Ocular Paraneoplastic Syndromes
Abstract
:1. Introduction
2. Clinical Evaluation
3. Ocular Paraneoplastic Syndromes
3.1. Cancer-Associated Retinopathy
3.2. Cancer-Associated Cone Dysfunction (CACD)
3.3. Paraneoplastic Vitelliform Maculopathy
3.4. Melanoma-Associated Retinopathy
3.5. Bilateral Diffuse Uveal Melanocytic Proliferation (BDUMP)
3.6. Paraneoplastic Optic Neuropathy
4. Ancillary Tests
4.1. Antiretinal Antibodies
- (A)
- AntirecoverinAverage age of onset is over 60 years, and patients are predominately female (female/male ratio = 2:1). This entry is highly symmetric, with acute (sudden) unexplained onset. Patient complaints are usually focused on photopsia, nyctalopia, and photophobia. Main presentation is severe central and peripheral vision loss, sometimes described as “ring scotoma”. During the course of the disease, rapid rod and cone loss is seen, often with vision acuity reduced to light perception or to no light perception in some cases. The ffERG is useful since there is a severe decrease in response of rods and cones, in addition to abnormal multifocal ERG [7,20,22,83].
- (B)
- AntienolaseAntienolase antibodies are associated with a subacute or even chronic presentation that is often symmetric. There is a variable central and global visual field loss with gradual and variable rate of visual acuity loss. Usually, the best corrected visual acuity is no better than 20/300 after several years of onset. There is characteristic nonequal dysfunction in cone and rod responses in ERG. The cones are more damaged than the rods with mild to severe abnormal mfERG [22,36,63].
- (C)
- AntitransducinThis is characterized as sudden but slowly progressive, symmetric, mild, patchy to global visual field loss with visual acuity loss secondary to the visual field symptoms. In electroretinography, primary scotopic defect (rod function) is found, and in mfERG, both decreased amplitudes and delayed timing is seen [22,77,84].
- (D)
- Anti-CAIIThis is usually a subacute, chronic, often symmetric presentation of paraneoplastic retinopathy with mild vitritis and mild to severe concentric constriction of visual field that occurs with color and visual acuity loss. In ERG, slight subnormal rod response can be found, but mfERG is of little use as it varies significantly from case to case [5,22,85].
- (E)
- Anti-Rab6The average age of onset is around 60 years, and patients are predominately female (female/male ratio = 2:1). Onset is sudden and often symmetric. Main patient complaints are photopsia and nyctalopia. Central and peripheral visual field loss can be found. In full-field ERG, the response of rods and cones are decreased, and severely abnormal mfERG can be found (which can progress over weeks to months) [7].
- (F)
- Anti-HSP27This is a type of paraneoplastic retinopathy that occurs in patients around 60 years of age with strong predominance of females over males (females/males = 3:1). Its characteristic can be described as subacute, slowly progressive, and mostly symmetric. Patient complaints are mostly focused on photopsia and nyctalopia with constriction of visual field, occurrence of central scotoma, or enlargement of blind spot. In ERG, generalized loss of response of rods and cones can be recorded [7].
- (G)
- Anti-GCAP1The anti-GCAP1 retinopathy occurs equally in males and females. Its onset can be characterized as subacute, chronic, and symmetric. The main patient complaint is photophobia. In additional tests, maculopathy can be seen with loss of color vision and visual acuity. Progressive cone loss with gradual cone dysfunction is characteristic in ERG [7].
4.2. Tumor-Expressed Growth Factors
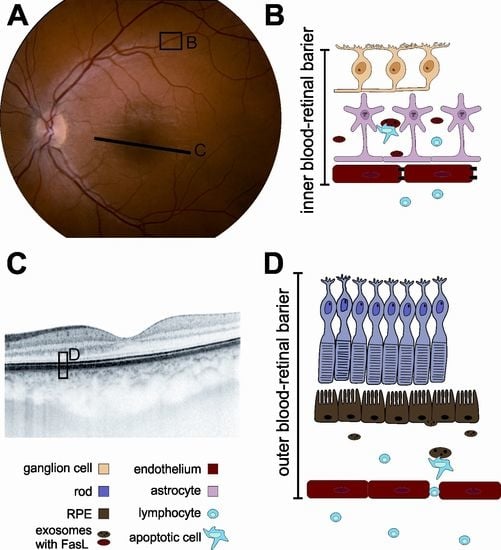
5. Possible Role of Extracellular Vesicles (EVs) in the Development of Paraneoplastic Syndromes
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AAbs | autoantibodies |
| AIPL1 | aryl hydrocarbon receptor interacting protein-like 1 |
| ANNA-1 | type 1 antineuronal nuclear antibody |
| BCVA | best-corrected visual acuity |
| BDUMP | bilateral diffuse uveal melanocytic proliferation |
| CA II | carbonic anhydrase II |
| CACD | cancer-associated cone dysfunction |
| CAR | cancer-associated retinopathy |
| CMEP factor | cultured melanocyte elongation and proliferation factor |
| CRMP5 | collapsin response mediator protein 5 |
| EOG | electrooculography |
| ERG | electroretinography |
| FAF | fundus autofluorescence |
| FFA | fundus fluorescein angiography |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| GCAPs | guanylyl cyclase-activating proteins |
| hsc-70 | heat shock cognate protein 70 |
| hsc-60 | heat shock cognate protein 60 |
| HSP27 | heat shock protein 27 |
| IRBP | interphotoreceptor retinoid binding protein |
| MAR | melanoma-associated retinopathy |
| MBP | myelin binding protein |
| MLSN1 | melastin 1 |
| OCT | optical coherent tomography |
| OPNS | ocular paraneoplastic syndromes |
| POEMS | polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes syndrome |
| PON | paraneoplastic optic neuritis |
| PNR | photoreceptor cell-specific nuclear receptor |
| PNS | paraneoplastic syndromes |
| PRDX3 | peroxiredoxin 3 (26-kDa) |
| PVM | paraneoplastic vitelliform maculopathy |
| Rab6A | Rab6A GTPase |
| ROS | rod outer segment protein (120-kDa) |
| RPE | retinal pigment epithelium |
| T-exGF | tumor-expressed growth factors |
| TRPM1 | transient receptor potential cation channel; subfamily M member 1 (that is labeled on ON-bipolar cells) |
| TULP1 | tubby-like protein 1 |
References
- Rahimy, E.; Sarraf, D. Paraneoplastic and non-paraneoplastic retinopathy and optic neuropathy: Evaluation and management. Surv. Ophthalmol. 2013, 58, 430–458. [Google Scholar] [CrossRef] [PubMed]
- Darnell, R.B.; Posner, J.B. Paraneoplastic syndromes involving the nervous system. N. Engl. J. Med. 2003, 349, 1543–1554. [Google Scholar] [CrossRef] [PubMed]
- De Salvo, G.; Prakash, P.; Rennie, C.A.; Lotery, A.J. Long-term survival in a case of bilateral diffuse uveal melanocytic proliferation. Eye 2011, 25, 1385–1386. [Google Scholar] [CrossRef]
- Adamus, G. Latest updates on antiretinal autoantibodies associated with vision loss and breast cancer. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1680–1688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalin, F.; Adamus, G.; Yang, S.; Landgren, E.; Palle, J.; Hallgren, Å.; Frost, B.-M.; Hugosson, T.; Landegren, N.; Eriksson, D.; et al. Aryl Hydrocarbon Receptor-Interacting Protein-Like 1 in Cancer-Associated Retinopathy. Ophthalmology 2016, 123, 1401–1404. [Google Scholar] [CrossRef]
- Weixler, B.; Oertli, D.; Nebiker, C.A. Cancer-associated retinopathy as the leading symptom in colon cancer. Clin. Case Rep. 2016, 4, 171–176. [Google Scholar] [CrossRef]
- Yang, S.; Dizhoor, A.; Wilson, D.J.; Adamus, G. GCAP1, Rab6, and HSP27: Novel Autoantibody Targets in Cancer-Associated Retinopathy and Autoimmune Retinopathy. Transl. Vis. Sci. Technol. 2016, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Yagyu, K.; Ueda, T.; Miyamoto, A.; Uenishi, R.; Matsushita, H.; Tanaka, T. Cancer-associated Retinopathy with Neuroendocrine Combined Large-cell Lung Carcinoma and Adenocarcinoma. Intern. Med. 2019, 58, 3289–3294. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Ruperto, L.; Busca-Arenzana, C.; Boto-de Los Bueis, A.; Schlincker, A.; Arnalich-Fernández, F.; Robles-Marhuenda, Á. Cancer-Associated Retinopathy and Treatment with Intravenous Immunoglobulin Therapy. A Seldom Used Approach? Ocul. Immunol. Inflamm. 2019, 1–4. [Google Scholar] [CrossRef]
- Dryja, T.P.; Demirs, J.T.; Twarog, M.; Lee, V. Complement Proteins in the Retina in Cancer-Associated Retinopathy. JAMA Ophthalmol. 2019. [Google Scholar] [CrossRef]
- Carrera, W.; Tsamis, K.A.; Shah, R. A case of cancer-associated retinopathy with chorioretinitis and optic neuritis associated with occult small cell lung cancer. BMC Ophthalmol. 2019, 19, 101. [Google Scholar] [CrossRef] [PubMed]
- Naramala, S.; Ahmad, J.; Adapa, S.; Gavini, F.; Konala, V.M. Case Series of Cancer-associated Retinopathy (CAR). Cureus 2019, 11, e4872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghadiri, N.; Yang, Y.; Burton, B.J. Cancer-associated retinopathy in ampullary pancreatic cancer. BMJ Case Rep. 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Kamei, M.; Fujitomi, Y.; Kondo, Y.; Adachi, T.; Shibata, K.; Takumi, Y.; Abe, M.; Sugio, K. Cancer-associated retinopathy after surgery for breast cancer: A case report and review of the literature. Surg. Case Rep. 2018, 4, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Igarashi, N.; Sawamura, H.; Kaburaki, T.; Aihara, M. Cancer-associated Retinopathy Developing After 10 Years of Complete Breast Cancer Remission. Neuroophthalmology 2019, 43, 36–42. [Google Scholar] [CrossRef]
- Javaid, Z.; Rehan, S.M.; Al-Bermani, A.; Payne, G. Unilateral cancer-associated retinopathy: A case report. Scott. Med. J. 2015. [Google Scholar] [CrossRef]
- Roels, D.; Ueno, S.; Talianu, C.D.; Draganova, D.; Kondo, M.; Leroy, B.P. Unilateral cancer-associated retinopathy: diagnosis, serology and treatment. Doc. Ophthalmol. 2017, 135, 233–240. [Google Scholar] [CrossRef]
- Hoogewoud, F.; Butori, P.; Blanche, P.; Brézin, A.P. Cancer-associated retinopathy preceding the diagnosis of cancer. BMC Ophthalmol. 2018, 18, 285. [Google Scholar] [CrossRef]
- Makiyama, Y.; Kikuchi, T.; Otani, A.; Oishi, A.; Guo, C.; Nakagawa, S.; Ogino, K.; Kojima, H.; Kurimoto, M.; Yoshimura, N. Clinical and immunological characterization of paraneoplastic retinopathy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5424–5431. [Google Scholar] [CrossRef] [Green Version]
- Stanwyck, L.K.; Chan, W.; Sood, A.; Susarla, G.; Romano, J.; Pefkianaki, M.; Jayasundera, K.T.; Heckenlively, J.R.; Lundy, S.K.; Sobrin, L. Correlation of Immunological Markers with Disease and Clinical Outcome Measures in Patients with Autoimmune Retinopathy. Transl. Vis. Sci. Technol. 2020, 9. [Google Scholar] [CrossRef]
- Braithwaite, T.; Vugler, A.; Tufail, A. Autoimmune Retinopathy. OPH 2012, 228, 131–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grewal, D.S.; Fishman, G.A.; Jampol, L.M. Autoimmune retinopathy and antiretinal antibodies: A review. Retina 2014, 34, 827–845. [Google Scholar] [CrossRef] [PubMed]
- Adamus, G.; Brown, L.; Schiffman, J.; Iannaccone, A. Diversity in autoimmunity against retinal, neuronal, and axonal antigens in acquired neuro-retinopathy. J. Ophthalmic Inflamm. Infect. 2011, 1, 111–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamus, G. Are Anti-Retinal Autoantibodies a Cause or a Consequence of Retinal Degeneration in Autoimmune Retinopathies? Front. Immunol. 2018, 9, 765. [Google Scholar] [CrossRef] [Green Version]
- Klingeborn, M.; Dismuke, W.M.; Rickman, C.B.; Stamer, W.D. Roles of Exosomes in the Normal and Diseased Eye. Prog. Retin. Eye Res. 2017, 59, 158–177. [Google Scholar] [CrossRef]
- Perez, V.L.; Caspi, R.R. Immune mechanisms in inflammatory and degenerative eye disease. Trends Immunol. 2015, 36, 354–363. [Google Scholar] [CrossRef] [Green Version]
- Cao, R.; Cao, Y. Cancer-associated retinopathy: A new mechanistic insight on vascular remodeling. Cell Cycle 2010, 9, 1882–1885. [Google Scholar] [CrossRef]
- Knickelbein, J.E.; Liu, B.; Arakelyan, A.; Zicari, S.; Hannes, S.; Chen, P.; Li, Z.; Grivel, J.-C.; Chaigne-Delalande, B.; Sen, H.N.; et al. Modulation of Immune Responses by Extracellular Vesicles From Retinal Pigment Epithelium. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4101–4107. [Google Scholar] [CrossRef] [Green Version]
- Gangalum, R.K.; Atanasov, I.C.; Zhou, Z.H.; Bhat, S.P. AlphaB-crystallin is found in detergent-resistant membrane microdomains and is secreted via exosomes from human retinal pigment epithelial cells. J. Biol. Chem. 2011, 286, 3261–3269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ousman, S.S.; Tomooka, B.H.; Van Noort, J.M.; Wawrousek, E.F.; O’Conner, K.; Hafler, D.A.; Sobel, R.A.; Robinson, W.H.; Steinman, L. Protective and therapeutic role for alphaB-crystallin in autoimmune demyelination. Nature 2007, 448, 474–479. [Google Scholar] [CrossRef]
- Sreekumar, P.G.; Kannan, R.; Kitamura, M.; Spee, C.; Barron, E.; Ryan, S.J.; Hinton, D.R. αB crystallin is apically secreted within exosomes by polarized human retinal pigment epithelium and provides neuroprotection to adjacent cells. PLoS ONE 2010, 5, e12578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebrahimi, K.B.; Fijalkowski, N.; Cano, M.; Handa, J.T. Decreased membrane complement regulators in the retinal pigmented epithelium contributes to age-related macular degeneration. J. Pathol. 2013, 229, 729–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamus, G. Impact of Autoantibodies against Glycolytic Enzymes on Pathogenicity of Autoimmune Retinopathy and Other Autoimmune Disorders. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mrejen, S.; Audo, I.; Bonnel, S.; Sahel, J.-A. Retinitis Pigmentosa and Other Dystrophies. Dev. Ophthalmol. 2017, 58, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Weleber, R.G.; Watzke, R.C.; Shults, W.T.; Trzupek, K.M.; Heckenlively, J.R.; Egan, R.A.; Adamus, G. Clinical and electrophysiologic characterization of paraneoplastic and autoimmune retinopathies associated with antienolase antibodies. Am. J. Ophthalmol. 2005, 139, 780–794. [Google Scholar] [CrossRef]
- Magrys, A.; Anekonda, T.; Ren, G.; Adamus, G. The role of anti-alpha-enolase autoantibodies in pathogenicity of autoimmune-mediated retinopathy. J. Clin. Immunol. 2007, 27, 181–192. [Google Scholar] [CrossRef]
- Li, D.Q.; Golding, J.; Glittenberg, C.; Choudhry, N. Multimodal Imaging Features in Acute Exudative Paraneoplastic Polymorphous Vitelliform Maculopathy. Ophthalmic Surg. Lasers Imaging Retina 2016, 47, 1143–1146. [Google Scholar] [CrossRef]
- Murtagh, P.; Treacy, M.; Stephenson, K.; Dooley, I. Acute Exudative Polymorphous Vitelliform Maculopathy Syndrome; natural history and evolution of fundal and OCT images over time. BMJ Case Rep. 2018, 2018. [Google Scholar] [CrossRef]
- Gündüz, K.; Çöndü, G.; Shields, C.L. Acute Exudative Polymorphous Paraneoplastic Vitelliform Maculopathy Managed With Intravitreal Aflibercept. Ophthalmic Surg. Lasers Imaging Retina 2017, 48, 844–850. [Google Scholar] [CrossRef] [Green Version]
- Barbazetto, I.; Dansingani, K.K.; Dolz-Marco, R.; Giovannini, A.; Piccolino, F.C.; Agarwal, A.; Lima, L.H.; Vianna, R.N.; Yannuzzi, L.A. Idiopathic Acute Exudative Polymorphous Vitelliform Maculopathy: Clinical Spectrum and Multimodal Imaging Characteristics. Ophthalmology 2018, 125, 75–88. [Google Scholar] [CrossRef] [Green Version]
- Lincoff, N.; Nadeem, M.; Younus, Z.; Thirkill, C.E. Exudative Polymorphous Vitelliform Retinopathy: Importance of Early Recognition of the Condition in Patients with Metastatic Melanoma. Ophthalmol. Ther. 2016, 5, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Eksandh, L.; Adamus, G.; Mosgrove, L.; Andréasson, S. Autoantibodies against bestrophin in a patient with vitelliform paraneoplastic retinopathy and a metastatic choroidal malignant melanoma. JAMA Ophthalmol. 2008, 126, 432–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsheikh, S.; Gurney, S.P.; Burdon, M.A. Melanoma-associated retinopathy. Clin. Exp. Dermatol. 2020, 45, 147–152. [Google Scholar] [CrossRef]
- Heberton, M.; Azher, T.; Council, M.L.; Khanna, S. Metastatic Cutaneous Melanoma Presenting With Melanoma-Associated Retinopathy. Dermatol. Surg. 2019, 45, 606–607. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.J.; Coupland, S.; Karanjia, R.; Sadun, A.A. Melanoma-Associated Retinopathy 28 Years After Diagnosis. JAMA Ophthalmol. 2017, 135, 1276–1277. [Google Scholar] [CrossRef] [PubMed]
- Aronow, M.E.; Adamus, G.; Abu-Asab, M.; Wang, Y.; Chan, C.-C.; Zakov, Z.N.; Singh, A.D. Paraneoplastic vitelliform retinopathy: Clinicopathologic correlation and review of the literature. Surv. Ophthalmol. 2012, 57, 558–564. [Google Scholar] [CrossRef] [Green Version]
- Varin, J.; Reynolds, M.M.; Bouzidi, N.; Tick, S.; Wohlschlegel, J.; Becquart, O.; Michiels, C.; Dereure, O.; Duvoisin, R.M.; Morgans, C.W.; et al. Identification and characterization of novel TRPM1 autoantibodies from serum of patients with melanoma-associated retinopathy. PLoS ONE 2020, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittal, R.; Cherepanoff, S.; Thornton, S.; Kalirai, H.; Damato, B.; Coupland, S.E. Bilateral Diffuse Uveal Melanocytic Proliferation: Molecular Genetic Analysis of a Case and Review of the Literature. Ocul. Oncol. Pathol. 2015, 2, 94–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, J.C.G.; Van Calster, J.; Pulido, J.S.; Miles, S.L.; Vile, R.G.; Van Bergen, T.; Cassiman, C.; Spielberg, L.H.; Leys, A.M. Early diagnosis and successful treatment of paraneoplastic melanocytic proliferation. Br. J. Ophthalmol. 2015, 99, 943–948. [Google Scholar] [CrossRef] [Green Version]
- Padrón-Pérez, N.; Caminal, J.M.; Lorenzo, D.; Català-Mora, J. Bilateral multiple iridociliary cysts in diffuse uveal melanocytic proliferation. Can. J. Ophthalmol. 2017, 52, e225–e228. [Google Scholar] [CrossRef] [Green Version]
- van Noort, B.C.; Keunen, J.E.E.; Schlingemann, R.O.; Marinkovic, M. Long Survival and Preservation of Good Visual Acuity in a Patient with Bilateral Diffuse Uveal Melanocytic Proliferation. Ocul. Oncol. Pathol. 2019, 5, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Lavine, J.A.; Ramos, M.S.; Wolk, A.M.; Baynes, K.; Sharma, S.; Rachitskaya, A.V.; Anand-Apte, B.; Srivastava, S.K.; Yuan, A. Heterogeneity of cultured melanocyte elongation and proliferation factor in bilateral diffuse uveal melanocytic proliferation. Exp. Eye Res. 2019, 184, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Raval, V.; Pathengay, A.; Narayanan, R. Bilateral diffuse uveal melanocytic proliferation secondary to thyroid carcinoma. Indian J. Ophthalmol. 2019, 67, 2094–2097. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F. Unilateral diffuse uveal melanocytic proliferation. Retin. Cases Brief Rep. 2018, 12, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Shiraki, A.; Winegarner, A.; Hashida, N.; Nishi, O.; Nishi, Y.; Maruyama, K.; Nishida, K. Diagnostic evaluation of optical coherence tomography angiography and fundus autofluorescence in bilateral diffuse uveal melanocytic proliferation. Am. J. Ophthalmol. Case Rep. 2018, 11, 32–34. [Google Scholar] [CrossRef]
- Alasil, T.; Coady, P.A.; Koudsi, S.; Mathur, M.; Materin, M.A. Bilateral diffuse uveal melanocytic proliferation: A case report. Retin. Cases Brief Rep. 2017, 11, 71–74. [Google Scholar] [CrossRef]
- Niffenegger, J.H.; Soltero, A.; Niffenegger, J.S.; Yang, S.; Adamus, G. Prevalence of Hepatocyte Growth Factor and Autoantibodies to α-HGF as a New Etiology for Bilateral Diffuse Uveal Melanocytic Proliferation Masquerading as Neovascular Age-Related Macular Degeneration. J. Clin. Exp. Ophthalmol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Kniggendorf, V.F.; Neto, E.T.; Maia, E.M.; Grando, J.P.S.; Bardal, A.M.C.; Beato, P.M.M.; Torres, C.C.; Maia, M. Bilateral diffuse uveal melanocytic proliferation associated with renal cancer: The importance of indocyanine green angiography and early diagnosis. Retin. Cases Brief Rep. 2018, 12, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Dolz-Marco, R.; Vilaplana, F.; Gallego-Pinazo, R.; Freund, K.B. Delayed-onset bilateral diffuse uveal melanocytic proliferation associated with gastric adenocarcinoma. Retin. Cases Brief Rep. 2017, 11 (Suppl. 1), S182–S186. [Google Scholar] [CrossRef]
- Tanaka, M.; Kamoi, K.; Nagaoka, N.; Ishida, T.; Karube, H.; Takase, H.; Ohno-Matsui, K. Bilateral diffuse retinal pigment epithelium proliferation induced by choroidal inflammation. Medicine 2019, 98. [Google Scholar] [CrossRef] [PubMed]
- Rahimy, E.; Coffee, R.E.; McCannel, T.A. Bilateral diffuse uveal melanocytic proliferation as a precursor to multiple systemic malignancies. Semin. Ophthalmol. 2015, 30, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Chen, Z.; Luo, Y.; Zhao, L.; Dai, R.; Zhong, Y. Diagnosis of bilateral diffuse uveal melanocytic proliferation unveils primary gastric adenocarcinoma: A case report. BMC Ophthalmol. 2020, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, M.; Saito, W.; Kanda, A.; Ohguro, H.; Ishida, S. A case of paraneoplastic optic neuropathy and outer retinitis positive for autoantibodies against collapsin response mediator protein-5, recoverin, and α-enolase. BMC Ophthalmol. 2014, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.K.; Woo, S.J.; Park, W.-Y.; Hwang, J.-M. Paraneoplastic neuromyelitis optica associated with ANNA-1 antibodies in invasive thymoma. BMC Ophthalmol. 2014, 14, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueroa, M.; Guo, Y.; Tselis, A.; Pittock, S.J.; Lennon, V.A.; Lucchinetti, C.F.; Lisak, R.P. Paraneoplastic neuromyelitis optica spectrum disorder associated with metastatic carcinoid expressing aquaporin-4. JAMA Neurol. 2014, 71, 495–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyer, A.; Elsone, L.; Appleton, R.; Jacob, A. A review of the current literature and a guide to the early diagnosis of autoimmune disorders associated with neuromyelitis optica. Autoimmunity 2014, 47, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Verschuur, C.V.M.; van der Kooi, A.J.; Troost, D. Anti-aquaporin 4 related paraneoplastic neuromyelitis optica in the presence of adenocarcinoma of the lung. Clin. Neuropathol. 2015, 34, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, T.; Al-Sarawi, A.; Binfalah, M.; Dermime, S. Paraneoplastic neuromyelitis optica spectrum disorder associated with stomach carcinoid tumor. Hematol. Oncol. Stem Cell Ther. 2014, 7, 116–119. [Google Scholar] [CrossRef] [Green Version]
- Dubey, D.; Lennon, V.A.; Gadoth, A.; Pittock, S.J.; Flanagan, E.P.; Schmeling, J.E.; McKeon, A.; Klein, C.J. Autoimmune CRMP5 neuropathy phenotype and outcome defined from 105 cases. Neurology 2018, 90, e103–e110. [Google Scholar] [CrossRef]
- Durrani, A.; Shah, R.J.; Kim, S.J. Successful long-term treatment of paraneoplastic optic neuropathy with mycophenolate mofetil, prednisone, and plasmapheresis. Am. J. Ophthalmol. Case Rep. 2017, 8, 31–34. [Google Scholar] [CrossRef]
- Micieli, J.A.; Margolin, E.A. Paraneoplastic Optic Neuropathy Associated With Purkinje Cell Antibody-2 in a Patient With Small Cell Lung Cancer. J. Neuroophthalmol. 2017, 37, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.Y.; Pula, J.H.; Khan, S.; Lee, J.M. Paraneoplastic Optic Neuropathy and Pineal Germinoma With Collapsin Response-Mediating Protein Antibodies. J. Neuroophthalmol. 2018, 38, 198–199. [Google Scholar] [CrossRef]
- Xu, Q.; Du, W.; Zhou, H.; Zhang, X.; Liu, H.; Song, H.; Wang, X.; Wei, S. Distinct clinical characteristics of paraneoplastic optic neuropathy. Br. J. Ophthalmol. 2019, 103, 797–801. [Google Scholar] [CrossRef]
- Nakajima, M.; Uchibori, A.; Ogawa, Y.; Miyazaki, T.; Ichikawa, Y.; Kaneko, K.; Takahashi, T.; Nakashima, I.; Shiraishi, H.; Motomura, M.; et al. CV2/CRMP5-antibody-related Paraneoplastic Optic Neuropathy Associated with Small-cell Lung Cancer. Intern. Med. 2018, 57, 1645–1649. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Bel, L.; Puchades-Gimeno, F.; Fernandez-Diaz, A.; Mata-Moret, L.; Beltrán-Catalán, E.; Hernandez-Garfella, M.L.; Cervera-Taulet, E. CRMP-5-IgG Antibody: Role in the bilateral uveitis with swollen disc. Rom. J. Ophthalmol. 2020, 64, 217–221. [Google Scholar] [CrossRef]
- Adamus, G.; Webb, S.; Shiraga, S.; Duvoisin, R.M. Anti-recoverin antibodies induce an increase in intracellular calcium, leading to apoptosis in retinal cells. J. Autoimmun. 2006, 26, 146–153. [Google Scholar] [CrossRef]
- Adamus, G.; Brown, L.; Weleber, R.G. Molecular biomarkers for autoimmune retinopathies: Significance of anti-transducin-alpha autoantibodies. Exp. Mol. Pathol. 2009, 87, 195–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamus, G. Autoantibody Targets and their Cancer Relationship in the Pathogenicity of Paraneoplastic Retinopathy. Autoimmun. Rev. 2009, 8, 410–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grange, L.; Dalal, M.; Nussenblatt, R.B.; Sen, H.N. Autoimmune retinopathy. Am. J. Ophthalmol. 2014, 157, 266–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ten Berge, J.C.; van Rosmalen, J.; Vermeer, J.; Hellström, C.; Lindskog, C.; Nilsson, P.; Qundos, U.; Rothova, A.; Schreurs, M.W.J. Serum Autoantibody Profiling of Patients with Paraneoplastic and Non-Paraneoplastic Autoimmune Retinopathy. PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- Ohguro, H.; Yokoi, Y.; Ohguro, I.; Mamiya, K.; Ishikawa, F.; Yamazaki, H.; Metoki, T.; Takano, Y.; Ito, T.; Nakazawa, M. Clinical and immunologic aspects of cancer-associated retinopathy. Am. J. Ophthalmol. 2004, 137, 1117–1119. [Google Scholar] [CrossRef] [PubMed]
- Narayan, D.S.; Wood, J.P.M.; Chidlow, G.; Casson, R.J. A review of the mechanisms of cone degeneration in retinitis pigmentosa. Acta Ophthalmol. 2016, 94, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Machida, S.; Ohguro, H.; Ishida, K.; Suzuki, M.; Kawagishi, K. Recoverin-associated retinopathy secondary to Warthin tumor of parotid gland. Doc. Ophthalmol. 2014, 129, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Berson, D.M.; Dunn, F.A.; Takao, M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 2002, 295, 1070–1073. [Google Scholar] [CrossRef] [Green Version]
- Adamus, G.; Yang, S.; Weleber, R.G. Unique epitopes for carbonic anhydrase II autoantibodies related to autoimmune retinopathy and cancer-associated retinopathy. Exp. Eye Res. 2016, 147, 161–168. [Google Scholar] [CrossRef]
- Zhou, R.; Horai, R.; Mattapallil, M.J.; Caspi, R.R. A new look at immune privilege of the eye: Dual role for the vision-related molecule, retinoic acid. J. Immunol. 2011, 187, 4170–4177. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Horai, R.; Silver, P.B.; Mattapallil, M.J.; Zárate-Bladés, C.R.; Chong, W.P.; Chen, J.; Rigden, R.C.; Villasmil, R.; Caspi, R.R. The living eye “disarms” uncommitted autoreactive T cells by converting them to Foxp3(+) regulatory cells following local antigen recognition. J. Immunol. 2012, 188, 1742–1750. [Google Scholar] [CrossRef] [Green Version]
- Maeda, A.; Maeda, T.; Liang, Y.; Yenerel, M. Effects of cytotoxic T lymphocyte antigen 4 (CTLA4) signaling and locally applied steroid on retinal dysfunction by recoverin, cancer-associated retinopathy antigen. Mol. Vis. 2006, 885–891. [Google Scholar]
- Narbute, K.; Pilipenko, V.; Pupure, J.; Klinovičs, T.; Auders, J.; Jonavičė, U.; Kriaučiūnaitė, K.; Pivoriūnas, A.; Kluša, V. Time-Dependent Memory and Gait Improvement by Intranasally-Administered Extracellular Vesicles in Parkinson’s Disease Model Rats. Cell. Mol. Neurobiol. 2020. [Google Scholar] [CrossRef]
- Tarasov, V.V.; Svistunov, A.A.; Chubarev, V.N.; Dostdar, S.A.; Sokolov, A.V.; Brzecka, A.; Sukocheva, O.; Neganova, M.E.; Klochkov, S.G.; Somasundaram, S.G.; et al. Extracellular vesicles in cancer nanomedicine. Semin. Cancer Biol. 2019. [Google Scholar] [CrossRef]
- Roberson, C.D.; Atay, S.; Gercel-Taylor, C.; Taylor, D.D. Tumor-derived exosomes as mediators of disease and potential diagnostic biomarkers. Cancer Biomark. 2010, 8, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Javeed, N.; Sagar, G.; Dutta, S.K.; Smyrk, T.C.; Lau, J.S.; Bhattacharya, S.; Truty, M.; Petersen, G.M.; Kaufman, R.J.; Chari, S.T.; et al. Pancreatic Cancer–Derived Exosomes Cause Paraneoplastic β-cell Dysfunction. Clin. Cancer Res. 2015, 21, 1722–1733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tawil, N.; Spinelli, C.; Bassawon, R.; Rak, J. Genetic and epigenetic regulation of cancer coagulome – lessons from heterogeneity of cancer cell populations. Thrombosis Res. 2020, 191, S99–S105. [Google Scholar] [CrossRef]


| Clinical Features | CAR | CACD | PVM | MAR | BDUMP |
|---|---|---|---|---|---|
| Onset | Acute, sudden (few days to several months) | Subacute | Acute or subacute (few weeks to several years) | Acute (few weeks to months), may be sudden | Acute, sudden (several months) |
| Ocular symmetry | Bilateral with asymmetric presentation | Often symmetric | Bilateral with asymmetric presentation | Bilateral | Bilateral with asymmetric presentation |
| Photosensitivity | +++ | +++ | − | − | − |
| Photopsias | +++ | + | − | +++ | − |
| Glare | +++ prolonged | − | ++ | − | − |
| Halo | − | − | +++ | − | − |
| Starburst | − | − | − | − | − |
| Color discrimination problems (basic colors | ++ | +++ | − | − | − |
| Disturbed color vision (color desaturation) | ++ | +++ | − | ++ | − |
| Night blindness | +++ | − | − | +++ | − |
| Prolonged adaptation to darkness | +++ | − | − | + | − |
| Improvement of visual acuity while wearing sunglasses | − | +++ | − | − | − |
| Significant decrease of visual acuity during the day | − | + | +++ | − | − |
| Phosphenes (visual hallucinations) | +++ | − | +++ | ++ | − |
| Sudden shimmering | − | − | + | +++ | − |
| Sudden flickering | ++ | − | − | +++ | − |
| Increased contrast sensitivity (hyperphotosensitivity) | − | − | − | +++ | − |
| Increased color contrast sensitivity (hyperphotosensitivity) | − | − | − | + | − |
| Pain of the eye | − | − | − | − | +/− |
| Feeling of “full” eyes | − | − | − | − | +/− |
| Clinical Work-Up | CAR | CACD | PVM | MAR | BDUMP |
|---|---|---|---|---|---|
| BCVA | Severely decreased | Mildly to moderately decreased | Blurred vision, mildly decreased | Mildly to severely decreased | Moderately to severely decreased |
| Visual field | Central or ring scotoma, peripheral scotomas | Central scotoma | Central/paracentral scotoma | Central/paracentral scotoma | Nonspecific |
| OCT signs | Loss of outer retinal structures, including ellipsoid and interdigitation zone (central and peripheral) | Central loss of outer retinal structures, including ellipsoid and interdigitation zone with normal periphery | Vitelliform submacular deposits on the pigment epithelium that elevate the neurosensory retina | Macular atrophy with thinning of the inner retina | Diffuse thickening of the uveal tract with multiple elevated pigment and nonpigment uveal melanocytic tumors, possible atrophy of choroidal vasculature in Enhanced Depth Imaging (EDI) scans was observed |
| FAF | Ring macular hyperautofluorescence with surrounding hypoautofluorescence | Nonspecific | Nonspecific | Nonspecific | Hypo-/hyperautofluorescence characteristic “giraffe-like” pattern lesions |
| FFA | Normal/periphlebitis | Nonspecific | Blocking effect on the choroid with late-phase contrast uptake | Normal/vasculitis with vascular diffusion | Peripheral arterial nonperfusion area, nummular or dermal loss of retinal pigment epithelium, exudative retinal detachment in the late phases |
| ICGA | Normal | Normal | Normal | Normal | Normal/atrophy of choroidal vasculature |
| Full-field ERG | Rods and cones equally affected, firstly affecting the a-wave, progression to the “flat ERG” | Affected only cone response | Nonspecific, variable results | Scotopic—disappearance or microvoltage of b-wave, normal a-wave. Dysfunction of bipolar cells | Reduction of scotopic and photopic a- and b-wave amplitude |
| mfERG | Severely abnormal | Partially abnormal | Nonspecific | Mildly abnormal | Mildly abnormal |
| EOG | Normal Arden ratio | Normal Arden ratio | Variable Arden ratio | Reduced Arden ratio | Normal Arden ratio |
| OPNS | Neoplasm | Mediator * | Target Cell |
|---|---|---|---|
| CAR | Small-cell lung carcinoma, other lung neoplasm, breast cancer, cancers of the cervix, ovary, uterus and thymus, osteosarcoma, Warthin tumor of parotid gland, prostate, pancreatic neuroendocrine, small bowel, bladder and laryngeal neoplasms, lymphomas (systemic follicular cell lymphoma), and colon adenomas | Recoverin, retinal enolase, TULP1, hsc-70 and 60, AIPL1, IRBP, PNR, GAPDH, aldolase C, transducin-α, GCAPs, HSP27 and Rab6A, CA II, CRMP5, antiretinal autoantibodies against arrestin and 64-kDa and 94-kDa, C3, and C9 | Rod, cone, bipolar cell, retinal pigment epithelium |
| CACD | small-cell endometrial cancer, primary cervical intraepithelial neoplasia, occult small cell lung carcinoma, and laryngeal carcinoma | Recoverin, retinal enolase, and protein whose molecular weight is 50 and 40 kDa | L and M than S cones |
| PVM | cutaneous and mucosal melanoma, lymphoma | PRDX3, ROS, bestropin-1, CA II, IRBP, proteins 35-kDa and 68-kDa | Probably cones, bipolar cells, and rods |
| MAR | cutaneous and mucosal melanoma | TRPM1, MLSN1 α-enolase, recoverin or hsc-70, CA II, IRBP, bestrophin, myelin basic protein, mitofilin, titin, and rod outer segment proteins | Bipolar cells (preferably ON-bipolar cell) |
| BDUMP | ovarian, cervix, uterus, colon and rectum cancer, gallbladder cancer, neoplasm of the retroperitoneal space, and a variety of lung cancers | CMEP factor; AAbs against 35-kDa, 46-kDa, 30-kDa, 50-kDa, and 70-kDa proteins; α-HGF and HGF | Pigment epithelium |
| PON | adenocarcinoma and small-cell carcinoma of the lung, prostate carcinoma, stomach carcinoid tumor, colon adenocarcinoma, cutaneous melanoma, occult pancreatic nonsecretory neuroendocrine tumor, thymoma | CRMP5, aquaporin 4, MBP, ANNA-1, recoverin, enolase | Photoreceptors, ganglion cells, and their axons |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przeździecka-Dołyk, J.; Brzecka, A.; Ejma, M.; Misiuk-Hojło, M.; Torres Solis, L.F.; Solís Herrera, A.; Somasundaram, S.G.; Kirkland, C.E.; Aliev, G. Ocular Paraneoplastic Syndromes. Biomedicines 2020, 8, 490. https://doi.org/10.3390/biomedicines8110490
Przeździecka-Dołyk J, Brzecka A, Ejma M, Misiuk-Hojło M, Torres Solis LF, Solís Herrera A, Somasundaram SG, Kirkland CE, Aliev G. Ocular Paraneoplastic Syndromes. Biomedicines. 2020; 8(11):490. https://doi.org/10.3390/biomedicines8110490
Chicago/Turabian StylePrzeździecka-Dołyk, Joanna, Anna Brzecka, Maria Ejma, Marta Misiuk-Hojło, Luis Fernando Torres Solis, Arturo Solís Herrera, Siva G. Somasundaram, Cecil E. Kirkland, and Gjumrakch Aliev. 2020. "Ocular Paraneoplastic Syndromes" Biomedicines 8, no. 11: 490. https://doi.org/10.3390/biomedicines8110490
APA StylePrzeździecka-Dołyk, J., Brzecka, A., Ejma, M., Misiuk-Hojło, M., Torres Solis, L. F., Solís Herrera, A., Somasundaram, S. G., Kirkland, C. E., & Aliev, G. (2020). Ocular Paraneoplastic Syndromes. Biomedicines, 8(11), 490. https://doi.org/10.3390/biomedicines8110490