Looking Inside the World of Granulosa Cells: The Noxious Effects of Cigarette Smoke
Abstract
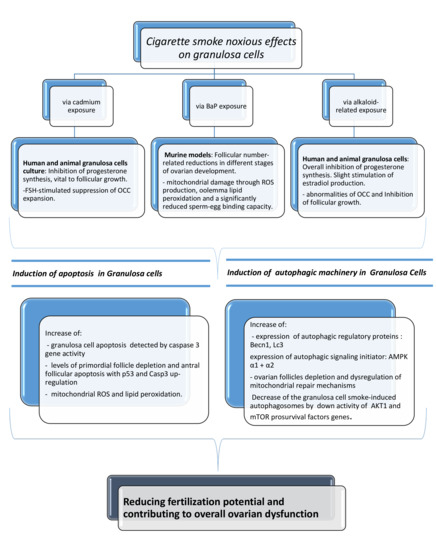
:1. Introduction
2. Cigarette Smoke Chemical Components and Their Effects on Granulosa Cells
2.1. Cadmium Exposure
2.2. Benzoapyrene Exposure
2.3. Alkaloid-Related Exposure
3. The Impact of Tobacco Smoke on the Proliferation of Mammal Granulosa Cells
3.1. Induction of Apoptosis in Granulosa Cells
3.2. Activation of Autophagy-Mediated Pathways and Granulosa Cell Death
4. Electronic Cigarettes and Female Reproduction
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- U.S. Department of Health and Human Services; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. A Report of the Surgeon General: How Tobacco Smoke Causes Disease: What It Means to You; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, GA, USA, 2010.
- Annamalai, J.; Namasivayam, V. Endocrine disrupting chemicals in the atmosphere: Their effects on humans and wildlife. Environ. Int. 2015, 76, 78–97. [Google Scholar] [CrossRef]
- De Angelis, C.; Nardone, A.; Garifalos, F.; Pivonello, C.; Sansone, A.; Conforti, A.; Di Dato, C.; Sirico, F.; Alviggi, C.; Isidori, A.; et al. Smoke, alcohol and drug addiction and female fertility. Reprod. Biol. Endocrinol. 2020, 18, 21. [Google Scholar] [CrossRef]
- Budani, M.C.; D’Aurora, M.; Stuppia, L.; Gatta, V.; Tiboni, G.M. Whole-body exposure to cigarette smoke alters oocyte miRNAs expression in C57BL/6 mice. Mol. Reprod. Dev. 2019, 86, 1741–1757. [Google Scholar] [CrossRef] [PubMed]
- Mikhael, S.; Punjala-Patel, A.; Gavrilova-Jordan, L. Hypothalamic-pituitary-ovarian axis disorders impacting female fertility. Biomedicines 2019, 7, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harlev, A.; Agarwal, A.; Gunes, S.O.; Shetty, A.; du Plessis, S.S. Smoking and male infertility: An evidence-based review. World J. Men’s Health 2015, 33, 143–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henson, M.C.; Chedrese, P.J. Endocrine disruption by cadmium, a common environmental toxicant with paradoxical effects on reproduction. Exp. Biol. Med. 2004, 229, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Mlynarcikova, A.; Fickova, M.; Scsukova, S. Ovarian intrafollicular processes as a target for cigarette smoke components and selected environmental reproductive disruptors. Endocr. Regul. 2005, 39, 20–31. [Google Scholar]
- Centers for Disease Control and Prevention (US); National Center for Chronic Disease Prevention and Health Promotion (US); Office on Smoking and Health (US). How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2010.
- Borja-Aburto, V.H.; Hertz-Picciotto, I.; Rojas Lopez, M.; Farias, P.; Rios, C.; Blanco, J. Blood lead levels measured prospectively and risk of spontaneous abortion. Am. J. Epidemiol. 1999, 150, 590–597. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Chen, D.; Niu, T.; Wang, Z.; Wang, L.; Ryan, L.; Smith, T.; Christiani, D.C.; Zuckerman, B.; Xu, X. Genetic susceptibility to benzene and shortened gestation: Evidence of gene-Environment interaction. Am. J. Epidemiol. 2000, 152, 693–700. [Google Scholar] [CrossRef]
- D’Aurora, M.; Sperduti, S.; Di Emidio, G.; Stuppia, L.; Artini, P.G.; Gatta, V. Inside the granulosa transcriptome. Gynecol. Endocrinol. 2016, 32, 951–956. [Google Scholar] [CrossRef]
- Gannon, A.M.; Stämpfli, M.R.; Foster, W.G. Cigarette smoke exposure leads to follicle loss via an alternative ovarian cell death pathway in a mouse model. Toxicol. Sci. 2012, 125, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Varga, B.; Zsolnai, B.; Paksy, K.; Náray, M.; Ungváry, G.Y. Age dependent accumulation of cadmium in the human ovary. Reprod. Toxicol. 1993, 7, 225–228. [Google Scholar] [CrossRef]
- Piasek, M.; Blanusa, M.; Kostial, K.; Laskey, J.W. Placental cadmium and progesterone concentrations in cigarette smokers. Reprod. Toxicol. 2001, 15, 673–681. [Google Scholar] [CrossRef]
- Paksy, K.; Rajczy, K.; Forgacs, Z.; Lazar, P.; Bernard, A.; Gati, I.; Kaali, G.S. Effect of cadmium on morphology and steroidogenesis of cultured human ovarian granulosa cells. J. Appl. Toxicol. 1997, 17, 321–327. [Google Scholar] [CrossRef]
- Piasek, M.; Laskey, J.W. Effects of in vitro cadmium exposure on ovarian steroidogenesis in rats. J. Appl. Toxicol. 1999, 19, 211–217. [Google Scholar] [CrossRef]
- Oonk, R.B.; Krasnow, J.S.; Beattie, W.G.; Richards, J.S. Cyclic AMP- dependent and -independent regulation of cholesterol side chain cleavage cytochrome P-450 (P-450scc) in rat ovarian granulosa cells and corpora lutea. J. Biol. Chem. 1989, 264, 21934–21942. [Google Scholar]
- Vrsanska, S.; Nagyova, E.; Mlynarcikova, A.; Fickova, M.; Kolena, L. Components of cigarette smoke inhibit expansion of oocyte-cumulus complexes from porcine follicles. Physiol. Res. 2003, 52, 383–387. [Google Scholar]
- Uyar, A.; Torrealday, S.; Seli, E. Cumulus and granulosa cell markers of oocyte and embryo quality. Fertil. Steril. 2013, 99, 979–997. [Google Scholar] [CrossRef] [Green Version]
- Fragouli, E.; Lalioti, M.D.; Wells, D. The transcriptome of follicular cells: Biological insights and clinical implications for the treatment of infertility. Hum. Reprod. Update 2014, 20, 1–11. [Google Scholar] [CrossRef]
- Artini, P.G.; Tatone, C.; Sperduti, S.; D’Aurora, M.; Franchi, S.; Di Emidio, G.; Ciriminna, R.; Vento, M.; Di Pietro, C.; Stuppia, L.; et al. Cumulus cells surrounding oocytes with high developmental competence exhibit down-regulation of phosphoinositol 1,3 kinase/protein kinase B (PI3K/AKT) signalling genes involved in proliferation and survival. Hum. Reprod. 2017, 32, 2474–2484. [Google Scholar] [CrossRef]
- Gatta, V.; Tatone, C.; Ciriminna, R.; Vento, M.; Franchi, S.; D’Aurora, M.; Sperduti, S.; Cela, V.; Borzì, P.; Palermo, R.; et al. Gene expression profiles of cumulus cells obtained from women treated with recombinant human luteinizing hormone + recombinant human follicle-stimulating hormone or highly purified human menopausal gonadotropin versus recombinant human follicle-stimulating hormone alone. Fertil. Steril. 2013, 99, 2000–2008. [Google Scholar] [PubMed]
- Vanderhyden, B.C.; Armstrong, D.T. Role of cumulus cells and serum on the in vitro maturation, fertilization, and subsequent development of rat oocytes. Biol. Reprod. 1989, 40, 720–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sagredo, C.; Ovrebo, S.; Haugen, A.; Fujii-Kuriyama, Y.; Baera, R.; Botnen, I.V.; Mollerup, S. Quantitative analysis of benzo[a]pyrene biotransformation and adduct formation in Ahr knockout mice. Toxicol. Lett. 2006, 167, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Lodovici, M.; Akpan, V.; Evangelisti, C.; Dolara, P. Sidestream tobacco smoke as the main predictor of exposure to polycyclic aromatic hydrocarbons. J. Appl. Toxicol. 2004, 24, 277–281. [Google Scholar] [CrossRef]
- Jurisicova, A.; Taniuchi, A.; Li, H.; Shang, Y.; Antenos, M.; Detmar, J.; Xu, J.; Matikainen, T.; Benito Hernandez, A.; Nunez, G.; et al. Maternal exposure to polycyclic aromatic hydrocarbons diminishes murine ovarian reserve via induction of Harakiri. J. Clin. Investig. 2007, 117, 3971–3978. [Google Scholar] [CrossRef] [Green Version]
- Neal, M.S.; Zhu, J.; Foster, W.G. Quantification of benzo[a]pyrene and other PAHs in the serum and follicular fluid of smokers versus non-smokers. Reprod. Toxicol. 2008, 25, 100–106. [Google Scholar] [CrossRef]
- Zenzes, M.T. Smoking and reproduction: Gene damage to human gametes and embryos. Hum. Reprod. Update 2000, 6, 122–131. [Google Scholar] [CrossRef]
- Tuttle, A.M.; Stämpfli, M.; Foster, W.G. Cigarette smoke causes follicle loss in mice ovaries at concentrations representative of human exposure. Hum. Reprod. 2009, 24, 1452–1459. [Google Scholar] [CrossRef]
- Sobinoff, A.P.; Pye, V.; Nixon, B.; Roman, S.D.; McLaughlin, E.A. Jumping the gun: Smoking constituent BaP causes premature primordial follicle activation and impairs oocyte fusibility through oxidative stress. Toxicol. Appl. Pharmacol. 2012, 260, 70–80. [Google Scholar] [CrossRef]
- Le Quesne, P.W. Alkaloids: Biochemistry, Ecology, and Medicinal Applications Edited by Margaret F. Roberts (University of London) and Michael Wink (University of Heidelberg); Plenum Press: New York, NY, USA, 1999; Volume 62, p. 664. [Google Scholar]
- Kumar, A.; Premoli, M.; Aria, F.; Bonini, S.A.; Maccarinelli, G.; Gianoncelli, A.; Memo, M.; Mastinu, A. Cannabimimetic plants: Are they new cannabinoidergic modulators? Planta 2019, 249, 1681–1694. [Google Scholar] [CrossRef]
- Jarvis, M.J.; Russell, M.A.; Benowitz, N.L.; Feyerabend, C. Elimination of cotinine from body fluids: Implications for noninvasive measurement of tobacco smoke exposure. Am. J. Public Health 1988, 78, 696–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benowitz, N.L.; Kuyt, F.; Jacob, P.; Jones, R.T.; Osman, A.L. Cotinine disposition and effects. Clin. Pharmacol. Ther. 1983, 34, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Miceli, F.; Minici, F.; Tropea, A.; Catino, S.; Orlando, M.; Lamanna, G.; Sagnella, F.; Tiberi, F.; Bompiani, A.; Mancuso, S.; et al. Effects of nicotine on human luteal cells in vitro: A possible role on reproductive outcome for smoking women. Biol. Reprod. 2005, 72, 628–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gocze, P.M.; Sbazo, I.; Freeman, D.A. Influence of nicotine, cotinine, anabasine and cigarette smoke extract on human granulosa cell progesterone and estradiol synthesis. Gynecol. Endocrinol. 1999, 13, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Sanders, S.R.; Cuneo, S.P.; Turzillo, A.M. Effects of nicotine and cotinine on bovine theca interna and granulosa cells. Reprod. Toxicol. 2002, 16, 795–800. [Google Scholar] [CrossRef]
- Racowsky, C.; Hendricks, R.C.; Baldwin, K.V. Direct effects of nicotine on the meiotic maturation of hamster oocytes. Reprod. Toxicol. 1989, 3, 13–21. [Google Scholar] [CrossRef]
- Bordel, R.; Laschke, M.W.; Menger, M.D.; Vollmar, B. Nicotine does not affect vascularization but inhibits growth of freely transplanted ovarian follicles by inducing granulosa cell apoptosis. Hum. Reprod. 2006, 21, 610–617. [Google Scholar] [CrossRef] [Green Version]
- Allworth, A.E.; Albertini, D.F. Meiotic maturation in cultured bovine oocytes is accompanied by remodeling of the cumulus cell cytoskeleton. Dev. Biol. 1993, 158, 101–112. [Google Scholar] [CrossRef]
- Barrett, S.L.; Albertini, D.F. Cumulus cell contact during oocyte maturation in mice regulates meiotic spindle positioning and enhances developmental competence. J. Assist. Reprod. Genet. 2010, 27, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Assidi, M.; Dieleman, S.J.; Sirard, M.A. Cumulus cell gene expression following the LH surge in bovine preovulatory follicles: Potential early markers of oocyte competence. Reproduction 2010, 140, 835–852. [Google Scholar] [CrossRef] [Green Version]
- Tatemoto, H.; Sakurai, N.; Muto, N. Protection of porcine oocytes against apoptotic cell death caused by oxidative stress during in vitro maturation: Role of cumulus cells. Biol. Reprod. 2000, 63, 805–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cetica, P.D.; Pintos, L.N.; Dalvit, G.C.; Beconi, M.T. Antioxidant enzyme activity and oxidative stress in bovine oocyte in vitro maturation. IUBMB Life 2001, 51, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; McKenzie, L.J.; Matzuk, M.M. Revisiting oocyte-somatic cell interactions: In search of novel intrafollicular predictors and regulators of oocyte developmental competence. Mol. Hum. Reprod. 2008, 14, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Assou, S.; Haouzi, D.; De Vos, J.; Hamamah, S. Human cumulus cells as biomarkers for embryo and pregnancy outcomes. Mol. Hum. Reprod. 2010, 16, 531–538. [Google Scholar] [CrossRef] [Green Version]
- Jancar, N.; Kopitar, A.N.; Ihan, A.; Virant Klun, I.; Bokal, E.V. Effect of apoptosis and reactive oxygen species production in human granulosa cells on oocyte fertilization and blastocyst development. J. Assist. Reprod. Genet. 2007, 24, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Hao, Z.D.; Liu, J.; Wu, Y.; Yang, L.; Liu, G.S.; Tian, J.H.; Zhu, S.E.; Zeng, S.M. Heat shock at the germinal vesicle breakdown stage induces apoptosis in surrounding cumulus cells and reduces maturation rates of porcine oocytes in vitro. Theriogenology 2008, 70, 168–178. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, X.L.; Liu, J.H.; Bao, Z.J.; Tang, D.W.; Wu, Y.; Zeng, S.M. BIM EL-mediated apoptosis in cumulus cells contributes to degenerative changes in aged porcine oocytes via a paracrine action. Theriogenology 2011, 76, 1487–1495. [Google Scholar] [CrossRef]
- Grupen, C.G.; Armstrong, D.T. Relationship between cumulus cell apoptosis, progesterone production and porcine oocyte developmental competence: Temporal effects of follicular fluid during IVM. Reprod. Fertil. Dev. 2010, 22, 1100–1109. [Google Scholar] [CrossRef]
- Kaneko, T.; Saito, H.; Takahashi, T.; Ohta, N.; Saito, T.; Hiroi, M. Effects of controlled ovarian hyperstimulation on oocyte quality in terms of the incidence of apoptotic granulosa cells. J. Assist. Reprod. Genet. 2000, 17, 580–585. [Google Scholar] [CrossRef]
- Kilic, S.; Yuksel, B.; Lortlar, N.; Sertyel, S.; Aksu, T.; Batioglu, S. Environmental tobacco smoke exposure during intrauterine period promotes granulosa cell apoptosis: A prospective, randomized study. J. Matern. Fetal Neonatal Med. 2012, 25, 1904–1908. [Google Scholar] [CrossRef]
- Sobinoff, A.P.; Beckett, E.L.; Jarnicki, A.G.; Sutherland, J.M.; McCluskey, A.; Hansbro, P.M.; McLaughlin, E.A. Scrambled and fried: Cigarette smoke exposure causes antral follicle destruction and oocyte dysfunction through oxidative stress. Toxicol. Appl. Pharmacol. 2013, 271, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Emr, S.D. Autophagy as a regulated pathway of cellular degradation. Science 2000, 290, 1717–1721. [Google Scholar] [CrossRef]
- Levine, B.; Klionsky, D.J. Development by self-digestion: Molecular mechanisms and biological functions of autophagy. Dev. Cell. 2004, 6, 463–477. [Google Scholar] [CrossRef]
- Vazquez-Nin, G.H.; Escobar, M.L.; De Felici, M.; Echeverria, O.M.; Kilinger, F.G. Cell Death in Mammalian Ovary; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Inguscio, V.; Panzarini, E.; Dini, L. Autophagy contributes to the death/survival balance in cancer photodynamic therapy. Cells 2012, 1, 464–491. [Google Scholar] [CrossRef] [PubMed]
- Mathew, R.; Karantza-Wadsworth, V.; White, E. Role of autophagy in cancer. Nat. Rev. Cancer 2007, 7, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Jo, M.W.; Lee, E.Y.; Yoon, B.K.; Choi, D.S. The role of autophagy in follicular development and atresia in rat granulosa cells. Fertil. Steril. 2010, 93, 2532–2537. [Google Scholar] [CrossRef]
- Billig, H.; Furuta, I.; Hsueh, A.J. Estrogens inhibit and androgens enhance ovarian granulosa cell apoptosis. Endocrinology 1993, 133, 2204–2212. [Google Scholar] [CrossRef]
- Gannon, A.M.; Stämpfli, M.R.; Foster, W.G. Cigarette smoke exposure elicits increased autophagy and dysregulation of mitochondrial dynamics in murine granulosa cells. Biol. Reprod. 2013, 88, 63. [Google Scholar] [CrossRef]
- Furlong, H.C.; Stämpfli, M.R.; Gannon, A.M.; Foster, W.G. Cigarette smoke exposure triggers the autophagic cascade via activation of the AMPK pathway in mice. Biol. Reprod. 2015, 93, 93. [Google Scholar] [CrossRef]
- Furlong, H.C.; Wessels, J.M.; Guerra, M.T.; Stämpfli, M.R.; Foster, W.G. Hydroxychloroquine attenuates cigarette smoke induced autophagic signaling in the mouse ovary. Reprod. Toxicol. 2016, 61, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Benowitz, N.L. Emerging nicotine delivery products. Implications for public health. Ann. Am. Thorac. Soc. 2014, 11, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Nitzkin, J.L. The case in favor of E-cigarettes for tobacco harm reduction. Int. J. Environ. Res. Public Health 2014, 11, 6459–6471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarthy, M. US plan gives greater role to electronic cigarettes in tobacco harm reduction. BMJ 2017, 358, 3689. [Google Scholar] [CrossRef]
- Cheng, T. Chemical evaluation of electronic cigarettes. Tob. Control 2014, 23, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.P.; Da Mata, F.A.; Figueiredo, A.C.; de Andrade, K.R.; Pereira, M.G. Maternal active smoking during pregnancy and low birth weight in the Americas: A systematic review and meta-analysis. Nicotine Tob. Res. 2017, 19, 497–505. [Google Scholar] [CrossRef]
- Ion, R.; Bernal, A.L. Smoking and preterm birth. Reprod. Sci. 2015, 22, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Tong, V.T.; Dietz, P.M.; Morrow, B.; D’Angelo, D.V.; Farr, S.L.; Rockhill, K.M.; England, L.J. Trends in smoking before, during, and after pregnancy–pregnancy risk assessment monitoring system, United States, 40 sites, 2000–2010. MMWR Surveill. Summ. 2013, 62, 1–19. [Google Scholar] [PubMed]
- Cui, Y.; Shooshtari, S.; Forget, E.L.; Clara, I.; Cheung, K.F. Smoking during pregnancy: Findings from the 2009-2010 Canadian community health survey. PLoS ONE 2014, 9, 84640. [Google Scholar] [CrossRef]
- Drake, P.; Driscoll, A.K.; Mathews, T.J. Cigarette smoking during pregnancy: United States, 2016. NCHS Data Brief 2018, 305, 1–8. [Google Scholar]
- Curtin, S.C.; Matthews, T.J. Smoking prevalence and cessation before and during pregnancy: Data from the birth certificate, 2014. Natl. Vital Stat. Rep. 2016, 65, 1–14. [Google Scholar]
- Lange, S.; Probst, C.; Rehm, J.; Popova, S. National, regional, and global prevalence of smoking during pregnancy in the general population: A systematic review and meta-analysis. Lancet Glob. Health 2018, 6, 769–776. [Google Scholar] [CrossRef] [Green Version]
- Oncken, C.; Ricci, K.A.; Kuo, C.L.; Dornelas, E.; Kranzler, H.R.; Sankey, H.Z. Correlates of electronic cigarettes use before and during pregnancy. Nicotine Tob. Res. 2017, 19, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Wetendorf, M.; Randall, L.T.; Lemma, M.T.; Hurr, S.H.; Pawlak, J.B.; Tarran, R.; Doerschuk, C.M.; Caron, K.M. E-cigarette exposure delays implantation and causes reduced weight gain in female offspring exposed in utero. J. Endocr. Soc. 2019, 3, 1907–1916. [Google Scholar] [CrossRef] [Green Version]
- Raez-Villanueva, S.; Ma, C.; Kleiboer, S.; Holloway, A.C. The effects of electronic cigarette vapor on placental trophoblast cell function. Reprod. Toxicol. 2018, 81, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Kondracki, A.J. Prevalence and patterns of cigarette smoking before and during early and late pregnancy according to maternal characteristics: The first national data based on the 2003 birth certificate revision, United States, 2016. Reprod. Health 2019, 1, 142. [Google Scholar] [CrossRef]
- Stuppia, L.; Franzago, M.; Ballerini, P.; Gatta, V.; Antonucci, I. Epigenetics and male reproduction: The consequences of paternal lifestyle on fertility, embryo development, and children lifetime health. Clin. Epigenetics 2015, 7, 120. [Google Scholar] [CrossRef] [Green Version]
- Zong, D.; Liu, X.; Li, J.; Ouyang, R.; Chen, P. The role of cigarette smoke-induced epigenetic alterations in inflammation. Epigenetics Chromatin 2019, 12, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konstantinidou, F.; Stuppia, L.; Gatta, V. Looking Inside the World of Granulosa Cells: The Noxious Effects of Cigarette Smoke. Biomedicines 2020, 8, 309. https://doi.org/10.3390/biomedicines8090309
Konstantinidou F, Stuppia L, Gatta V. Looking Inside the World of Granulosa Cells: The Noxious Effects of Cigarette Smoke. Biomedicines. 2020; 8(9):309. https://doi.org/10.3390/biomedicines8090309
Chicago/Turabian StyleKonstantinidou, Fani, Liborio Stuppia, and Valentina Gatta. 2020. "Looking Inside the World of Granulosa Cells: The Noxious Effects of Cigarette Smoke" Biomedicines 8, no. 9: 309. https://doi.org/10.3390/biomedicines8090309
APA StyleKonstantinidou, F., Stuppia, L., & Gatta, V. (2020). Looking Inside the World of Granulosa Cells: The Noxious Effects of Cigarette Smoke. Biomedicines, 8(9), 309. https://doi.org/10.3390/biomedicines8090309