M.; Lagori, G.; Merigo, E. Laser and Platelet-Rich Plasma to treat Medication-Related Osteonecrosis of the Jaws (MRONJ): A case report. Laser Ther. 2017, 26, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Jornet, P.; Sanchez Perez, A.; Amaral Mendes, R.; Tobias, A. Medication-related osteonecrosis of the jaw: Is autologous platelet concentrate application effective for prevention and treatment? A systematic review. J. Cranio-Maxillo-Fac. Surg. 2016, 44, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, M.J.; Ha, S.W.; Kim, H.K. Autologous Platelet-rich Plasma Eye Drops in the Treatment of Recurrent Corneal Erosions. Korean J. Ophthalmol. 2016, 30, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Zapata, M.J.; Martí-Carvajal, A.; Solà, I.; Bolibar, I.; Angel Expósito, J.; Rodriguez, L.; García, J. Efficacy and safety of the use of autologous plasma rich in platelets for tissue regeneration: A systematic review. Transfusion 2009, 49, 44–56. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merigo, E.; Oppici, A.; Parlatore, A.; Cella, L.; Clini, F.; Fontana, M.; Fornaini, C. Platelet-Rich Plasma (PRP) Rinses for the Treatment of Non-Responding Oral Lichen Planus: A Case Report. Biomedicines 2018, 6, 15. https://doi.org/10.3390/biomedicines6010015
Merigo E, Oppici A, Parlatore A, Cella L, Clini F, Fontana M, Fornaini C. Platelet-Rich Plasma (PRP) Rinses for the Treatment of Non-Responding Oral Lichen Planus: A Case Report. Biomedicines. 2018; 6(1):15. https://doi.org/10.3390/biomedicines6010015
Chicago/Turabian StyleMerigo, Elisabetta, Aldo Oppici, Anna Parlatore, Luigi Cella, Fabio Clini, Matteo Fontana, and Carlo Fornaini. 2018. "Platelet-Rich Plasma (PRP) Rinses for the Treatment of Non-Responding Oral Lichen Planus: A Case Report" Biomedicines 6, no. 1: 15. https://doi.org/10.3390/biomedicines6010015
APA StyleMerigo, E., Oppici, A., Parlatore, A., Cella, L., Clini, F., Fontana, M., & Fornaini, C. (2018). Platelet-Rich Plasma (PRP) Rinses for the Treatment of Non-Responding Oral Lichen Planus: A Case Report. Biomedicines, 6(1), 15. https://doi.org/10.3390/biomedicines6010015