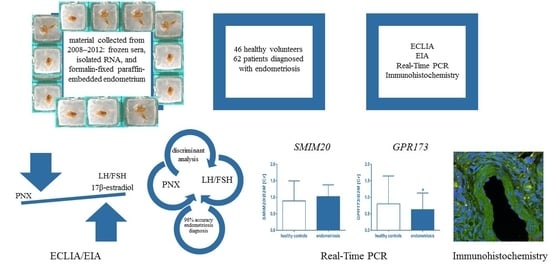
Phoenixin as a New Target in the Development of Strategies for Endometriosis Diagnosis and Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Materials
2.2. Electrochemiluminescence Immunoassay (ECLIA)
2.3. Enzyme-Linked Immunoassay (EIA)
2.4. SMIM20 and GPR173 Expression
2.4.1. RNA Quality and Quantity Assessment
2.4.2. Reverse Transcription
2.4.3. Real-Time PCR
2.5. Immunohistochemistry
2.5.1. SMIM20 Immunostaining
2.5.2. PNX-14 and GPR173 Protein Co-Localization
2.6. Statistical Analyses
3. Results
3.1. Analysis of PNX, FSH, LH, 17β-Estradiol Serum Levels
3.2. Real-Time PCR: SMIM20 and GPR173 Expression
3.3. Immunohistochemistry SMIM20, PNX-14, and GPR173
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alimi, Y.; Iwanaga, J.; Loukas, M.; Tubbs, R.S. The Clinical Anatomy of Endometriosis: A Review. Cureus 2018, 10, e3361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cramer, D.W.; Missmer, S.A. The epidemiology of endometriosis. Ann. N. Y. Acad. Sci. 2002, 955, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.A. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am. J. Obstet. Gynecol. 1927, 14, 422–469. [Google Scholar] [CrossRef]
- Jensen, J.R.; Coddington, C.C. Evolving spectrum: The pathogenesis of endometriosis. Clin. Obstet. Gynecol. 2010, 53, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Gruber, T.M.; Mechsner, S. Pathogenesis of Endometriosis: The Origin of Pain and Subfertility. Cells 2021, 10, 1381. [Google Scholar] [CrossRef] [PubMed]
- Stein, L.M.; Haddock, C.J.; Samson, W.K.; Kolar, G.R.; Yosten, G.L.C. The phoenixins: From discovery of the hormone to identification of the receptor and potential physiologic actions. Peptides 2018, 106, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Yosten, G.L.C.; Lyu, R.M.; Hsueh, A.J.W.; Avsian-Kretchmer, O.; Chang, J.K.; Tullock, C.W.; Dun, S.L.; Dun, N.; Samson, W.K. A Novel Reproductive Peptide, Phoenixin. J. Neuroendocrinol. 2013, 25, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Treen, A.K.; Luo, V.; Belsham, D.D. Phoenixin activates immortalized GnRH and kisspeptin neurons through the novel receptor GPR173. Mol. Endocrinol. 2016, 30, 872–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gershon, E.; Dekel, N. Newly identified regulators of ovarian folliculogenesis and ovulation. Int. J. Mol. Sci. 2020, 21, 4565. [Google Scholar] [CrossRef] [PubMed]
- Ullah, K.; ur Rahman, T.; Wu, D.D.; Lin, X.H.; Liu, Y.; Guo, X.Y.; Leung, P.C.K.; Zhang, R.J.; Huang, H.F.; Sheng, J.Z. Phoenixin-14 concentrations are increased in association with luteinizing hormone and nesfatin-1 concentrations in women with polycystic ovary syndrome. Clin. Chim. Acta 2017, 471, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Kalamon, N.; Błaszczyk, K.; Szlaga, A.; Billert, M.; Skrzypski, M.; Pawlicki, P.; Górowska-Wójtowicz, E.; Kotula-Balak, M.; Błasiak, A.; Rak, A. Levels of the neuropeptide phoenixin-14 and its receptor GRP173 in the hypothalamus, ovary and periovarian adipose tissue in rat model of polycystic ovary syndrome. Biochem. Biophys. Res. Commun. 2020, 528, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Suszka-Świtek, A.; Pałasz, A.; Filipczyk, Ł.; Menezes, I.C.; Mordecka-Chamera, K.; Angelone, T.; Bogus, K.; Bacopoulou, F.; Worthington, J.J.; Wiaderkiewicz, R. The GnRH analogues affect novel neuropeptide SMIM20/phoenixin and GPR173 receptor expressions in the female rat hypothalamic–pituitary–gonadal (HPG) axis. Clin. Exp. Pharmacol. Physiol. 2019, 46, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Papatheodorou, I.; Moreno, P.; Manning, J.; Fuentes, A.M.P.; George, N.; Fexova, S.; Fonseca, N.A.; Füllgrabe, A.; Green, M.; Huang, N.; et al. Expression Atlas update: From tissues to single cells. Nucleic Acids Res. 2020, 48, D77–D83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thul, P.J.; Akesson, L.; Wiking, M.; Mahdessian, D.; Geladaki, A.; Ait Blal, H.; Alm, T.; Asplund, A.; Björk, L.; Breckels, L.M.; et al. A subcellular map of the human proteome. Science 2017, 356, eaal3321. [Google Scholar] [CrossRef] [PubMed]
- Podfigurna, A.; Stellmach, A.; Szeliga, A.; Czyzyk, A.; Meczekalski, B. Metabolic profile of patients with premature ovarian insufficiency. J. Clin. Med. 2018, 7, 374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrusiewicz, M.; Słowikowski, B.; Skibińska, I.; Wołuń-Cholewa, M.; Dera-Szymanowska, A. Selection of reliable reference genes in eutopic and ectopic endometrium for quantitative expression studies. Biomed. Pharmacother. 2016, 78, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Field, A. Discovering Statistics Using SPSS, 5th ed.; Sage Publications Ltd.: New York, NY, USA, 2017. [Google Scholar]
- Lyu, R.M.; Huang, X.F.; Zhang, Y.; Dun, S.L.; Luo, J.J.; Chang, J.K.; Dun, N.J. Phoenixin: A novel peptide in rodent sensory ganglia. Neuroscience 2013, 250, 622–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imai, A.; Takagi, A.; Tamaya, T. Gonadotropin-releasing hormone analog repairs reduced endometrial cell apoptosis in endometriosis in vitro. Am. J. Obstet. Gynecol. 2000, 182, 1142S–1146S. [Google Scholar] [CrossRef] [PubMed]
- Stein, L.M.; Tullock, C.W.; Mathews, S.K.; Garcia-Galiano, D.; Elias, C.F.; Samson, W.K.; Yosten, G.L.C. Hypothalamic action of phoenixin to control reproductive hormone secretion in females: Importance of the orphan G protein-coupled receptor Gpr173. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2016, 311, R489–R496. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Gene Symbol | NCBI Accession N° | Orientation | Primer Sequence 5′ →3′ |
|---|---|---|---|
| SMIM20 | NM_001145432.2 | F | CGGCTTCATCTCCCTGATCG |
| R | ACAGCCCTCTCATTTCCTGC | ||
| GPR173 | NM_018969.6 | F | CCCGGGCTGTGATTTACCTG |
| R | TCCTGCTACATTGCACCTTGG | ||
| B2M | NM_004048.4 | F | GATGAGTATGCCTGCCGTGT |
| R | CTGCTTACATGTCTCGATCCCA | ||
| GAPDH | NM_002046.7 | F | CGCTCTCTGCTCCTCCTGTT |
| R | CCATGGTGTCTGAGCGATGT | ||
| HPRT1 | NM_000194.3 | F | TGACCTTGATTTATTTTGCATACC |
| R | CGAGCAAGACGTTCAGTCCT |
| Rank Sum (Controls) | Rank Sum (Cases) | Controls (N) | Cases (N) | p-Value | |
|---|---|---|---|---|---|
| PNX [pg/mL] | 942 | 598 | 23 | 32 | <0.001 |
| FSH [mIU/mL] | 400 | 590 | 19 | 25 | >0.05 |
| FSH [mIU/mL] | 381 | 609 | 19 | 25 | >0.05 |
| 17β-estradiol [pg/mL] | 304 | 686 | 19 | 25 | <0.01 |
| LH/FSH | 294 | 696 | 19 | 25 | <0.01 |
| Wilks `Lambda | F | p-Value | |
|---|---|---|---|
| PNX14 [pg/mL] | 0.57 | 30.2 | <0.001 |
| LH/FSH | 0.51 | 19.4 | <0.001 |
| Rank Sum (Controls) | Rank Sum (Cases) | Controls (N) | Cases (N) | p-Value | |
|---|---|---|---|---|---|
| GPR173 | 2713.5 | 2746.5 | 45 | 59 | <0.05 |
| SMIM20 | 2459.5 | 3426.5 | 46 | 62 | >0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulinska, K.I.; Andrusiewicz, M.; Dera-Szymanowska, A.; Billert, M.; Skrzypski, M.; Szymanowski, K.; Nowak-Markwitz, E.; Kotwicka, M.; Wołuń-Cholewa, M. Phoenixin as a New Target in the Development of Strategies for Endometriosis Diagnosis and Treatment. Biomedicines 2021, 9, 1427. https://doi.org/10.3390/biomedicines9101427
Kulinska KI, Andrusiewicz M, Dera-Szymanowska A, Billert M, Skrzypski M, Szymanowski K, Nowak-Markwitz E, Kotwicka M, Wołuń-Cholewa M. Phoenixin as a New Target in the Development of Strategies for Endometriosis Diagnosis and Treatment. Biomedicines. 2021; 9(10):1427. https://doi.org/10.3390/biomedicines9101427
Chicago/Turabian StyleKulinska, Karolina Iwona, Mirosław Andrusiewicz, Anna Dera-Szymanowska, Maria Billert, Marek Skrzypski, Krzysztof Szymanowski, Ewa Nowak-Markwitz, Małgorzata Kotwicka, and Maria Wołuń-Cholewa. 2021. "Phoenixin as a New Target in the Development of Strategies for Endometriosis Diagnosis and Treatment" Biomedicines 9, no. 10: 1427. https://doi.org/10.3390/biomedicines9101427
APA StyleKulinska, K. I., Andrusiewicz, M., Dera-Szymanowska, A., Billert, M., Skrzypski, M., Szymanowski, K., Nowak-Markwitz, E., Kotwicka, M., & Wołuń-Cholewa, M. (2021). Phoenixin as a New Target in the Development of Strategies for Endometriosis Diagnosis and Treatment. Biomedicines, 9(10), 1427. https://doi.org/10.3390/biomedicines9101427