New Model of Ventral Spinal Cord Lesion Induced by Balloon Compression in Rats
Abstract
:1. Introduction
2. Materials and Methods
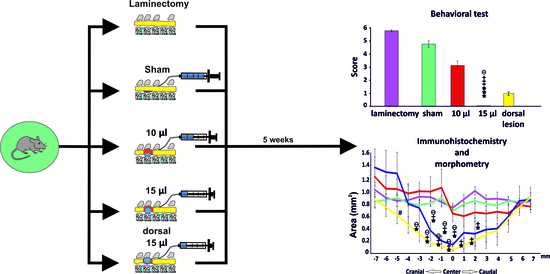
2.1. Spinal Cord Injury Procedure
2.2. Behavioral Analysis
2.2.1. Basso, Beattie and Bresnahan (BBB)
2.2.2. Ladder Rung
2.2.3. Plantar Analysis
2.3. Histological and Immunohistochemical Analysis
2.3.1. Cresyl Violet-Luxol Staining
2.3.2. GFAP Staining
2.3.3. Motoneurons
2.4. Statistics
3. Results
3.1. BBB Test
3.2. Ladder Rung Test
3.3. Plantar Test
3.4. White and Grey Matter Sparing
3.5. Astrogliosis) and Number of Protoplasmic Astrocytes
3.6. Motoneurons
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hachem, L.D.; Ahuja, C.S.; Fehlings, M.G. Assessment and management of acute spinal cord injury: From point of injury to rehabilitation. J. Spinal Cord Med. 2017, 40, 665–675. [Google Scholar] [CrossRef]
- Machova Urdzikova, L.; Karova, K.; Ruzicka, J.; Kloudova, A.; Shannon, C.; Dubisova, J.; Murali, R.; Kubinova, S.; Sykova, E.; Jhanwar-Uniyal, M.; et al. The Anti-Inflammatory Compound Curcumin Enhances Locomotor and Sensory Recovery after Spinal Cord Injury in Rats by Immunomodulation. Int. J. Mol. Sci. 2016, 17, 49. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, R.; Hickey, M.J.; Espinosa, J.M.; Nistor, G.; Lane, T.E.; Keirstead, H.S. Therapeutic neutralization of CXCL10 decreases secondary degeneration and functional deficit after spinal cord injury in mice. Regen. Med. 2007, 2, 771–783. [Google Scholar] [CrossRef]
- Lima, R.; Monteiro, S.; Lopes, J.P.; Barradas, P.; Vasconcelos, N.L.; Gomes, E.D.; Assuncao-Silva, R.C.; Teixeira, F.G.; Morais, M.; Sousa, N.; et al. Systemic Interleukin-4 Administration after Spinal Cord Injury Modulates Inflammation and Promotes Neuroprotection. Pharmaceuticals 2017, 10, 83. [Google Scholar] [CrossRef] [Green Version]
- Krupa, P.; Svobodova, B.; Dubisova, J.; Kubinova, S.; Jendelova, P.; Machova Urdzikova, L. Nano-formulated curcumin (Lipodisq) modulates the local inflammatory response, reduces glial scar and preserves the white matter after spinal cord injury in rats. Neuropharmacology 2019, 155, 54–64. [Google Scholar] [CrossRef]
- Dumont, C.M.; Margul, D.J.; Shea, L.D. Tissue Engineering Approaches to Modulate the Inflammatory Milieu following Spinal Cord Injury. Cells Tissues Organs 2016, 202, 52–66. [Google Scholar] [CrossRef] [Green Version]
- Krupa, P.; Vackova, I.; Ruzicka, J.; Zaviskova, K.; Dubisova, J.; Koci, Z.; Turnovcova, K.; Urdzikova, L.M.; Kubinova, S.; Rehak, S.; et al. The Effect of Human Mesenchymal Stem Cells Derived from Wharton’s Jelly in Spinal Cord Injury Treatment Is Dose-Dependent and Can Be Facilitated by Repeated Application. Int. J. Mol. Sci. 2018, 19, 1503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.M.; He, X.Y.; Liu, J.; Xu, Y.; Xu, F.F.; Tan, Y.X.; Zhang, Z.B.; Wang, T.H. Neural Stem Cell Transplantation Improves Locomotor Function in Spinal Cord Transection Rats Associated with Nerve Regeneration and IGF-1 R Expression. Cell Transplant. 2019, 28, 1197–1211. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Leung, G.K. Oligodendrocyte Precursor Cells in Spinal Cord Injury: A Review and Update. BioMed Res. Int. 2015, 2015, 235195. [Google Scholar] [CrossRef] [Green Version]
- Ruzicka, J.; Machova-Urdzikova, L.; Gillick, J.; Amemori, T.; Romanyuk, N.; Karova, K.; Zaviskova, K.; Dubisova, J.; Kubinova, S.; Murali, R.; et al. A Comparative Study of Three Different Types of Stem Cells for Treatment of Rat Spinal Cord Injury. Cell Transplant. 2017, 26, 585–603. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.S.; Leem, J.W.; Lee, K.H.; Kim, S.S.; Suh-Kim, H.; Jung, S.J.; Kim, U.J.; Lee, B.H. Effects of human mesenchymal stem cell transplantation combined with polymer on functional recovery following spinal cord hemisection in rats. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2012, 16, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Lynskey, J.V.; Sandhu, F.A.; Dai, H.N.; McAtee, M.; Slotkin, J.R.; MacArthur, L.; Bregman, B.S. Delayed intervention with transplants and neurotrophic factors supports recovery of forelimb function after cervical spinal cord injury in adult rats. J. Neurotrauma 2006, 23, 617–634. [Google Scholar] [CrossRef]
- Tsai, E.C.; Dalton, P.D.; Shoichet, M.S.; Tator, C.H. Matrix inclusion within synthetic hydrogel guidance channels improves specific supraspinal and local axonal regeneration after complete spinal cord transection. Biomaterials 2006, 27, 519–533. [Google Scholar] [CrossRef]
- Brock, J.H.; Rosenzweig, E.S.; Blesch, A.; Moseanko, R.; Havton, L.A.; Edgerton, V.R.; Tuszynski, M.H. Local and remote growth factor effects after primate spinal cord injury. J. Neurosci. 2010, 30, 9728–9737. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wu, F.; Kong, X.; Yang, J.; Chen, H.; Deng, L.; Cheng, Y.; Ye, L.; Zhu, S.; Zhang, X. Nerve growth factor improves functional recovery by inhibiting endoplasmic reticulum stress-induced neuronal apoptosis in rats with spinal cord injury. J. Transl. Med. 2014, 12, 130. [Google Scholar] [CrossRef] [Green Version]
- Ansorena, E.; De Berdt, P.; Ucakar, B.; Simón-Yarza, T.; Jacobs, D.; Schakman, O.; Jankovski, A.; Deumens, R.; Blanco-Prieto, M.J.; Préat, V. Injectable alginate hydrogel loaded with GDNF promotes functional recovery in a hemisection model of spinal cord injury. Int. J. Pharm. 2013, 455, 148–158. [Google Scholar] [CrossRef] [Green Version]
- Ichiyama, R.; Gerasimenko, Y.P.; Zhong, H.; Roy, R.; Edgerton, V.R. Hindlimb stepping movements in complete spinal rats induced by epidural spinal cord stimulation. Neurosci. Lett. 2005, 383, 339–344. [Google Scholar] [CrossRef]
- Lavrov, I.; Musienko, P.E.; Selionov, V.A.; Zdunowski, S.; Roy, R.R.; Edgerton, V.R.; Gerasimenko, Y. Activation of spinal locomotor circuits in the decerebrated cat by spinal epidural and/or intraspinal electrical stimulation. Brain Res. 2015, 1600, 84–92. [Google Scholar] [CrossRef]
- Krupa, P.; Siddiqui, A.M.; Grahn, P.J.; Islam, R.; Chen, B.K.; Madigan, N.N.; Windebank, A.J.; Lavrov, I.A. The Translesional Spinal Network and Its Reorganization after Spinal Cord Injury. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2020. [Google Scholar] [CrossRef]
- Metz, G.A.; Curt, A.; van de Meent, H.; Klusman, I.; Schwab, M.E.; Dietz, V. Validation of the weight-drop contusion model in rats: A comparative study of human spinal cord injury. J. Neurotrauma 2000, 17, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Tator, C.H. Update on the pathophysiology and pathology of acute spinal cord injury. Brain Pathol. 1995, 5, 407–413. [Google Scholar] [CrossRef]
- Dumont, R.J.; Okonkwo, D.O.; Verma, S.; Hurlbert, R.J.; Boulos, P.T.; Ellegala, D.B.; Dumont, A.S. Acute spinal cord injury, part I: Pathophysiologic mechanisms. Clin. Neuropharmacol. 2001, 24, 254–264. [Google Scholar] [CrossRef] [Green Version]
- Fehlings, M.G.; Smith, J.S.; Kopjar, B.; Arnold, P.M.; Yoon, S.T.; Vaccaro, A.R.; Brodke, D.S.; Janssen, M.E.; Chapman, J.R.; Sasso, R.C. Perioperative and delayed complications associated with the surgical treatment of cervical spondylotic myelopathy based on 302 patients from the AOSpine North America Cervical Spondylotic Myelopathy Study: Presented at the 2011 Spine Section Meeting. J. Neurosurg. Spine 2012, 16, 425–432. [Google Scholar] [CrossRef]
- Scheff, S.W.; Rabchevsky, A.G.; Fugaccia, I.; Main, J.A.; Lumpp, J.E., Jr. Experimental modeling of spinal cord injury: Characterization of a force-defined injury device. J. Neurotrauma 2003, 20, 179–193. [Google Scholar] [CrossRef]
- Gruner, J.A. A monitored contusion model of spinal cord injury in the rat. J. Neurotrauma 1992, 9, 123–128. [Google Scholar] [CrossRef]
- Vanicky, I.; Urdzikova, L.; Saganova, K.; Cizkova, D.; Galik, J. A simple and reproducible model of spinal cord injury induced by epidural balloon inflation in the rat. J. Neurotrauma 2001, 18, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Andrews, E.M.; Richards, R.J.; Yin, F.Q.; Viapiano, M.S.; Jakeman, L.B. Alterations in chondroitin sulfate proteoglycan expression occur both at and far from the site of spinal contusion injury. Exp. Neurol. 2012, 235, 174–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amemori, T.; Ruzicka, J.; Romanyuk, N.; Jhanwar-Uniyal, M.; Sykova, E.; Jendelova, P. Comparison of intraspinal and intrathecal implantation of induced pluripotent stem cell-derived neural precursors for the treatment of spinal cord injury in rats. Stem Cell Res. Ther. 2015, 6, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urdzikova, L.M.; Ruzicka, J.; LaBagnara, M.; Karova, K.; Kubinova, S.; Jirakova, K.; Murali, R.; Sykova, E.; Jhanwar-Uniyal, M.; Jendelova, P. Human mesenchymal stem cells modulate inflammatory cytokines after spinal cord injury in rat. Int. J. Mol. Sci. 2014, 15, 11275–11293. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.F.; Lin, C.L.; Lee, K.S.; Tsai, T.H.; Wu, S.C.; Hwang, S.L.; Chen, S.C.; Kwan, A.L. A modified compression model of spinal cord injury in rats: Functional assessment and the expression of nitric oxide synthases. Spinal Cord 2015, 53, 432–435. [Google Scholar] [CrossRef] [Green Version]
- Chung, W.H.; Lee, J.H.; Chung, D.J.; Yang, W.J.; Lee, A.J.; Choi, C.B.; Chang, H.S.; Kim, D.H.; Chung, H.J.; Suh, H.J.; et al. Improved rat spinal cord injury model using spinal cord compression by percutaneous method. J. Vet. Sci. 2013, 14, 329–335. [Google Scholar] [CrossRef] [Green Version]
- Quertainmont, R.; Cantinieaux, D.; Botman, O.; Sid, S.; Schoenen, J.; Franzen, R. Mesenchymal stem cell graft improves recovery after spinal cord injury in adult rats through neurotrophic and pro-angiogenic actions. PLoS ONE 2012, 7, e39500. [Google Scholar] [CrossRef]
- Perrin, F.E.; Boniface, G.; Serguera, C.; Lonjon, N.; Serre, A.; Prieto, M.; Mallet, J.; Privat, A. Grafted human embryonic progenitors expressing neurogenin-2 stimulate axonal sprouting and improve motor recovery after severe spinal cord injury. PLoS ONE 2010, 5, e15914. [Google Scholar] [CrossRef] [PubMed]
- Bosch, A.; Stauffer, E.S.; Nickel, V.L. Incomplete traumatic quadriplegia. A ten-year review. JAMA 1971, 216, 473–478. [Google Scholar] [CrossRef]
- McKinley, W.; Santos, K.; Meade, M.; Brooke, K. Incidence and outcomes of spinal cord injury clinical syndromes. J. Spinal Cord Med. 2007, 30, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Adedigba, J.A.; Oremakinde, A.A.; Huang, B.; Maulucci, C.M.; Malomo, A.O.; Shokunbi, T.M.; Adeolu, A.A. Preliminary Findings After Nonoperative Management of Traumatic Cervical Spinal Cord Injury on a Background of Degenerative Disc Disease: Providing Optimum Patient Care and Costs Saving in a Nigerian Setting. World Neurosurg. 2020, 142, 246–254. [Google Scholar] [CrossRef]
- Pikija, S.; Mutzenbach, J.S.; Kunz, A.B.; Nardone, R.; Leis, S.; Deak, I.; McCoy, M.R.; Trinka, E.; Sellner, J. Delayed Hospital Presentation and Neuroimaging in Non-surgical Spinal Cord Infarction. Front. Neurol. 2017, 8, 143. [Google Scholar] [CrossRef] [Green Version]
- Kwiecien, J.M.; Dabrowski, W.; Dąbrowska-Bouta, B.; Sulkowski, G.; Oakden, W.; Kwiecien-Delaney, C.J.; Yaron, J.R.; Zhang, L.; Schutz, L.; Marzec-Kotarska, B.; et al. Prolonged inflammation leads to ongoing damage after spinal cord injury. PloS ONE 2020, 15, e0226584. [Google Scholar] [CrossRef] [Green Version]
- Schucht, P.; Raineteau, O.; Schwab, M.E.; Fouad, K. Anatomical correlates of locomotor recovery following dorsal and ventral lesions of the rat spinal cord. Exp. Neurol. 2002, 176, 143–153. [Google Scholar] [CrossRef]
- Schrimsher, G.W.; Reier, P.J. Forelimb motor performance following dorsal column, dorsolateral funiculi, or ventrolateral funiculi lesions of the cervical spinal cord in the rat. Exp. Neurol. 1993, 120, 264–276. [Google Scholar] [CrossRef]
- Ijima, Y.; Furuya, T.; Koda, M.; Matsuura, Y.; Saito, J.; Kitamura, M.; Miyamoto, T.; Orita, S.; Inage, K.; Suzuki, T.; et al. Experimental rat model for cervical compressive myelopathy. Neuroreport 2017, 28, 1239–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benzel, E.C.; Lancon, J.A.; Thomas, M.M.; Beal, J.A.; Hoffpauir, G.M.; Kesterson, L. A new rat spinal cord injury model: A ventral compression technique. J. Spinal Disord. 1990, 3, 334–338. [Google Scholar]
- al-Mefty, O.; Harkey, H.L.; Marawi, I.; Haines, D.E.; Peeler, D.F.; Wilner, H.I.; Smith, R.R.; Holaday, H.R.; Haining, J.L.; Russell, W.F. Experimental chronic compressive cervical myelopathy. J. Neurosurg. 1993, 79, 550–561. [Google Scholar] [CrossRef]
- Shinomiya, K.; Mutoh, N.; Furuya, K. Study of experimental cervical spondylotic myelopathy. Spine 1992, 17, S383–S387. [Google Scholar] [CrossRef]
- Lee, J.; Satkunendrarajah, K.; Fehlings, M.G. Development and characterization of a novel rat model of cervical spondylotic myelopathy: The impact of chronic cord compression on clinical, neuroanatomical, and neurophysiological outcomes. J. Neurotrauma 2012, 29, 1012–1027. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, R.S.; Parker, J.; Syed, Y.A.; Edgley, S.; Young, A.; Fawcett, J.W.; Jeffery, N.D.; Franklin, R.J.M.; Kotter, M.R.N. Axonal plasticity underpins the functional recovery following surgical decompression in a rat model of cervical spondylotic myelopathy. Acta Neuropathol. Commun. 2016, 4, 89. [Google Scholar] [CrossRef] [Green Version]
- Urdzikova, L.; Vanicky, I. Post-traumatic moderate systemic hyperthermia worsens behavioural outcome after spinal cord injury in the rat. Spinal Cord 2006, 44, 113–119. [Google Scholar] [CrossRef]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef]
- Metz, G.A.; Whishaw, I.Q. The ladder rung walking task: A scoring system and its practical application. J. Vis. Exp. 2009. [Google Scholar] [CrossRef] [Green Version]
- May, Z.; Fouad, K.; Shum-Siu, A.; Magnuson, D.S.K. Challenges of animal models in SCI research: Effects of pre-injury task-specific training in adult rats before lesion. Behav. Brain Res. 2015, 291, 26–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruzicka, J.; Urdzikova, L.M.; Svobodova, B.; Amin, A.G.; Karova, K.; Dubisova, J.; Zaviskova, K.; Kubinova, S.; Schmidt, M.; Jhanwar-Uniyal, M.; et al. Does combined therapy of curcumin and epigallocatechin gallate have a synergistic neuroprotective effect against spinal cord injury? Neural Regen. Res. 2018, 13, 119–127. [Google Scholar]
- Svobodova, B.; Kloudova, A.; Ruzicka, J.; Kajtmanova, L.; Navratil, L.; Sedlacek, R.; Suchy, T.; Jhanwar-Uniyal, M.; Jendelova, P.; Machova Urdzikova, L. The effect of 808 nm and 905 nm wavelength light on recovery after spinal cord injury. Sci. Rep. 2019, 9, 7660. [Google Scholar] [CrossRef]
- Ruzicka, J.; Urdzikova, L.M.; Kloudova, A.; Amin, A.G.; Vallova, J.; Kubinova, S.; Schmidt, M.H.; Jhanwar-Uniyal, M.; Jendelova, P. Anti-inflammatory compound curcumin and mesenchymal stem cells in the treatment of spinal cord injury in rats. Acta Neurobiol. Exp. 2018, 78, 358–374. [Google Scholar] [CrossRef] [Green Version]
- Amemori, T.; Romanyuk, N.; Jendelova, P.; Herynek, V.; Turnovcova, K.; Prochazka, P.; Kapcalova, M.; Cocks, G.; Price, J.; Sykova, E. Human conditionally immortalized neural stem cells improve locomotor function after spinal cord injury in the rat. Stem Cell Res. Ther. 2013, 4, 68. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.H.; Lee, J.K. Animal models of axon regeneration after spinal cord injury. Neurosci. Bull. 2013, 29, 436–444. [Google Scholar] [CrossRef]
- Kwon, B.K.; Hillyer, J.; Tetzlaff, W. Translational research in spinal cord injury: A survey of opinion from the SCI community. J. Neurotrauma 2010, 27, 21–33. [Google Scholar] [CrossRef]
- Young, W. MASCIS spinal cord contusion model. In Animal Models of Acute Neurological Injuries; Springer: Berlin/Heidelberg, Germany, 2009; pp. 411–421. [Google Scholar]
- Khan, T.; Havey, R.M.; Sayers, S.T.; Patwardhan, A.; King, W.W. Animal models of spinal cord contusion injuries. Lab. Anim. Sci. 1999, 49, 161–172. [Google Scholar]
- Talac, R.; Friedman, J.A.; Moore, M.J.; Lu, L.; Jabbari, E.; Windebank, A.J.; Currier, B.L.; Yaszemski, M.J. Animal models of spinal cord injury for evaluation of tissue engineering treatment strategies. Biomaterials 2004, 25, 1505–1510. [Google Scholar] [CrossRef]
- Krishna, V.; Andrews, H.; Jin, X.; Yu, J.; Varma, A.; Wen, X.; Kindy, M. A contusion model of severe spinal cord injury in rats. J. Vis. Exp. 2013. [Google Scholar] [CrossRef] [Green Version]
- Rivlin, A.; Tator, C. Effect of duration of acute spinal cord compression in a new acute cord injury model in the rat. Surg. Neurol. 1978, 10, 38–43. [Google Scholar]
- Aslan, A.; Cemek, M.; Eser, O.; Altunbaş, K.; Buyukokuroglu, M.E.; Cosar, M.; Baş, O.; Ela, Y.; Fidan, H. Does dexmedetomidine reduce secondary damage after spinal cord injury? An experimental study. Eur. Spine J. 2009, 18, 336. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, S.; Nakamura, T.; Kishigami, Y.; Endo, K.; Azuma, T.; Fujikawa, T.; Tsutsumi, S.; Shimizu, Y. New canine spinal cord injury model free from laminectomy. Brain Res. Protoc. 2005, 14, 171–180. [Google Scholar] [CrossRef] [Green Version]
- Nesathurai, S.; Graham, W.A.; Mansfield, K.; Magill, D.; Sehgal, P.; Westmoreland, S.V.; Prusty, S.; Rosene, D.L.; Sledge, J.B. Model of traumatic spinal cord injury in Macaca fascicularis: Similarity of experimental lesions created by epidural catheter to human spinal cord injury. J. Med. Primatol. 2006, 35, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Hukuda, S.; Wilson, C.B. Experimental cervical myelopathy: Effects of compression and ischemia on the canine cervical cord. J. Neurosurg. 1972, 37, 631–652. [Google Scholar] [CrossRef] [PubMed]
- Wolfla, C.E.; Snell, B.E.; Honeycutt, J.H. Cervical ventral epidural pressure response to graded spinal canal compromise and spinal motion. Spine 2004, 29, 1524–1529. [Google Scholar] [CrossRef]
- Šedý, J.; Zicha, J.; Kuneš, J.; Jendelová, P.; Syková, E. Rapid but not slow spinal cord compression elicits neurogenic pulmonary edema in the rat. Physiol. Res. 2009, 58, 269–277. [Google Scholar]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krupa, P.; Stepankova, K.; Kwok, J.C.; Fawcett, J.W.; Cimermanova, V.; Jendelova, P.; Machova Urdzikova, L. New Model of Ventral Spinal Cord Lesion Induced by Balloon Compression in Rats. Biomedicines 2020, 8, 477. https://doi.org/10.3390/biomedicines8110477
Krupa P, Stepankova K, Kwok JC, Fawcett JW, Cimermanova V, Jendelova P, Machova Urdzikova L. New Model of Ventral Spinal Cord Lesion Induced by Balloon Compression in Rats. Biomedicines. 2020; 8(11):477. https://doi.org/10.3390/biomedicines8110477
Chicago/Turabian StyleKrupa, Petr, Katerina Stepankova, Jessica CF. Kwok, James W. Fawcett, Veronika Cimermanova, Pavla Jendelova, and Lucia Machova Urdzikova. 2020. "New Model of Ventral Spinal Cord Lesion Induced by Balloon Compression in Rats" Biomedicines 8, no. 11: 477. https://doi.org/10.3390/biomedicines8110477
APA StyleKrupa, P., Stepankova, K., Kwok, J. C., Fawcett, J. W., Cimermanova, V., Jendelova, P., & Machova Urdzikova, L. (2020). New Model of Ventral Spinal Cord Lesion Induced by Balloon Compression in Rats. Biomedicines, 8(11), 477. https://doi.org/10.3390/biomedicines8110477