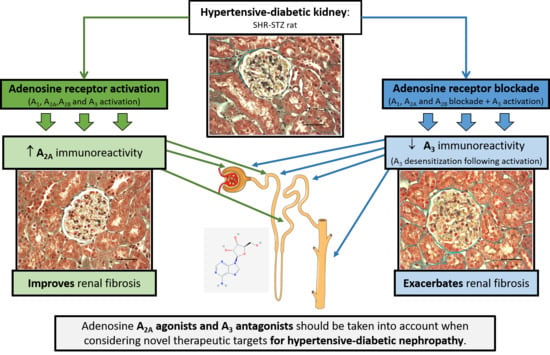
Adenosine A2A and A3 Receptors as Targets for the Treatment of Hypertensive-Diabetic Nephropathy
Abstract
:1. Introduction
2. Experimental Section
2.1. Drugs
2.2. Animals and Treatments
2.3. Metabolic Parameters, Renal Function, and Blood Pressure
2.4. Immunohistochemistry
2.5. Imaging
2.6. Histomorphometry
2.7. Histology
2.8. Urinary and renal Oxidative Status Parameters
2.9. Statistical Analysis
3. Results
3.1. Metabolic Parameters, Renal Function, and Blood Pressure in SHR-STZ Rats Treated with CADO or DPSPX
3.2. Effect of Endogenous and Exogenous Adenosine on the Distribution Profile and Expression of Adenosine A1 and A2B Receptors in SHR-STZ Rats
3.3. Effect of Endogenous and Exogenous Adenosine on the Distribution Profile and Expression of Adenosine A2A and A3 Receptors in SHR-STZ Rats
3.4. Effect of Endogenous and Exogenous Adenosine on the Glomeruli of SHR-STZ Rats Treated with CADO or DPSPX
3.5. Urinary and Renal Oxidative Status Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Oyarzun, C.; Garrido, W.; Alarcon, S.; Yanez, A.; Sobrevia, L.; Quezada, C.; San Martin, R. Adenosine contribution to normal renal physiology and chronic kidney disease. Mol. Aspects Med. 2017, 55, 75–89. [Google Scholar]
- Olivera, A.; Lamas, S.; Rodriguez-Puyol, D.; Lopez-Novoa, J.M. Adenosine induces mesangial cell contraction by an a1-type receptor. Kidney Int. 1989, 35, 1300–1305. [Google Scholar]
- Faulhaber-Walter, R.; Chen, L.; Oppermann, M.; Kim, S.M.; Huang, Y.; Hiramatsu, N.; Mizel, D.; Kajiyama, H.; Zerfas, P.; Briggs, J.P.; et al. Lack of a1 adenosine receptors augments diabetic hyperfiltration and glomerular injury. J. Am. Soc. Nephrol. 2008, 19, 722–730. [Google Scholar] [PubMed] [Green Version]
- Truong, L.D.; Trostel, J.; McMahan, R.; Chen, J.F.; Garcia, G.E. Macrophage a2a adenosine receptors are essential to protect from progressive kidney injury. Am. J. Pathol. 2016, 186, 2601–2613. [Google Scholar] [PubMed] [Green Version]
- Min, H.S.; Cha, J.J.; Kim, K.; Kim, J.E.; Ghee, J.Y.; Kim, H.; Lee, J.E.; Han, J.Y.; Jeong, L.S.; Cha, D.R.; et al. Renoprotective effects of a highly selective a3 adenosine receptor antagonist in a mouse model of adriamycin-induced nephropathy. J. Korean Med. Sci. 2016, 31, 1403–1412. [Google Scholar] [PubMed] [Green Version]
- Awad, A.S.; Rouse, M.; Liu, L.; Vergis, A.L.; Rosin, D.L.; Linden, J.; Sedor, J.R.; Okusa, M.D. Activation of adenosine 2a receptors preserves structure and function of podocytes. J. Am. Soc. Nephrol. 2008, 19, 59–68. [Google Scholar] [PubMed] [Green Version]
- Nemeth, Z.H.; Bleich, D.; Csoka, B.; Pacher, P.; Mabley, J.G.; Himer, L.; Vizi, E.S.; Deitch, E.A.; Szabo, C.; Cronstein, B.N.; et al. Adenosine receptor activation ameliorates type 1 diabetes. FASEB J. 2007, 21, 2379–2388. [Google Scholar] [PubMed] [Green Version]
- Dubey, R.K.; Gillespie, D.G.; Mi, Z.; Jackson, E.K. Adenosine inhibits pdgf-induced growth of human glomerular mesangial cells via a(2b) receptors. Hypertension 2005, 46, 628–634. [Google Scholar]
- Valladares, D.; Quezada, C.; Montecinos, P.; Concha, I.I.; Yanez, A.J.; Sobrevia, L.; San Martin, R. Adenosine a(2b) receptor mediates an increase on vegf-a production in rat kidney glomeruli. Biochem. Biophys. Res. Commun. 2008, 366, 180–185. [Google Scholar]
- Wilkinson, P.F.; Farrell, F.X.; Morel, D.; Law, W.; Murphy, S. Adenosine signaling increases proinflammatory and profibrotic mediators through activation of a functional adenosine 2b receptor in renal fibroblasts. Ann. Clin. Lab. Sci. 2016, 46, 339–345. [Google Scholar]
- Zhao, Z.; Kapoian, T.; Shepard, M.; Lianos, E.A. Adenosine-induced apoptosis in glomerular mesangial cells. Kidney Int. 2002, 61, 1276–1285. [Google Scholar] [PubMed] [Green Version]
- Ghaderian, S.B.; Hayati, F.; Shayanpour, S.; Beladi Mousavi, S.S. Diabetes and end-stage renal disease; a review article on new concepts. J. Renal Inj. Prev. 2015, 4, 28–33. [Google Scholar] [PubMed]
- Calle, P.; Hotter, G. Macrophage phenotype and fibrosis in diabetic nephropathy. Int. J. Mol. Sci. 2020, 21, 2806. [Google Scholar]
- Kopel, J.; Pena-Hernandez, C.; Nugent, K. Evolving spectrum of diabetic nephropathy. World J. Diabetes 2019, 10, 269–279. [Google Scholar]
- Tsimihodimos, V.; Gonzalez-Villalpando, C.; Meigs, J.B.; Ferrannini, E. Hypertension and diabetes mellitus: Coprediction and time trajectories. Hypertension 2018, 71, 422–428. [Google Scholar]
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.; Yang, C.W. Chronic kidney disease: Global dimension and perspectives. Lancet 2013, 382, 260–272. [Google Scholar]
- Magee, G.M.; Bilous, R.W.; Cardwell, C.R.; Hunter, S.J.; Kee, F.; Fogarty, D.G. Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia 2009, 52, 691–697. [Google Scholar]
- Roa, H.; Gajardo, C.; Troncoso, E.; Fuentealba, V.; Escudero, C.; Yanez, A.; Sobrevia, L.; Pastor-Anglada, M.; Quezada, C.; San Martin, R. Adenosine mediates transforming growth factor-beta 1 release in kidney glomeruli of diabetic rats. FEBS Lett. 2009, 583, 3192–3198. [Google Scholar]
- Quezada, C.; Alarcon, S.; Jaramillo, C.; Munoz, D.; Oyarzun, C.; San Martin, R. Targeting adenosine signaling to treatment of diabetic nephropathy. Curr. Drug Targets 2013, 14, 490–496. [Google Scholar]
- Patinha, D.; Afonso, J.; Sousa, T.; Morato, M.; Albino-Teixeira, A. Activation of adenosine receptors improves renal antioxidant status in diabetic wistar but not shr rats. Ups. J. Med. Sci. 2014, 119, 10–18. [Google Scholar]
- Patinha, D.; Fasching, A.; Pinho, D.; Albino-Teixeira, A.; Morato, M.; Palm, F. Angiotensin ii contributes to glomerular hyperfiltration in diabetic rats independently of adenosine type i receptors. Am. J. Physiol. Renal Physiol. 2013, 304, F614–F622. [Google Scholar] [PubMed] [Green Version]
- Pawelczyk, T.; Grden, M.; Rzepko, R.; Sakowicz, M.; Szutowicz, A. Region-specific alterations of adenosine receptors expression level in kidney of diabetic rat. Am. J. Pathol. 2005, 167, 315–325. [Google Scholar] [PubMed] [Green Version]
- Elsherbiny, N.M.; Al-Gayyar, M.M. Adenosine receptors: New therapeutic targets for inflammation in diabetic nephropathy. Inflamm. Allergy Drug Targets 2013, 12, 153–161. [Google Scholar] [PubMed]
- Awad, A.S.; Huang, L.; Ye, H.; Duong, E.T.; Bolton, W.K.; Linden, J.; Okusa, M.D. Adenosine a2a receptor activation attenuates inflammation and injury in diabetic nephropathy. Am. J. Physiol. Renal Physiol. 2006, 290, F828–F837. [Google Scholar]
- Roberts, V.S.; Cowan, P.J.; Alexander, S.I.; Robson, S.C.; Dwyer, K.M. The role of adenosine receptors a2a and a2b signaling in renal fibrosis. Kidney Int. 2014, 86, 685–692. [Google Scholar]
- Smith, J.A.; Sivaprasadarao, A.; Munsey, T.S.; Bowmer, C.J.; Yates, M.S. Immunolocalisation of adenosine a(1) receptors in the rat kidney. Biochem. Pharmacol. 2001, 61, 237–244. [Google Scholar]
- Weaver, D.R.; Reppert, S.M. Adenosine receptor gene expression in rat kidney. Am. J. Physiol. 1992, 263, F991–F995. [Google Scholar]
- Vitzthum, H.; Weiss, B.; Bachleitner, W.; Kramer, B.K.; Kurtz, A. Gene expression of adenosine receptors along the nephron. Kidney Int. 2004, 65, 1180–1190. [Google Scholar]
- Patinha, D.; Carvalho, C.; Abreu, C.; Cunha, O.M.; Mota, M.C.; Afonso, J.; Albino-Teixeira, A.; Diniz, C.; Morato, M. Diabetes downregulates renal adenosine a2a receptors in an experimental model of hypertension. PLoS ONE 2019, 14, e0217552. [Google Scholar]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The arrive guidelines for reporting animal research. J. Pharmacol. Pharmacother. 2010, 1, 94–99. [Google Scholar]
- Daly, J.W.; Padgett, W.L.; Secunda, S.I.; Thompson, R.D.; Olsson, R.A. Structure-activity relationships for 2-substituted adenosines at a1 and a2 adenosine receptors. Pharmacology 1993, 46, 91–100. [Google Scholar] [PubMed]
- Daly, J.W.; Padgett, W.; Shamim, M.T.; Butts-Lamb, P.; Waters, J. 1,3-dialkyl-8-(p-sulfophenyl)xanthines: Potent water-soluble antagonists for a1- and a2-adenosine receptors. J. Med. Chem. 1985, 28, 487–492. [Google Scholar] [PubMed]
- Moreira-Rodrigues, M.; Quelhas-Santos, J.; Serrao, P.; Fernandes-Cerqueira, C.; Sampaio-Maia, B.; Pestana, M. Glycaemic control with insulin prevents the reduced renal dopamine d1 receptor expression and function in streptozotocin-induced diabetes. Nephrol. Dial. Transplant. 2010, 25, 2945–2953. [Google Scholar] [PubMed] [Green Version]
- Leal, S.; Sa, C.; Goncalves, J.; Fresco, P.; Diniz, C. Immunohistochemical characterization of adenosine receptors in rat aorta and tail arteries. Microsc. Res. Technol. 2008, 71, 703–709. [Google Scholar]
- Sousa-Oliveira, A.; Brandao, A.; Vojtek, M.; Goncalves-Monteiro, S.; Sousa, J.B.; Diniz, C. Vascular impairment of adenosinergic system in hypertension: Increased adenosine bioavailability and differential distribution of adenosine receptors and nucleoside transporters. Histochem. Cell Biol. 2019, 51, 407–418. [Google Scholar]
- Zizzo, M.G.; Bonomo, A.; Belluardo, N.; Mule, F.; Serio, R. A1 receptors mediate adenosine inhibitory effects in mouse ileum via activation of potassium channels. Life Sci. 2009, 84, 772–778. [Google Scholar]
- Thakur, S.; Du, J.; Hourani, S.; Ledent, C.; Li, J.M. Inactivation of adenosine a2a receptor attenuates basal and angiotensin ii-induced ros production by nox2 in endothelial cells. J. Biol. Chem. 2010, 285, 40104–40113. [Google Scholar]
- Eckle, T.; Faigle, M.; Grenz, A.; Laucher, S.; Thompson, L.F.; Eltzschig, H.K. A2b adenosine receptor dampens hypoxia-induced vascular leak. Blood 2008, 111, 2024–2035. [Google Scholar]
- Carreira, M.C.; Camina, J.P.; Diaz-Rodriguez, E.; Alvear-Perez, R.; Llorens-Cortes, C.; Casanueva, F.F. Adenosine does not bind to the growth hormone secretagogue receptor type-1a (ghs-r1a). J. Endocrinol. 2006, 191, 147–157. [Google Scholar]
- Leal, S.; Diniz, C.; Sa, C.; Goncalves, J.; Soares, A.S.; Rocha-Pereira, C.; Fresco, P. Semiautomated computer-assisted image analysis to quantify 3,3’-diaminobenzidine tetrahydrochloride-immunostained small tissues. Anal. Biochem. 2006, 357, 137–143. [Google Scholar]
- Diniz, C. Microscopic image analysis using computer-assisted methodology to Quantify immunostained receptors. In Microscopy: Science, Technology, Applications and Education; Méndez-Vilas, A., Díaz, J., Eds.; Formatex Research Center: Badajoz, Spain, 2010; Volume 2, pp. 1516–1525. [Google Scholar]
- Guirado, R.; Carceller, H.; Castillo-Gomez, E.; Castren, E.; Nacher, J. Automated analysis of images for molecular quantification in immunohistochemistry. Heliyon 2018, 4, e00669. [Google Scholar] [PubMed]
- Laforest, S.; Pelletier, M.; Michaud, A.; Daris, M.; Descamps, J.; Soulet, D.; Jensen, M.D.; Tchernof, A. Histomorphometric analyses of human adipose tissues using intact, flash-frozen samples. Histochem. Cell Biol. 2018, 149, 209–218. [Google Scholar] [PubMed]
- Lu, Z.; Liu, Y.; Xu, J.; Yin, H.; Yuan, H.; Gu, J.; Chen, Y.H.; Shi, L.; Chen, D.; Xie, B. Immunohistochemical quantification of expression of a tight junction protein, claudin-7, in human lung cancer samples using digital image analysis method. Comput. Methods Programs. Biomed. 2018, 155, 179–187. [Google Scholar] [PubMed]
- Sousa, T.; Pinho, D.; Morato, M.; Marques-Lopes, J.; Fernandes, E.; Afonso, J.; Oliveira, S.; Carvalho, F.; Albino-Teixeira, A. Role of superoxide and hydrogen peroxide in hypertension induced by an antagonist of adenosine receptors. Eur. J. Pharmacol. 2008, 588, 267–276. [Google Scholar]
- Patinha, D.; Afonso, J.; Sousa, T.; Morato, M.; Albino-Teixeira, A. Diabetes-induced increase of renal medullary hydrogen peroxide and urinary angiotensinogen is similar in normotensive and hypertensive rats. Life Sci. 2014, 108, 71–79. [Google Scholar]
- Farris, A.B.; Adams, C.D.; Brousaides, N.; Della Pelle, P.A.; Collins, A.B.; Moradi, E.; Smith, R.N.; Grimm, P.C.; Colvin, R.B. Morphometric and visual evaluation of fibrosis in renal biopsies. J. Am. Soc. Nephrol. 2011, 22, 176–186. [Google Scholar]
- Garcia, G.E.; Truong, L.D.; Chen, J.F.; Johnson, R.J.; Feng, L. Adenosine a(2a) receptor activation prevents progressive kidney fibrosis in a model of immune-associated chronic inflammation. Kidney Int. 2011, 80, 378–388. [Google Scholar]
- Caruso-Neves, C.; Francisco-Pedro, L.G.; Souza, L.P.; Chagas, C.; Lopes, A.G. Effect of adenosine on the ouabain-insensitive na+-atpase activity from basolateral membrane of the proximal tubule. Biochim. Biophys. Acta 1997, 1329, 336–344. [Google Scholar]
- Vallon, V.; Osswald, H. Adenosine receptors and the kidney. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 443–470. [Google Scholar]
- Kang, H.S.; Kerstan, D.; Dai, L.J.; Ritchie, G.; Quamme, G.A. Adenosine modulates mg(2+) uptake in distal convoluted tubule cells via a(1) and a(2) purinoceptors. Am. J. Physiol. Renal Physiol. 2001, 281, F1141–F1147. [Google Scholar]
- Dousdampanis, P.; Trigka, K.; Fourtounas, C. Hypomagnesemia, chronic kidney disease and cardiovascular mortality: Pronounced association but unproven causation. Hemodial. Int. 2014, 18, 730–739. [Google Scholar]
- Muller, C.E.; Jacobson, K.A. Xanthines as adenosine receptor antagonists. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 151–199. [Google Scholar]
- Yu, J.; Kim, G.; Jarhad, D.B.; Lee, H.W.; Lee, J.; Park, C.W.; Ha, H.; Jeong, L.S. Correlation study between a3 adenosine receptor binding affinity and anti-renal interstitial fibrosis activity of truncated adenosine derivatives. Arch. Pharm. Res. 2019, 42, 773–779. [Google Scholar] [PubMed]
- Garrido, W.; Jara, C.; Torres, A.; Suarez, R.; Cappelli, C.; Oyarzun, C.; Quezada, C.; San Martin, R. Blockade of the adenosine a3 receptor attenuates caspase 1 activation in renal tubule epithelial cells and decreases interleukins il-1beta and il-18 in diabetic rats. Int. J. Mol. Sci. 2019, 20, 4531. [Google Scholar]
- Trincavelli, M.L.; Ciampi, O.; Martini, C. The desensitisation as a3 adenosine receptor regulation: Physiopathological implications. In A3 Adenosine Receptors from Cell Biology to Pharmacology and Therapeutics; Borea, P.A., Ed.; Springer: Dordrecht, The Netherlands, 2010; pp. 75–90. [Google Scholar]
- Effendi, W.I.; Nagano, T.; Kobayashi, K.; Nishimura, Y. Focusing on adenosine receptors as a potential targeted therapy in human diseases. Cells 2020, 9, 785. [Google Scholar]
- Jacobson, K.A.; Merighi, S.; Varani, K.; Borea, P.A.; Baraldi, S.; Aghazadeh Tabrizi, M.; Romagnoli, R.; Baraldi, P.G.; Ciancetta, A.; Tosh, D.K.; et al. A3 adenosine receptors as modulators of inflammation: From medicinal chemistry to therapy. Med. Res. Rev. 2018, 38, 1031–1072. [Google Scholar]
- Kost, C.K., Jr.; Herzer, W.A.; Rominski, B.R.; Mi, Z.; Jackson, E.K. Diuretic response to adenosine a(1) receptor blockade in normotensive and spontaneously hypertensive rats: Role of pertussis toxin-sensitive g-proteins. J. Pharmacol. Exp. Ther. 2000, 292, 752–760. [Google Scholar]
- Cardenas, A.; Toledo, C.; Oyarzun, C.; Sepulveda, A.; Quezada, C.; Guillen-Gomez, E.; Diaz-Encarnacion, M.M.; Pastor-Anglada, M.; San Martin, R. Adenosine a(2b) receptor-mediated vegf induction promotes diabetic glomerulopathy. Lab. Invest. 2013, 93, 135–144. [Google Scholar]
- Klaasse, E.C.; Ijzerman, A.P.; de Grip, W.J.; Beukers, M.W. Internalization and desensitization of adenosine receptors. Purinergic. Signal. 2008, 4, 21–37. [Google Scholar]
- Mundell, S.; Kelly, E. Adenosine receptor desensitization and trafficking. Biochim. Biophys. Acta 2011, 1808, 1319–1328. [Google Scholar]
- Saura, C.A.; Mallol, J.; Canela, E.I.; Lluis, C.; Franco, R. Adenosine deaminase and a1 adenosine receptors internalize together following agonist-induced receptor desensitization. J. Biol. Chem. 1998, 273, 17610–17617. [Google Scholar]
- Klaasse, E.C.; van den Hout, G.; Roerink, S.F.; de Grip, W.J.; Ijzerman, A.P.; Beukers, M.W. Allosteric modulators affect the internalization of human adenosine a1 receptors. Eur. J. Pharmacol. 2005, 522, 1–8. [Google Scholar]
- Ferguson, G.; Watterson, K.R.; Palmer, T.M. Subtype-specific kinetics of inhibitory adenosine receptor internalization are determined by sensitivity to phosphorylation by g protein-coupled receptor kinases. Mol. Pharmacol. 2000, 57, 546–552. [Google Scholar] [PubMed]








| SHR-STZ Control | SHR-STZ + CADO | SHR-STZ + DPSPX | ||
|---|---|---|---|---|
| Adenosine A1 receptor | ||||
| SG | 73.73 (59.53–80.82) | 59.30 (41.53–71.27) | 68.40 (43.68–76.85) | |
| DG | 54.31 (27.32–68.69) | 43.36 (28.18–62.38) | 48.46 (23.23–65.24) | |
| PCT | 28.05 (8.82–54.82) | 17.71 (9.35–37.98) | 17.11 (6.71–41.13) | |
| DCT | 48.04 (23.43–63.49) | 52.43 (34.12–65.90) | 53.86 (27.15–64.80) | |
| LH | 47.24 (39.53–58.24) | 52.04 (40.39–59.67) | 47.38 (38.20–59.76) | |
| CT | 49.71 (42.95–61.29) | 47.54 (33.40–53.83) | 48.31 (41.70–58.19) | |
| Adenosine A2B receptor | ||||
| SG | 74.94 (61.10–80.16) | 72.91 (68.24–76.89) | 72.07 (62.75–77.70) | |
| DG | 69.02 (60.16–74.04) | 72.47 (66.47–74.48) | 65.83 (58.95–73.72) | |
| PCT | 8.06 (1.82–42.92) | 17.74 (10.89–29.38) | 14.01 (6.77–23.34) | |
| DCT | 52.38 (21.83–69.54) | 51.76 (42.08–60.35) | 40.42 (26.19–62.96) | |
| LH | 39.81 (33.22–50.03) | 44.40 (41.29–46.54) | 41.03 (34.66–47.14) | |
| CT | 45.74 (34.95–57.54) | 43.78 (41.67–51.64) | 44.00 (36.38–54.81) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patinha, D.; Abreu, C.; Carvalho, C.; Cunha, O.M.; Mota, M.; Afonso, J.; Sousa, T.; Albino-Teixeira, A.; Diniz, C.; Morato, M. Adenosine A2A and A3 Receptors as Targets for the Treatment of Hypertensive-Diabetic Nephropathy. Biomedicines 2020, 8, 529. https://doi.org/10.3390/biomedicines8110529
Patinha D, Abreu C, Carvalho C, Cunha OM, Mota M, Afonso J, Sousa T, Albino-Teixeira A, Diniz C, Morato M. Adenosine A2A and A3 Receptors as Targets for the Treatment of Hypertensive-Diabetic Nephropathy. Biomedicines. 2020; 8(11):529. https://doi.org/10.3390/biomedicines8110529
Chicago/Turabian StylePatinha, Daniela, Carla Abreu, Carla Carvalho, Olga Mariana Cunha, Mariana Mota, Joana Afonso, Teresa Sousa, António Albino-Teixeira, Carmen Diniz, and Manuela Morato. 2020. "Adenosine A2A and A3 Receptors as Targets for the Treatment of Hypertensive-Diabetic Nephropathy" Biomedicines 8, no. 11: 529. https://doi.org/10.3390/biomedicines8110529
APA StylePatinha, D., Abreu, C., Carvalho, C., Cunha, O. M., Mota, M., Afonso, J., Sousa, T., Albino-Teixeira, A., Diniz, C., & Morato, M. (2020). Adenosine A2A and A3 Receptors as Targets for the Treatment of Hypertensive-Diabetic Nephropathy. Biomedicines, 8(11), 529. https://doi.org/10.3390/biomedicines8110529