A Brief Review about the Role of Nanomaterials, Mineral-Organic Nanoparticles, and Extra-Bone Calcification in Promoting Carcinogenesis and Tumor Progression
Abstract
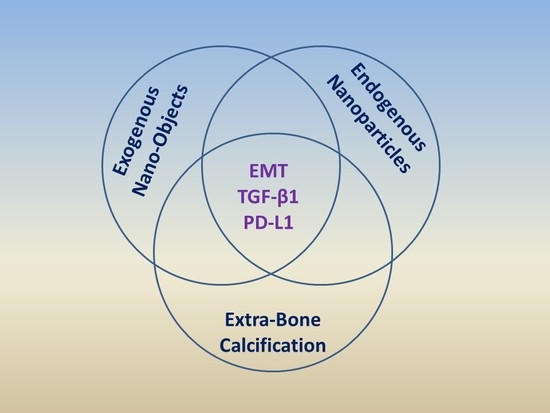
:1. Introduction
2. General Characteristics of Nano-Objects
3. The Effect of Nanoparticles on the Respiratory System
4. Interaction of Ingested Nanoparticles with the Gastrointestinal Tract
5. Toxic Effects of Nanoparticles on the Gastrointestinal Tract
5.1. In Vitro Studies of Nanoparticle Toxicity
5.2. In Vivo Study of Nanoparticle Toxicity
5.3. Bioavailability of Nanoparticles
6. Nanoparticles and Carcinogenesis
7. Endogenous Nanoparticles and Their Role in Physiological Processes and Pathology
8. Mineral-Organic Nanoparticles and Extra-Bone Calcification
Funding
Acknowledgments
Conflicts of Interest
References
- Hobson, D.W.; Roberts, S.M.; Shvedova, A.A.; Warheit, D.B.; Hinkley, G.K.; Guy, R.C. Applied Nanotoxicology. Int. J. Toxicol. 2016, 35, 5–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.W.; Cambre, M.; Lee, H.J. The Toxicity of Nanoparticles Depends on Multiple Molecular and Physicochemical Mechanisms. Int. J. Mol. Sci. 2017, 18, 2702. [Google Scholar] [CrossRef] [PubMed]
- Griffin, S.; Masood, M.I.; Nasim, M.J.; Sarfraz, M.; Ebokaiwe, A.P.; Schäfer, K.H.; Keck, C.M.; Jacob, C. Natural Nanoparticles: A Particular Matter Inspired by Nature. Antioxidants 2017, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Willhite, C.C.; Karyakina, N.A.; Yokel, R.A.; Yenugadhati, N.; Wisniewski, T.M.; Arnold, I.M.; Momoli, F.; Krewski, D. Systematic review of potential health risks posed by pharmaceutical, occupational and consumer exposures to metallic and nanoscale aluminum, aluminum oxides, aluminum hydroxide and its soluble salts. Crit. Rev. Toxicol. 2014, 44 (Suppl. 4), 1–80. [Google Scholar] [CrossRef] [PubMed]
- Grande, F.; Tucci, P. Titanium Dioxide Nanoparticles: A Risk for Human Health? Mini Rev. Med. Chem. 2016, 16, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Singh, S. Zinc Oxide Nanoparticles Impacts: Cytotoxicity, Genotoxicity, Developmental toxicity, and Neurotoxicity. Toxicol. Mech. Methods 2018, 29, 300–311. [Google Scholar] [CrossRef]
- Huang, Y.; Mei, L.; Chen, X.; Wang, Q. Recent Developments in Food Packaging Based on Nanomaterials. Nanomaterials 2018, 8, 830. [Google Scholar] [CrossRef] [PubMed]
- Sutunkova, M.P.; Privalova, L.I.; Minigalieva, I.A.; Gurvich, V.B.; Panov, V.G.; Katsnelson, B.A. The most important inferences from the Ekaterinburg nanotoxicology team’s animal experiments assessing adverse health effects of metallic and metal oxide nanoparticles. Toxicol. Rep. 2018, 5, 363–376. [Google Scholar] [CrossRef]
- Kermanizadeh, A.; Balharry, D.; Wallin, H.; Loft, S.; Møller, P. Nanomaterial translocation--the biokinetics, tissue accumulation, toxicity and fate of materials in secondary organs—A review. Crit. Rev. Toxicol. 2015, 45, 837–872. [Google Scholar] [CrossRef]
- Brown, D.M.; Wilson, M.R.; MacNee, W.; Stone, V.; Donaldson, K. Size-dependent proinflammatory effects of ultrafine polystyrene particles: A role for surface area and oxidative stress in the enhanced activity of ultrafines. Toxicol. Appl. Pharmacol. 2001, 175, 191–199. [Google Scholar] [CrossRef]
- Lahde, A.; Gudmundsdottir, S.S.; Joutsensaari, J.; Tapper, U.; Ruusunen, J.; Ihalainen, M.; Karhunen, T.; Torvela, T.; Jokiniemi, J.; Jarvinen, K.; et al. In vitro evaluation of pulmonary deposition of airborne volcanic ash. Atmos. Environ. 2013, 70, 18–27. [Google Scholar] [CrossRef]
- Porter, D.W.; Hubbs, A.F.; Chen, B.T.; McKinney, W.; Mercer, R.R.; Wolfarth, M.G.; Battelli, L.; Wu, N.; Sriram, K.; Leonard, S.; et al. Acute pulmonary dose-responses to inhaled multi-walled carbon nanotubes. Nanotoxicology 2013, 7, 1179–1194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ji, Q.; Yang, Y.; Li, Q.; Wang, Z. Exosome: Function and Role in Cancer Metastasis and Drug Resistance. Technol. Cancer Res. Treat. 2018, 17, 1533033818763450. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, M.; Stigler, J.; Elia, G.; Lynch, I.; Cedervall, T.; Dawson, K.A. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. USA 2008, 105, 14265–14270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karmali, P.P.; Simberg, D. Interactions of nanoparticles with plasma proteins: Implication on clearance and toxicity of drug delivery systems. Expert Opin. Drug Deliv. 2011, 8, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Ault, A.P.; Stark, D.I.; Axson, J.L.; Keeney, J.N.; Maynard, A.D.; Bergin, I.L.; Philbert, M.A. Protein corona-induced modification of silver nanoparticle aggregation in simulated gastric fluid. Environ. Sci. Nano 2016, 3, 1510–1520. [Google Scholar] [CrossRef] [PubMed]
- Shvedova, A.A.; Kisin, E.; Murray, A.R.; Johnson, V.J.; Gorelik, O.; Arepalli, S.; Hubbs, A.F.; Mercer, R.R.; Keohavong, P.; Sussman, N.; et al. Inhalation vs. aspiration of single-walled carbon nanotubes in C57BL/6 mice: Inflammation, fibrosis, oxidative stress, and mutagenesis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 295, L552–L565. [Google Scholar] [CrossRef] [PubMed]
- Shvedova, A.A.; Tkach, A.V.; Kisin, E.R.; Khaliullin, T.; Stanley, S.; Gutkin, D.W.; Star, A.; Chen, Y.; Shurin, G.V.; Kagan, V.E.; et al. Carbon nanotubes enhance metastatic growth of lung carcinoma via up-regulation of myeloid-derived suppressor cells. Small 2013, 9, 1691–1695. [Google Scholar] [CrossRef]
- Shvedova, A.A.; Kisin, E.R.; Yanamala, N.; Tkach, A.V.; Gutkin, D.W.; Star, A.; Shurin, G.V.; Kagan, V.E.; Shurin, M.R. MDSC and TGFβ Are Required for Facilitation of Tumor Growth in the Lungs of Mice Exposed to Carbon Nanotubes. Cancer Res. 2015, 75, 1615–1623. [Google Scholar] [CrossRef]
- Khaliullin, T.O.; Kisin, E.R.; Murray, A.R.; Yanamala, N.; Shurin, M.R.; Gutkin, D.W.; Fatkhutdinova, L.M.; Kagan, V.E.; Shvedova, A.A. Mediation of the single-walled carbon nanotubes induced pulmonary fibrogenic response by osteopontin and TGF-β1. Exp. Lung Res. 2017, 43, 311–326. [Google Scholar] [CrossRef]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; Von Goetz, N. Titanium dioxide nanoparticles in food and personal care products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.J.B.; van Bemmel, G.; Herrera-Rivera, Z.; Helsper, H.P.F.G.; Marvin, H.J.P.; Weigel, S.; Tromp, P.C.; Oomen, A.G.; Rietveld, A.; Bouwmeester, H. Characterization of titanium dioxide nanoparticles in food products: Analytical methods to define nanoparticles. J. Agric. Food Chem. 2014, 62, 6285–6293. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, P.; Yoon, J.; Lee, B.; Choi, K.; Kil, K.H.; Park, K. Serum kinetics, distribution and excretion of silver in rabbits following 28 days after a single intravenous injection of silver nanoparticles. Nanotoxicology 2013, 7, 1120–1130. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Morishita, M.; Wagner, J.G.; Fatouraie, M.; Wooldridge, M.; Eagle, W.E.; Barres, J.; Carlander, U.; Emond, C.; Jolliet, O. In Vivo biodistribution and physiologically based pharmacokinetic modeling of inhaled fresh and aged cerium oxide nanoparticles in rats. Part. Fibre Toxicol. 2016, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Pietroiusti, A.; Bergamaschi, E.; Campagna, M.; Campagnolo, L.; De Palma, G.; Iavicoli, S.; Leso, V.; Magrini, A.; Miragoli, M.; Pedata, P.; et al. The unrecognized occupational relevance of the interaction between engineered nanomaterials and the gastro-intestinal tract: A consensus paper from a multidisciplinary working group. Part. Fibre Toxicol. 2017, 14, 47. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Martel, J.; Wong, T.Y.; Young, D.; Liu, C.C.; Lin, C.W.; Young, J.D. Formation and characteristics of biomimetic mineralo-organic particles in natural surface water. Sci. Rep. 2016, 6, 28817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böhmert, L.; Girod, M.; Hansen, U.; Maul, R.; Knappe, P.; Niemann, B.; Weidner, S.M.; Thünemann, A.F.; Lampen, A. Analytically monitored digestion of silver nanoparticles and their toxicity on human intestinal cells. Nanotoxicology 2014, 8, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Axson, J.L.; Stark, D.I.; Bondy, A.L.; Capracotta, S.S.; Maynard, A.D.; Philbert, M.A.; Bergin, I.L.; Ault, A.P. Rapid kinetics of size and pH-dependent dissolution and aggregation of silver nanoparticles in simulated gastric fluid. J. Phys. Chem. C Nanomater. Interfaces 2015, 119, 20632–20641. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Ba, T.; Pu, J.; Chen, T.; Song, Y.; Gu, Y.; Qian, Q.; Xu, Y.; Xiang, K.; et al. Susceptibility of young and adult rats to the oral toxicity of titanium dioxide nanoparticles. Small 2013, 9, 1742–1752. [Google Scholar] [CrossRef]
- Brun, E.; Barreau, F.; Veronesi, G.; Fayard, B.; Sorieul, S.; Chanéac, C.; Carapito, C.; Rabilloud, T.; Mabondzo, A.; Herlin-Boime, N.; et al. Titanium dioxide nanoparticle impact and translocation through ex vivo, in vivo and in vitro gut epithelia. Part. Fibre Toxicol. 2014, 11, 13. [Google Scholar] [CrossRef]
- Koeneman, B.A.; Zhang, Y.; Westerhoff, P.; Chen, Y.; Crittenden, J.C.; Capco, D.G. Toxicity and cellular responses of intestinal cells exposed to titanium dioxide. Cell Biol. Toxicol. 2010, 26, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Howe, S.E.; Licktieg, D.J.; Plunkett, K.N.; Ryerse, J.S.; Konjufca, V. The uptake of soluble and particulate antigens by epithelial cells in the mouse small intestine. PLoS ONE 2014, 9, e86656. [Google Scholar] [CrossRef] [PubMed]
- Hansen, G.H.; Rasmussen, K.; Niels-Christiansen, L.L.; Danielsen, E.M. Endocytic trafficking from the small intestinal brush border probed with FM dye. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G708–G715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernando, I.; Zhou, Y. Impact of pH on the stability, dissolution and aggregation kinetics of silver nanoparticles. Chemosphere 2019, 216, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Ude, V.C.; Brown, D.M.; Viale, L.; Kanase, N.; Stone, V.; Johnston, H.J. Impact of copper oxide nanomaterials on differentiated and undifferentiated Caco-2 intestinal epithelial cells; assessment of cytotoxicity, barrier integrity, cytokine production and nanomaterial penetration. Part. Fibre Toxicol. 2017, 14, 31. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Gorey, B.; Casey, A. In vitro comparative cytotoxicity study of aminated polystyrene, zinc oxide and silver nanoparticles on a cervical cancer cell line. Drug Chem. Toxicol. 2019, 42, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Guan, R.; Lyu, F.; Kang, T.; Wu, Y.; Chen, X. In Vitro cytotoxicity of silver nanoparticles and zinc oxide nanoparticles to human epithelial colorectal adenocarcinoma (Caco-2) cells. Mutat. Res. 2014, 769, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Tarantini, A.; Lanceleur, R.; Mourot, A.; Lavault, M.T.; Casterou, G.; Jarry, G.; Hogeveen, K.; Fessard, V. Toxicity, genotoxicity and proinflammatory effects of amorphous nanosilica in the human intestinal Caco-2 cell line. Toxicol In Vitro 2015, 29, 398–407. [Google Scholar] [CrossRef]
- Gerloff, K.; Pereira, D.I.; Faria, N.; Boots, A.W.; Kolling, J.; Förster, I.; Albrecht, C.; Powell, J.J.; Schins, R.P. Influence of simulated gastrointestinal conditions on particle-induced cytotoxicity and interleukin-8 regulation in differentiated and undifferentiated Caco-2 cells. Nanotoxicology 2013, 7, 353–366. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, I.; Barone, F.; Zijno, A.; Bizzarri, L.; Russo, M.T.; Pozzi, R.; Franchini, F.; Giudetti, G.; Uboldi, C.; Ponti, J.; et al. Comparative study of ZnO and TiO2 nanoparticles: Physicochemical characterisation and toxicological effects on human colon carcinoma cells. Nanotoxicology 2013, 7, 1361–1372. [Google Scholar] [CrossRef]
- Zijno, A.; De Angelis, I.; De Berardis, B.; Andreoli, C.; Russo, M.T.; Pietraforte, D.; Scorza, G.; Degan, P.; Ponti, J.; Rossi, F.; et al. Different mechanisms are involved in oxidative DNA damage and genotoxicity induction by ZnO and TiO2 nanoparticles in human colon carcinoma cells. Toxicol. In Vitro 2015, 29, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Sakai-Kato, K.; Hidaka, M.; Un, K.; Kawanishi, T.; Okuda, H. Physicochemical properties and in vitro intestinal permeability properties and intestinal cell toxicity of silica particles, performed in simulated gastrointestinal fluids. Biochim. Biophys. Acta 2014, 1840, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Aueviriyavit, S.; Phummiratch, D.; Maniratanachote, R. Mechanistic study on the biological effects of silver and gold nanoparticles in Caco-2 cells--induction of the Nrf2/HO-1 pathway by high concentrations of silver nanoparticles. Toxicol. Lett. 2014, 224, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Susewind, J.; De Souza Carvalho-Wodarz, C.; Repnik, U.; Collnot, E.M.; Schneider-Daum, N.; Griffiths, G.W.; Lehr, C.M. A 3D co-culture of three human cell lines to model the inflamed intestinal mucosa for safety testing of nanomaterials. Nanotoxicology 2016, 10, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Tada-Oikawa, S.; Ichihara, G.; Fukatsu, H.; Shimanuki, Y.; Tanaka, N.; Watanabe, E.; Suzuki, Y.; Murakami, M.; Izuoka, K.; Chang, J.; et al. Titanium dioxide particle type and concentration influence the inflammatory response in Caco-2 cells. Int. J. Mol. Sci. 2016, 17, 576. [Google Scholar] [CrossRef] [PubMed]
- Ammendolia, M.G.; Iosi, F.; Maranghi, F.; Tassinari, R.; Cubadda, F.; Aureli, F.; Raggi, A.; Superti, F.; Mantovani, A.; De Berardis, B. Short-term oral exposure to low doses of nano-sized TiO2 and potential modulatory effects on intestinal cells. Food Chem. Toxicol. 2017, 102, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Tilton, S.C.; Karin, N.J.; Tolic, A.; Xie, Y.; Lai, X.; Hamilton, R.F., Jr.; Waters, K.M.; Holian, A.; Witzmann, F.A.; Orr, G. Three human cell types respond to multi-walled carbon nanotubes and titanium dioxide nanobelts with cell-specific transcriptomic and proteomic expression patterns. Nanotoxicology 2014, 8, 533–548. [Google Scholar] [CrossRef]
- Mahler, G.J.; Esch, M.B.; Tako, E.; Southard, T.L.; Archer, S.D.; Glahn, R.P.; Shuler, M.L. Oral exposure to polystyrene nanoparticles affects iron absorption. Nat. Nanotechnol. 2012, 7, 264–271. [Google Scholar] [CrossRef]
- Williams, K.M.; Gokulan, K.; Cerniglia, C.E.; Khare, S. Size and dose dependent effects of silver nanoparticle exposure on intestinal permeability in an in vitro model of the human gut epithelium. J. Nanobiotechnol. 2016, 14, 62. [Google Scholar] [CrossRef]
- Ruiz, P.A.; Morón, B.; Becker, H.M.; Lang, S.; Atrott, K.; Spalinger, M.R.; Scharl, M.; Wojtal, K.A.; Fischbeck-Terhalle, A.; Frey-Wagner, I.; et al. Titanium dioxide nanoparticles exacerbate DSS-induced colitis: Role of the NLRP3 inflammasome. Gut 2017, 66, 1216–1224. [Google Scholar] [CrossRef]
- Van der Zande, M.; Vandebriel, R.J.; Van Doren, E.; Kramer, E.; Herrera Rivera, Z.; Serrano-Rojero, C.S.; Gremmer, E.R.; Mast, J.; Peters, R.J.B.; Hollman, P.C.; et al. Distribution, elimination, and toxicity of silver nanoparticles and silver ions in rats after 28-day oral exposure. ACS Nano 2012, 6, 7427–7442. [Google Scholar] [CrossRef] [PubMed]
- Warheit, D.B.; Donner, E.M. How meaningful are risk determinations in the absence of a complete dataset? Making the case for publishing standardized test guideline and ‘no effect’ studies for evaluating the safety of nanoparticulates versus spurious ‘high effect’ results from single investigative studies. Sci. Technol. Adv. Mater. 2015, 16, 034603. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.N.; Jo, U.B.; Ryu, H.Y.; Kim, Y.S.; Song, K.S.; Yu, Y.J. Histochemical study of intestinal mucins after administration of silver nanoparticles in Sprague-Dawley rats. Arch. Toxicol. 2010, 84, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, C.M. Titanium dioxide induced inflammation in the small intestine. World J. Gastroenterol. 2012, 18, 4729. [Google Scholar] [CrossRef] [PubMed]
- Masyutin, A.G.; Erokhina, M.V.; Sychevskaya, K.A.; Gusev, A.A.; Vasyukova, I.A.; Tkachev, A.G.; Smirnova, E.A.; Onishchenko, G.E. Multiwalled carbon nanotubules induce pathological changes in the digestive organs of mice. Bull. Exp. Biol. Med. 2016, 161, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Urrutia-Ortega, I.M.; Garduno-Balderas, L.G.; Delgado-Buenrostra, N.L.; Pedraza-Chaverri, J.; Hernandez-Pando, R.; Rodriguez-Sosa, M.; Leon-Cabrera, S.; Terrazas, L.L.; van Loveren, H.; Chirino, Y.L. Food-grade titanium dioxide exposure exacerbates tumor formation in colitis associated cancer model. Food Chem. Toxicol. 2016, 93, 20–31. [Google Scholar] [CrossRef]
- Bettini, S.; Boutet-Robinet, E.; Cartier, C.; Coméra, C.; Gaultier, E.; Dupuy, J.; Naud, N.; Taché, S.; Grysan, P.; Reguer, S.; et al. Food-grade TiO2impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon. Sci. Rep. 2017, 7, 40373. [Google Scholar] [CrossRef]
- Sycheva, L.P.; Zhurkov, V.S.; Iurchenko, V.V.; Daugel-Dauge, N.O.; Kovalenko, M.A.; Krivtsova, E.K.; Durnev, A.D. Investigation of genotoxic and cytotoxic effects of micro- and nanosized titanium dioxide in six organs of mice in vivo. Mutat. Res. 2011, 726, 8–14. [Google Scholar] [CrossRef]
- Seok, S.H.; Cho, W.S.; Park, J.S.; Na, Y.; Jang, A.; Kim, H.; Cho, Y.; Kim, T.; You, J.R.; Ko, S.; et al. Rat pancreatitis produced by 13-week administration of zinc oxide nanoparticles: Biopersistence of nanoparticles and possible solutions. J. Appl. Toxicol. 2013, 33, 1089–1096. [Google Scholar] [CrossRef]
- Tassinari, R.; Cubadda, F.; Moracci, G.; Aureli, F.; D’Amato, M.; Valeri, M.; De Berardis, B.; Raggi, A.; Mantovani, A.; Passeri, D.; et al. Oral, short-term exposure to titanium dioxide nanoparticles in Sprague-Dawley rat: Focus on reproductive and endocrine systems and spleen. Nanotoxicology 2014, 8, 654–662. [Google Scholar] [CrossRef]
- Choi, J.; Kim, H.; Kim, P.; Jo, E.; Kim, H.M.; Lee, M.Y.; Jin, S.M.; Park, K. Toxicity of zinc oxide nanoparticles in rats treated by two different routes: Single intravenous injection and single oral administration. J. Toxicol. Environ. Health Part A 2015, 78, 226–243. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, D.E.; Venema, K.; Gombau, L.; Valerio, L.G., Jr.; Raju, J.; Bondy, G.S.; Bouwmeester, H.; Singh, R.P.; Clippinger, A.J.; Collnot, E.M.; et al. Utility of models of the gastrointestinal tract for assessment of the digestion and absorption of engineered nanomaterials released from food matrices. Nanotoxicology 2015, 9, 523–542. [Google Scholar] [CrossRef] [PubMed]
- Sansonetti, P.J.; Phalipon, A. M cells as ports of entry for enteroinvasive pathogens: Mechanisms of interaction, consequences for the disease process. Semin. Immunol. 1999, 11, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Jepson, M.A.; Clark, M.A. Studying M cells and their role in infection. Trends Microbiol. 1998, 6, 359–365. [Google Scholar] [CrossRef]
- Clark, M.A.; Jepson, M.A.; Hirst, B.H. Exploiting M cells for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2001, 50, 81–106. [Google Scholar] [CrossRef]
- Bouwmeester, H.; van der Zande, M.; Jepson, M.A. Effects of food-borne nanomaterials on gastrointestinal tissues and microbiota. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1481. [Google Scholar] [CrossRef] [PubMed]
- Ashwood, P.; Thompson, R.P.; Powell, J.J. Fine particles that adsorb lipopolysaccharide via bridging calcium cations may mimic bacterial pathogenicity towards cells. Exp. Biol. Med. 2007, 232, 107–117. [Google Scholar]
- Powell, J.J.; Faria, N.; Thomas-McKay, E.; Pele, L.C. Origin and fate of dietary nanoparticles and microparticles in the gastrointestinal tract. J. Autoimmun. 2010, 34, J226–J233. [Google Scholar] [CrossRef]
- Powell, J.J.; Thomas-McKay, E.; Thoree, V.; Robertson, J.; Hewitt, R.E.; Skepper, J.N.; Brown, A.; Hernandez-Garrido, J.C.; Midgley, P.A.; Gomez-Morilla, I.; et al. An endogenous nanomineral chaperones luminal antigen and peptidoglycan to intestinal immune cells. Nat. Nanotechnol. 2015, 10, 361–369. [Google Scholar] [CrossRef]
- Jani, P.U.; McCarthy, D.E.; Florence, A.T. Titanium dioxide (rutile) particle uptake from the rat GI tract and translocation to systemic organs after oral administration. Int. J. Pharm. 1994, 105, 157–168. [Google Scholar] [CrossRef]
- Zhou, M.; Ge, X.; Ke, D.M.; Tang, H.; Zhang, J.Z.; Calvaresi, M.; Gao, B.; Sun, L.; Su, Q.; Wang, H. The Bioavailability, Biodistribution, and Toxic Effects of Silica-Coated Upconversion Nanoparticles in vivo. Front. Chem. 2019, 7, 218. [Google Scholar] [CrossRef] [PubMed]
- Pele, L.C.; Thoree, V.; Bruggraber, S.F.; Koller, D.; Thompson, R.P.; Lomer, M.C.; Powell, J.J. Pharmaceutical/food grade titanium dioxide particles are absorbed into the bloodstream of human volunteers. Part. Fibre Toxicol. 2015, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Hinkley, G.K.; Carpinone, P.; Munson, J.W.; Powers, K.W.; Roberts, S.M. Oral absorption of PEG-coated versus uncoated gold nanospheres: Does agglomeration matter? Part. Fibre Toxicol. 2015, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Luo, F.; Zhu, B.; Wang, G.X. Mitochondrial impairment and oxidative stress mediated apoptosis induced by α-Fe2O3 nanoparticles in Saccharomyces cerevisiae. Toxicol. Res. 2017, 6, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, Z.; Du, J.; Qin, W.; Lu, M.; Cui, H.; Li, X.; Ding, S.; Li, R.; Yuan, J. Dermal exposure to nano-TiO2 induced cardiovascular toxicity through oxidative stress, inflammation and apoptosis. J. Toxicol. Sci. 2019, 44, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Singh, D.; Dubey, K.; Maurya, R.; Pandey, A.K. Zinc oxide nanoparticles induced gene mutation at the HGPRT locus and cell cycle arrest associated with apoptosis in V-79 cells. J. Appl. Toxicol. 2019, 39, 735–750. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, S.; Kasai, T.; Umeda, Y.; Ohnishi, M.; Sasaki, T.; Matsumoto, M. Carcinogenicity of multi-walled carbon nanotubes: Challenging issue on hazard assessment. J. Occup. Health 2018, 60, 10–30. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, K.; Murphy, F.A.; Duffin, R.; Poland, C.A. Asbestos, carbon nanotubes and the pleural mesothelium: A review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part. Fibre Toxicol. 2010, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Kasai, T.; Umeda, Y.; Ohnishi, M.; Mine, T.; Kondo, H.; Takeuchi, T.; Matsumoto, M.; Fukushima, S. Lung carcinogenicity of inhaled multi-walled carbon nanotube in rats. Part. Fibre Toxicol. 2016, 13, 53. [Google Scholar] [CrossRef]
- Sargent, L.M.; Porter, D.W.; Staska, L.M.; Hubbs, A.F.; Lowry, D.T.; Battelli, L.; Siegrist, K.J.; Kashon, M.L.; Mercer, R.R.; Bauer, A.K.; et al. Promotion of lung adenocarcinoma following inhalation exposure to multi-walled carbon nanotubes. Part. Fibre Toxicol. 2014, 11, 3. [Google Scholar] [CrossRef]
- Wang, P.; Voronkova, M.; Luanpitpong, S.; He, X.; Riedel, H.; Dinu, C.Z.; Wang, L.; Rojanasakul, Y. Induction of Slug by Chronic Exposure to Single-Walled Carbon Nanotubes Promotes Tumor Formation and Metastasis. Chem. Res. Toxicol. 2017, 30, 1396–1405. [Google Scholar] [CrossRef]
- Lindberg, H.K.; Falck, G.C.; Suhonen, S.; Vippola, M.; Vanhala, E.; Catalán, J.; Savolainen, K.; Norppa, H. Genotoxicity of nanomaterials: DNA damage and micronuclei induced by carbon nanotubes and graphite nanofibres in human bronchial epithelial cells in vitro. Toxicol. Lett. 2009, 186, 166–173. [Google Scholar] [CrossRef]
- Pacurari, M.; Yin, X.J.; Zhao, J.; Ding, M.; Leonard, S.S.; Schwegler-Berry, D.; Ducatman, B.S.; Sbarra, D.; Hoover, M.D.; Castranova, V.; et al. Raw single-wall carbon nanotubes induce oxidative stress and activate MAPKs, AP-1, NF-kappaB, and Akt in normal and malignant human mesothelial cells. Environ. Health Perspect. 2008, 116, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.; Fujitani, Y.; Furuyama, A.; Kanno, S. Uptake and cytotoxic effects of multi-walled carbon nanotubes in human bronchial epithelial cells. Toxicol. Appl. Pharmacol. 2010, 249, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. Biomed. Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef] [PubMed]
- Shvedova, A.A.; Yanamala, N.; Kisin, E.R.; Tkach, A.V.; Murray, A.R.; Hubbs, A.; Chirila, M.M.; Keohavong, P.; Sycheva, L.P.; Kagan, V.E.; et al. Long-term effects of carbon containing engineered nanomaterials and asbestos in the lung: One year postexposure comparisons. Am. J. Physiol. 2014, 306, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Song, W.J.; Jeong, M.S.; Choi, D.M.; Kim, K.N.; Wie, M.B. Zinc Oxide Nanoparticles Induce Autophagy and Apoptosis via Oxidative Injury and Pro-Inflammatory Cytokines in Primary Astrocyte Cultures. Nanomaterials 2019, 9, 1043. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.M.; Casey, L.M.; Hughes, K.R.; Wang, L.Z.; North, M.G.; Getts, D.R.; Miller, S.D.; She, L.D. Controlled Delivery of Single or Multiple Antigens in Tolerogenic Nanoparticles Using Peptide-Polymer Bioconjugates. Mol. Ther. 2017, 25, 1655–1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomić, S.; Janjetović, K.; Mihajlović, D.; Milenković, M.; Kravić-Stevović, T.; Marković, Z.; Todorović-Marković, B.; Spitalsky, Z.; Micusik, M.; Vučević, D.; et al. Graphene quantum dots suppress proinflammatory T cell responses via autophagy-dependent induction of tolerogenic dendritic cells. Biomaterials 2017, 146, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Stead, S.O.; Kireta, S.; McInnes, S.J.P.; Kette, F.D.; Sivanathan, K.N.; Kim, J.; Cueto-Diaz, E.J.; Cunin, F.; Durand, J.O.; Drogemuller, C.J.; et al. Murine and Non-Human Primate Dendritic Cell Targeting Nanoparticles for in Vivo Generation of Regulatory T-Cells. ACS Nano 2018, 12, 6637–6647. [Google Scholar] [CrossRef]
- Kuo, R.; Saito, E.; Miller, S.D.; Shea, L.D. Peptide-Conjugated Nanoparticles Reduce Positive Co-stimulatory Expression and T Cell Activity to Induce Tolerance. Mol. Ther. 2017, 25, 1676–1685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarthy, D.P.; Yap, J.W.; Harp, C.T.; Song, W.K.; Chen, J.; Pearson, R.M.; Miller, S.D.; Shea, L.D. An antigen-encapsulating nanoparticle platform for TH1/17 immune tolerance therapy. Nanomedicine 2017, 13, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Setyawati, M.I.; Tay, C.Y.; Bay, B.H.; Leong, D.T. Gold Nanoparticles Induced Endothelial Leakiness Depends on Particle Size and Endothelial Cell Origin. ACS Nano 2017, 11, 5020–5030. [Google Scholar] [CrossRef] [PubMed]
- Tay, C.Y.; Setyawati, M.I.; Leong, D.T. Nanoparticle Density: A Critical Biophysical Regulator of Endothelial Permeability. ACS Nano 2017, 11, 2764–2772. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Setyawati, M.I.; Tee, J.K.; Ding, X.; Wang, J.; Nga, M.E.; Ho, H.K.; Leong, D.T. Nanoparticles promote in vivo breast cancer cell intravasation and extravasation by inducing endothelial leakiness. Nat. Nanotechnol. 2019, 14, 279–286. [Google Scholar] [CrossRef]
- Bjarnadóttir, K.; Benkhoucha, M.; Merkler, D.; Weber, M.S.; Payne, N.L.; Bernard, C.C.; Molnarfi, N.; Lalive, P.H. B cell-derived transforming growth factor-β1 expression limits the induction phase of autoimmune neuroinflammation. Sci. Rep. 2016, 6, 34594. [Google Scholar] [CrossRef]
- Sanjabi, S.; Oh, S.A.; Li, M.O. Regulation of the Immune Response by TGF-β: From Conception to Autoimmunity and Infection. Cold Spring Harb. Perspect. Biol. 2017, 9, a022236. [Google Scholar] [CrossRef]
- Fuxe, J.; Karlsson, M.C. TGF-β-induced epithelial-mesenchymal transition: A link between cancer and inflammation. Semin. Cancer Biol. 2012, 22, 455–461. [Google Scholar] [CrossRef]
- Johansson, J.; Tabor, V.; Wikell, A.; Jalkanen, S.; Fuxe, J. TGF-β1-induced epithelial–mesenchymal transition promotes monocyte/macrophage properties in breast cancer cells. Front. Oncol. 2015, 5, 3. [Google Scholar] [CrossRef]
- Lee, H.; Pyo, M.J.; Bae, S.K.; Heo, Y.; Choudhary, I.; Hwang, D.; Yang, H.; Kim, J.H.; Chae, J.; Han, C.H.; et al. Nemopilema nomurai jellyfish venom exerts an anti-metastatic effect by inhibiting Smad- and NF-κB-mediated epithelial-mesenchymal transition in HepG2 cells. Sci. Rep. 2018, 8, 2808. [Google Scholar] [CrossRef]
- Yamagiwa, S.; Gray, J.D.; Hashimoto, S.; Horwitz, D.A. A Role for TGF-beta in the Generation and Expansion of CD4+CD25+ Regulatory T Cells from Human Peripheral Blood. J. Immunol. 2001, 166, 7282–7289. [Google Scholar] [CrossRef] [PubMed]
- Worthington, J.J.; Travis, M.A. Effector Tregs: Middle-men in TGFβ activation. Oncotarget 2015, 6, 19958–19959. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, P.G.; Moreno-Bueno, G.; Portillo, F.; Cano, A. EMT: Present and future in clinical oncology. Mol. Oncol. 2017, 11, 718–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Pang, N.; Du, R.; Zhu, Y.; Fan, L.; Cai, D.; Ding, Y.; Ding, J. Elevated Expression of Programmed Death-1 and Programmed Death Ligand-1 Negatively Regulates Immune Response against Cervical Cancer Cells. Mediat. Inflamm. 2016, 2016, 6891482. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, S.; Saeki, H.; Nakashima, Y.; Ito, S.; Oki, E.; Morita, M.; Oda, Y.; Okano, S.; Maehara, Y. Programmed death-ligand 1 expression at tumor invasive front is associated with epithelial-mesenchymal transition and poor prognosis in esophageal squamous cell carcinoma. Cancer Sci. 2017, 108, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Celada, L.J.; Kropski, J.A.; Herazo-Maya, J.D.; Luo, W.; Creecy, A.; Abad, A.T.; Chioma, O.S.; Lee, G.; Hassell, N.E.; Shaginurova, G.I.; et al. PD-1 up-regulation on CD4+ T cells promotes pulmonary fibrosis through STAT3-mediated IL-17A and TGF-β1 production. Sci. Transl. Med. 2018, 10, eaar8356. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Hölzel, M.; Knijnenburg, T.; Schlicker, A.; Roepman, P.; McDermott, U.; Garnett, M.; Grernrum, W.; Sun, C.; Prahallad, A.; et al. MED12 controls the response to multiple cancer drugs through regulation of TGF-β receptor signaling. Cell 2012, 151, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Oettle, H.; Hilbig, A.; Seufferlein, T.; Tsianakas, A.; Luger, T.; Schmid, R.M.; von Wichert, G.; Endlicher, E.; Garbe, C.; Kaehler, K.K.; et al. Phase I/II study with trabedersen (AP 12009) monotherapy for the treatment of patients with advanced pancreatic cancer, malignant melanoma, and colorectal carcinoma. J. Clin. Oncol. 2011, 29 (Suppl. 15), 2513. [Google Scholar] [CrossRef]
- Martel, J.; Young, J.D. Purported nanobacteria in human blood as calcium carbonate nanoparticles. Proc. Natl. Acad. Sci. USA 2008, 105, 5549–5554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, H.H.; Wu, C.Y.; Young, D.; Martel, J.; Young, A.; Ojcius, D.M.; Lee, Y.H.; Young, J.D. Physicochemical and biological properties of biomimetic mineralo-protein nanoparticles formed spontaneously in biological fluids. Small 2013, 9, 2297–2307. [Google Scholar] [CrossRef]
- Kutikhin, A.G.; Yuzhalin, A.E.; Borisov, V.V.; Velikanova, E.A.; Frolov, A.V.; Sakharova, V.M.; Brusina, E.B.; Golovkin, A.S. Calcifying nanoparticles: One face of distinct entities? Front. Microbiol. 2014, 5, 214. [Google Scholar] [CrossRef] [PubMed]
- Heiss, A.; Eckert, T.; Aretz, A.; Richtering, W.; van Dorp, W.; Schäfer, C.; Jahnen-Dechent, W. Hierarchical role of fetuin-A and acidic serum proteins in the formation and stabilization of calcium phosphate particles. J. Biol. Chem. 2008, 283, 4815–4825. [Google Scholar] [CrossRef] [PubMed]
- Jahnen-Dechent, W.; Heiss, A.; Schäfer, C.; Ketteler, M. Fetuin-A regulation of calcified matrix metabolism. Circ. Res. 2011, 108, 1494–1509. [Google Scholar] [CrossRef] [PubMed]
- Martel, J.; Peng, H.H.; Young, D.; Wu, C.Y.; Young, J.D. Of nanobacteria, nanoparticles, biofilms and their role in health and disease: Facts, fancy, and future. Nanomedicine 2014, 9, 483–499. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Martel, J.; Cheng, W.Y.; He, C.C.; Ojcius, D.M.; Young, J.D. Membrane vesicles nucleate mineralo-organic nanoparticles and induce carbonate apatite precipitation in human body fluids. J. Biol. Chem. 2013, 288, 30571–30584. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Hu, Y.; Yan, C.; Mu, L.; Huang, K.; Li, X.; Tao, D.; Wu, Y.; Qin, J. Fibroblast-Derived Exosomes Contribute to Chemoresistance through Priming Cancer Stem Cells in Colorectal Cancer. PLoS ONE 2015, 10, e0125625. [Google Scholar] [CrossRef] [PubMed]
- Zomer, A.; Maynard, C.; Verweij, F.J.; Kamermans, A.; Schäfer, R.; Beerling, E.; Schiffelers, R.M.; de Wit, E.; Berenguer, J.; Ellenbroek, S.I.J.; et al. In Vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell 2015, 161, 1046–1057. [Google Scholar] [CrossRef]
- Zhou, J.; Li, X.L.; Chen, Z.R.; Chng, W.J. Tumor-derived exosomes in colorectal cancer progression and their clinical applications. Oncotarget 2017, 8, 100781–100790. [Google Scholar] [CrossRef] [Green Version]
- Gopal, S.K.; Greening, D.W.; Rai, A.; Chen, M.; Xu, R.; Shafiq, A.; Mathias, R.A.; Zhu, H.J.; Simpson, R.J. Extracellular vesicles: Their role in cancer biology and epithelial-mesenchymal transition. Biochem. J. 2017, 474, 21–45. [Google Scholar] [CrossRef]
- Kajander, E.O.; Kuronen, I.; Akerman, K.; Pelttari, A.; Ciftcioglu, N. Nanobacteria from blood, the smallest culturable autonomously replicating agent on Earth. Proc. SPIE 1997, 3111, 420–428. [Google Scholar]
- Kajander, E.O.; Ciftcioglu, N. Nanobacteria: An alternative mechanism for pathogenic intra- and extracellular calcification and stone formation. Proc. Natl. Acad. Sci. USA 1998, 95, 8274–8279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumon, H.; Matsuura, E.; Nagaoka, N.; Yamamoto, T.; Uehara, S.; Araki, M.; Matsunami, Y.; Kobayashi, K.; Matsumoto, A. Ectopic calcification: Importance of common nanoparticle scaffolds containing oxidized acidic lipids. Nanomedicine 2014, 10, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Martel, J.; Wu, C.Y.; Huang, P.R.; Cheng, W.Y.; Young, J.D. Pleomorphic bacteria-like structures in human blood represent non-living membrane vesicles and protein particles. Sci. Rep. 2017, 7, 10650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martel, J.; Wu, C.Y.; Peng, H.H.; Young, J.D. Mineralo-organic nanoparticles in health and disease: An overview of recent findings. Nanomedicine 2018. [Google Scholar] [CrossRef] [PubMed]
- Chin, W.C.; Orellana, M.V.; Verdugo, P. Spontaneous assembly of marine dissolved organic matter into polymer gels. Nature 1998, 391, 568–572. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Pareek, V.K.; Liu, J. Synthesis of micro and nano-sized calcium carbonate particles and their applications. J. Mater. Chem. A 2014, 2, 14270–14288. [Google Scholar] [CrossRef]
- Koo, A.N.; Min, K.H.; Lee, H.J.; Jegal, J.H.; Lee, J.W.; Lee, S.C. Calcium carbonate mineralized nanoparticles as an intracellular transporter of cytochromec for cancer therapy. Chem. Asian J. 2015, 10, 2380–2387. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K.; Lotfipour, F. Calcium carbonate nanoparticles as cancer drug delivery system. Expert Opin. Drug Deliv. 2015, 12, 1649–1660. [Google Scholar] [CrossRef]
- Hua, K.H.; Wang, H.C.; Chung, R.S.; Hsu, J.C. Calcium carbonate nanoparticles can enhance plant nutrition and insect pest tolerance. J. Pestic. Sci. 2015, 40, 208–213. [Google Scholar] [CrossRef] [Green Version]
- Jeong, M.S.; Cho, H.S.; Park, S.J.; Song, K.S.; Ahn, K.S.; Cho, M.H.; Kim, J.S. Physicochemical characterization-based safety evaluation of nanocalcium. Food Chem. Toxicol. 2013, 62, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Kamba, A.S.; Ismail, M.; Ibrahim, T.A.; Zakaria, Z.A. Biocompatibility of bio based calcium carbonate nanocrystals aragonite polymorph on NIH 3T3 fibroblast cell line. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.J.; Liu, S.N.; Xu, G.; Guo, Y.N.; Fu, J.N.; Zhang, D.C. Cytotoxicity and apoptosis induced by nanobacteria in human breast cancer cells. Int. J. Nanomed. 2014, 9, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.B.; Balaravi Pillai, B.; Tha, K.K.; Ashaie, M.; Karim, M.E.; Chowdhury, E.H. Carbonate Apatite Nanoparticles-Facilitated Intracellular Delivery of siRNA(s) Targeting Calcium Ion Channels Efficiently Kills Breast Cancer Cells. Toxics 2018, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, N.; Zarei, L.; Esmaeili Govarchin Galeh, H.; Mansori Motlagh, B. Assessment of synergistic effect of combining hyperthermia with irradiation and calcium carbonate nanoparticles on proliferation of human breast adenocarcinoma cell line (MCF-7 cells). Artif. Cells Nanomed. Biotechnol. 2018, 46, 364–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, J.H.; Park, S.J.; Jeong, M.S.; Song, K.S.; Ahn, K.S.; Ryu, H.R.; Lee, H.; Song, M.R.; Cho, M.H.; Kim, J.S. Physicochemical analysis and repeated-dose 90-days oral toxicity study of nanocalcium carbonate in Sprague-Dawley rats. Nanotoxicology 2015, 9, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Kamba, A.S.; Ismail, M.; Ibrahim, T.A.; Zakaria, Z.A.; Gusau, L.H. In vitro ultrastructural changes of MCF-7 for metastasise bone cancer and induction of apoptosis via mitochondrial cytochrome C released by CaCO3/Dox nanocrystals. Biomed Res. Int. 2014, 2014, 391869. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wang, C.Q.; Zhuo, R.X.; Cheng, S.X. Modification of nanostructured calcium carbonate for efficient gene delivery. Colloids Surf. B Biointerfaces 2014, 118, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Q.; Wu, J.L.; Zhuo, R.X.; Cheng, S.X. Protamine sulfate-calcium carbonate-plasmid DNA ternary nanoparticles for efficient gene delivery. Mol. Biosyst. 2014, 10, 672–678. [Google Scholar] [CrossRef]
- Jaji, A.Z.; Zakaria, Z.A.B.; Mahmud, R.; Loqman, M.Y.; Hezmee, M.N.M.; Abba, Y.; Isa, T.; Mahmood, S.K. Safety assessments of subcutaneous doses of aragonite calcium carbonate nanocrystals in rats. J. Nanopart. Res. 2017, 19, 175. [Google Scholar] [CrossRef]
- Wu, J.H.; Deng, Y.L.; Liu, Q.; Yu, J.C.; Liu, Y.L.; He, Z.Q.; Guan, X.F. Induction of apoptosis and autophagy by calcifying nanoparticles in human bladder cancer cells. Tumour. Biol. 2017, 39, 1010428317707688. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Lee, J.A.; Jo, M.R.; Kim, M.K.; Kim, H.M.; Oh, J.M.; Song, N.W.; Choi, S.J. Cytotoxicity, Uptake Behaviors, and Oral Absorption of Food Grade Calcium Carbonate Nanomaterials. Nanomaterials 2015, 5, 1938–1954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horie, M.; Nishio, K.; Kato, H.; Endoh, S.; Fujita, K.; Nakamura, A.; Kinugasa, S.; Hagihara, Y.; Yoshida, Y.; Iwahashi, H. Evaluation of cellular influences caused by calcium carbonate nanoparticles. Chem. Biol. Interact. 2014, 210, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Kamba, A.S.; Zakaria, Z.A. Osteoblasts growth behaviour on bio-based calcium carbonate aragonite nanocrystal. Biomed Res. Int. 2014, 2014, 215097. [Google Scholar] [CrossRef]
- Senchukova, M.A.; Stadnikov, A.A.; Kozlov, A.A.; Bokov, D.A. Method of the Experimental Gastric Cancer Modeling. Patent for the Invention RU 2,401,463, 10 October 2010. (In Russian). [Google Scholar]
- Senchukova, M.; Tomchuk, O.; Shurygina, E.; Letuta, S.; Alidzhanov, E.; Nikiyan, H.; Razdobreev, D. Calcium Carbonate Nanoparticles Can Activate the Epithelial–Mesenchymal Transition in an Experimental Gastric Cancer Model. Biomedicines 2019, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Castellaro, A.M.; Tonda, A.; Cejas, H.H.; Ferreyra, H.; Caputto, B.L.; Pucci, O.A.; Gil, G.A. Oxalate induces breast cancer. BMC Cancer 2015, 15, 761. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Wu, C.Y.; Martel, J.; Lin, C.W.; Hsu, F.Y.; Ojcius, D.M.; Lin, P.Y.; Young, J.D. Detection and characterization of mineralo-organic nanoparticles in human kidneys. Sci. Rep. 2015, 5, 15272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, G.W.; Park, Y.M.; Yoon, H.K.; Jung, S.J.; Kim, T.H.; Lee, A.; Lee, S.M. Increased Malignant Microcalcifications after Neoadjuvant Chemotherapy in Advanced Breast Cancer. J. Breast Cancer 2016, 19, 459–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stabley, J.N.; Towler, D.A. Arterial calcification in diabetes mellitus: Preclinical models and translational implications. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Scimeca, M.; Giannini, E.; Antonacci, C.; Pistolese, C.A.; Spagnoli, L.G.; Bonanno, E. Microcalcifications in breast cancer: An active phenomenon mediated by epithelial cells with mesenchymal characteristics. BMC Cancer 2014, 14, 286. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chao, L.; Chen, L.; Tian, B.; Ma, G.; Zang, Y.; Hua, M.; Sun, J. Correlation of mammographic calcifications with Her-2/neu overexpression in primary breast carcinomas. J. Digit Imaging 2008, 21, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Hino, R.; Motoi, N.; Toda, K.; Ebina, A.; Yamada, K.; Higuchi, M.; Hirokawa, M.; Ishikawa, Y. Stromal tiny black dots, like “sugar-coated”, of von Kossa stain is a diagnostic clue to hyalinizing trabecular tumor of the thyroid gland. Pathol. Int. 2018, 68, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, N.C. Mechanisms of inhibition of calcification. Clin. Orthop. Relat. Res. 1989, 247, 279–289. [Google Scholar] [CrossRef]
- Terkeltaub, R.A.; Santoro, D.A.; Mandel, G.; Mandel, N. Serum and plasma inhibit neutrophil stimulation by hydroxyapatite crystals. Evidence that serum alpha 2-HS glycoprotein is a potent and specific crystal-bound inhibitor. Arthr. Rheum. 1988, 31, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Young, J.D.; Martel, J.; Young, L.; Wu, C.Y.; Young, A.; Young, D. Putative nanobacteria represent physiological remnants and culture by-products of normal calcium homeostasis. PLoS ONE 2009, 4, e4417. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lakatta, E.G. Role of inflammation in the pathogenesis of arterial stiffness. Yonsei Med. J. 2012, 53, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Leopold, J.A. Vascular calcification: Mechanisms of vascular smooth muscle cell calcification. Trends Cardiovasc. Med. 2015, 25, 267–274. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Ikeda, K.; Akakabe, Y.; Koide, M.; Uraoka, M.; Yutaka, K.T.; Kurimoto-Nakano, R.; Takahashi, T.; Matoba, S.; Yamada, H.; et al. Paracrine osteogenic signals via bone morphogenetic protein-2 accelerate the atherosclerotic intimal calcification in vivo. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 908–1915. [Google Scholar] [CrossRef]
- Yung, L.M.; Sánchez-Duffhues, G.; Ten Dijke, P.; Yu, P.B. Bone morphogenetic protein 6 and oxidized low-density lipoprotein synergistically recruit osteogenic differentiation in endothelial cells. Cardiovasc. Res. 2015, 108, 278–287. [Google Scholar] [CrossRef] [Green Version]
- Kowtharapu, B.S.; Prakasam, R.K.; Murín, R.; Koczan, D.; Stahnke, T.; Wree, A.; Jünemann, A.G.M.; Stachs, O. Role of Bone Morphogenetic Protein 7 (BMP7) in the Modulation of Corneal Stromal and Epithelial Cell Functions. Int. J. Mol. Sci. 2018, 19, 1415. [Google Scholar] [CrossRef]
- Scimeca, M.; Antonacci, C.; Toschi, N.; Giannini, E.; Bonfiglio, R.; Buonomo, C.O.; Pistolese, C.A.; Tarantino, U.; Bonanno, E. Breast Osteoblast-like Cells: A Reliable Early Marker for Bone Metastases from Breast Cancer. Clin. Breast Cancer 2018, 18, e659–e669. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Guihard, P.J.; Blazquez-Medela, A.M.; Guo, Y.; Moon, J.H.; Jumabay, M.; Boström, K.I.; Yao, Y. Serine protease activation essential for endothelial-mesenchymal transition in vascular calcification. Circ. Res. 2015, 117, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cen, D.; Liu, Z.; Liang, C. Presence of Intratumoral Calcifications and Vasculature Is Associated with Poor Overall Survival in Clear Cell Renal Cell Carcinoma. J. Comput. Assist. Tomogr. 2018, 42, 418–422. [Google Scholar] [CrossRef] [PubMed]


| Nano-Objects | Size (nm) | Cell Type/Animal/ Features of Experiment | Main Effects | Reference |
|---|---|---|---|---|
| In Vitro Studies | ||||
| SWCNT | 228 ± 77 | Pulmonary MDSCs from SWCNT-exposed Wild type mice bearing LLC – co-cultured with T-cells Pulmonary MDSCs – in LLC-conditioned medium |
| [19] |
| SWCNT | D1–4 x L100–1000 | Murine macrophage cells (RAW 264.7) and murine lung epithelial cells (MLE-15) |
| [20] |
| SWCNT | D0.8–0.2 x L100–1000 | Human bronchial epithelial cells (BEAS-2B) |
| [81] |
| SWCNTs | D0.8–2.0 | Normal mesothelial cell (NM) and malignant mesothelial cell (MM) cultures |
| [83] |
| MWCNT vs. TiO2 nanobelts | 458 ± 16 634 ± 86 | Human macrophages (THP-1), SAE and intestinal (Caco-2/HT29-MTX) cells |
| [47] |
| Graphene quantum dots | N/A | Monocyte-derived DCs, Human peripheral blood mononuclear cells – magnetic-activated cell sorting Mixed cell cultures – co-cultivation DCs and T cells |
| [89] |
| CNTs, GNFs | D1.1 x L500–100,000 D30–50 x L500–20,000 | Human bronchial epithelial BEAS 2B cells |
| [82] |
| CuO | 10 | Undifferentiated and differentiated Caco-2 intestinal cells |
| [35] |
| ZnO | N/A | Chinese hamster lung fibroblast cells (V-79) |
| [76] |
| Aminated polystyrene amine, ZnO, Ag | 17 ± 2, 107 ± 45 | HeLa cells |
| [36] |
| ZnO vs. Ag | 90 | Caco-2 cells |
| [37] |
| ZnO vs. TiO2 | N/A | Caco-2 cells |
| [40,41] |
| TiO2 | 30–50 | Human intestinal epithelial cells (IECs) and macrophages |
| [50] |
| SiO2 | 15, 55 | Caco-2 cell |
| [38] |
| CaCO3 | 40–60 | Mouse embryonic fibroblast NIH 3T3 cell line |
| [132] |
| Biomimetic calcium phosphate NPs | <100 | Neutrophils and macrophages isolated from whole blood of volunteers, Co-culture of neutrophils and macrophages |
| [110] |
| Food nano CaCO3 NPs vs. Food bulk CaCO3 NPs vs. reagent CaCO3 NPs (SS CaCO3) | 100 2000 110 | Human intestinal epithelial (INT-407) cells |
| [142] |
| CaCO3 | N/A | Human lung carcinoma A549 cells, Human keratinocyte HaCaT cells |
| [143] |
| CaCO3 | 35–60 | MC3T3-E1 and hFOB 1.19 osteoblast cell lines |
| [144] |
| CaCO3/CaP/ DNA vs. CaCO3/ DNA | N/A | 293T cells, HeLa cells – pGL3-Luc plasmid was used for gene transfection |
| [138] |
| Protamine sulfate -calcium carbonate-plasmid DNA (PS-CaCO3-DNA) NPs | N/A | 293T cells, HeLa cells – pGL3-Luc and pEGFP-C1 plasmids were used for gene transfection |
| [139] |
| In Vivo Studies | ||||
| MWCNT | D10 x L18 000 | C57BL/6J mice – MWCNT aerosol |
| [12] |
| MWCNT | N/A | C57BL/6 mice – inhalation exposures |
| [78] |
| SWCNT | D100 x L1000 | C57BL/6 mice – inhalation exposures vs. pharyngeal aspiration exposures |
| [17] |
| CNF vs. SWCNT vs. Asbestos | D80–60 x L5000–30,000 D65 x L1000–3000 D160–800 x L2000–30,000 | C57BL/6 mice – pharyngeal aspiration |
| [86] |
| Porous silicon NPs | 200 | C57BL/6 mice, Common marmosets (Callithrix jacchus) – intravenous injection |
| [90] |
| PLG(Ag) | 450–850 | Mouse model (SJL/J mice) of EAE – subcutaneous injection of PLP and after 7 days intravenous injection of PLG+PLP |
| [92] |
| Ag, Au, Fe3O4, SiO2, ZnO, CuO, NiO, MnO, PbO, Al2O3, TiO2 | 3.4–1000 | Outbred white rats – intratracheal instillation – intra-peritoneal injections of the same during 6–7 weeks |
| [8] |
| TiO2 | 30–50 | C57BL/6J and NLRP3-deficient mice – model of dextran sodium sulfate-induced colitis (DSS-treated mice) – by oral gavage administration |
| [50] |
| TiO2 | 66, 260 | Bl 57/6 male mice – by oral gavage administration |
| [54] |
| TiO2 | 300 | BALB/c male – colitis associated cancer (CAC model - DSS-treated mice) – by oral gavage administration |
| [56] |
| TiO2 (E-171) | 80–100 | Wistar rats – by oral gavage administration or with drinking water – induction of colon carcinogenesis by 1,2-dimethylhydrazine |
| [57] |
| TiO2 | 33, 160 | CBAB6F1 mice – by oral administration |
| [58] |
| TiO2 | 14–50 | Balb/c mice to – transdermal exposure |
| [75] |
| Ag | 60 | Sprague-Dawley rats – by oral administration |
| [53] |
| Ag-polymer conjugate NPs | 80, 400 | SJL/J mice, C57BL/6J mice – a subcutaneous administration |
| [88] |
| CaCO3 | 30 ± 5 | Sprague-Dawley rats – a single subcutaneous administration at a dose of 29,500 mg/m2 – a daily subcutaneous administration at a dose of 5900 mg/m2 for 28 days |
| [140] |
| Cancer Induction | ||||
| MWCNT | D30–80 x L500–5000 | B6C3F1 mice – intraperitoneal injection of MCA for carcinogenesis promotion and one week after that - the MWCNT inhalations |
| [80] |
| MWCNT-7 | D30–80 x L2500, D30–80 x L4200, D100 x L5000 | F344 rats and B6C3F1 mice – intratracheal instillation in rats – aerosol inhalation in mice + MCA – a single intraperitoneal injection in rats and mice –subcutaneous injection |
Results are presented for maximum NPs concentrations
| [77] |
| MWCNTs | D40–90 x L4000 | F344 rats -aerosol inhalation |
| [79] |
| SWCNT | 230 | C57BL/6 mice and TGF-β-deficient mice –model of LLC –pharyngeal aspiration |
| [18] |
| Calcium oxalate | N/A | BALB/c or BALB/c nude mice –7 injection in the mammary fat pad area in a period of 18 days |
| [147] |
| CaCO3 | 7.8 ± 10.8, 155.3 ± 86.5 | Wistar rats –suspension of CaCO3 in a mixture of formaldehyde and hydrogen peroxide by oral gavage administration |
| [146] |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senchukova, M. A Brief Review about the Role of Nanomaterials, Mineral-Organic Nanoparticles, and Extra-Bone Calcification in Promoting Carcinogenesis and Tumor Progression. Biomedicines 2019, 7, 65. https://doi.org/10.3390/biomedicines7030065
Senchukova M. A Brief Review about the Role of Nanomaterials, Mineral-Organic Nanoparticles, and Extra-Bone Calcification in Promoting Carcinogenesis and Tumor Progression. Biomedicines. 2019; 7(3):65. https://doi.org/10.3390/biomedicines7030065
Chicago/Turabian StyleSenchukova, Marina. 2019. "A Brief Review about the Role of Nanomaterials, Mineral-Organic Nanoparticles, and Extra-Bone Calcification in Promoting Carcinogenesis and Tumor Progression" Biomedicines 7, no. 3: 65. https://doi.org/10.3390/biomedicines7030065
APA StyleSenchukova, M. (2019). A Brief Review about the Role of Nanomaterials, Mineral-Organic Nanoparticles, and Extra-Bone Calcification in Promoting Carcinogenesis and Tumor Progression. Biomedicines, 7(3), 65. https://doi.org/10.3390/biomedicines7030065