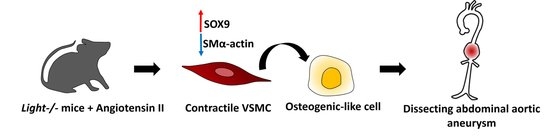
Dissecting Abdominal Aortic Aneurysm Is Aggravated by Genetic Inactivation of LIGHT (TNFSF14)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Approval and Mouse Models
2.2. Plasma Determinations
2.3. Blood Pressure Measurements
2.4. Aneurysm Measurements and Classification
2.5. Circulating Leukocyte Populations Analysis
2.6. Histological Characterization of Aneurysmal Lesions and Stainings
2.7. Aortic Human VSMC Cell Culture Experiments
2.8. Gene Expression Analysis by Quantitative Real-Time qPCR
2.9. Statistical Analysis
3. Results
3.1. Light Deficiency Promotes Severe Dissecting AAA in Apoe−/− Mice
3.2. Effect of Light Deficiency on Inflammation in Dissecting AAA in Apoe−/− Mice
3.3. Light Deficiency Modifies Lesion Characteristics and Gene Expression Pattern in Dissecting AngII-AAA
3.4. LIGHT-Dependent Signaling Modulates Aortic Human VSMC Phenotype
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAA | Abdominal aortic aneurysm |
| ah VSMC | Vascular smooth muscle cell |
| AngII | Angiotensin II |
| Apoe−/− | Apolipoprotein E-deficient |
| BW | Body weights |
| DBP | Diastolic blood pressure |
| HVEM | Herpesvirus Entry Mediator |
| JNK | c-Jun N-terminal kinase |
| LTβR | Lymphotoxin-β Receptor |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| SBP | Systolic blood pressure |
| SEM | Standard error of the mean |
| siRNA | Short interference ribonucleic acid |
| SM | Smooth muscle cell |
| TNF | Tumor necrosis factor |
References
- Quintana, R.A.; Taylor, W.R. Cellular Mechanisms of Aortic Aneurysm Formation. Circ. Res. 2019, 124, 607–618. [Google Scholar] [CrossRef]
- Petsophonsakul, P.; Furmanik, M.; Forsythe, R.; Dweck, M.; Schurink, G.W.; Natour, E.; Reutelingsperger, C.; Jacobs, M.; Mees, B.; Schurgers, L. Role of Vascular Smooth Muscle Cell Phenotypic Switching and Calcification in Aortic Aneurysm Formation. Arter. Thromb. Vasc. Biol. 2019, 39, 1351–1368. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Suetake, H.; Odaka, T.; Miyadai, T. Original Ligand for LTβR Is LIGHT: Insight into Evolution of the LT/LTβR System. J. Immunol. 2018, 201, 202–214. [Google Scholar] [CrossRef]
- Steinberg, M.W.; Cheung, T.C.; Ware, C.F. The signaling networks of the herpesvirus entry mediator (TNFRSF14) in immune regulation. Immunol. Rev. 2011, 244, 169–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, X.; Gu, Y.; Lai, X.; Gu, Q. LIGHT is increased in patients with coronary disease and regulates inflammatory response and lipid metabolism in oxLDL-induced THP-1 macrophages. Biochem. Biophys. Res. Commun. 2017, 490, 732–738. [Google Scholar] [CrossRef]
- Dahl, C.P.; Gullestad, L.; Fevang, B.; Holm, A.M.; Landrø, L.; Vinge, L.E.; Fiane, A.E.; Sandberg, W.J.; Otterdal, K.; Frøland, S.S.; et al. Increased expression of LIGHT/TNFSF14 and its receptors in experimental and clinical heart failure. Eur. J. Heart Fail. 2008, 10, 352–359. [Google Scholar] [CrossRef]
- Scholz, H.; Sandberg, W.; Damås, J.K.; Smith, C.; Andreassen, A.K.; Gullestad, L.; Frøland, S.S.; Yndestad, A.; Aukrust, P.; Halvorsen, B. Enhanced Plasma Levels of LIGHT in Unstable Angina. Circulation 2005, 112, 2121–2129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owens, A.W.; Matulevicius, S.; Rohatgi, A.; Ayers, C.R.; Das, S.R.; Khera, A.; McGuire, D.K.; de Lemos, J.A. Circulating lymphotoxin β receptor and atherosclerosis: Observations from the Dallas Heart Study. Atherosclerosis 2010, 212, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Grandoch, M.; Feldmann, K.; Göthert, J.R.; Dick, L.S.; Homann, S.; Klatt, C.; Bayer, J.K.; Waldheim, J.N.; Rabausch, B.; Nagy, N.; et al. Deficiency in Lymphotoxin β Receptor Protects From Atherosclerosis in apoE-Deficient Mice. Circ. Res. 2015, 116, e57–e68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrés-Blasco, I.; Vinué, À.; Herrero-Cervera, A.; Martínez-Hervás, S.; Nuñez, L.; Piqueras, L.; Ascaso, J.F.; Sanz, M.J.; Burks, D.J.; González-Navarro, H. Hepatic lipase inactivation decreases atherosclerosis in insulin resistance by reducing LIGHT/Lymphotoxin β-Receptor pathway. Thromb. Haemost. 2016, 116, 379–393. [Google Scholar] [CrossRef]
- Hu, D.; Mohanta, S.K.; Yin, C.; Peng, L.; Ma, Z.; Srikakulapu, P.; Grassia, G.; MacRitchie, N.; Dever, G.; Gordon, P.; et al. Artery Tertiary Lymphoid Organs Control Aorta Immunity and Protect against Atherosclerosis via Vascular Smooth Muscle Cell Lymphotoxin β Receptors. Immunity 2015, 42, 1100–1115. [Google Scholar] [CrossRef] [Green Version]
- Gräbner, R.; Lötzer, K.; Döpping, S.; Hildner, M.; Radke, D.; Beer, M.; Spanbroek, R.; Lippert, B.; Reardon, C.A.; Getz, G.S.; et al. Lymphotoxin β receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged ApoE−/− mice. J. Exp. Med. 2009, 206, 233–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daugherty, A.; Manning, M.W.; Cassis, L.A. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E–deficient mice. J. Clin. Investig. 2000, 105, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Martorell, S.; Hueso, L.; Gonzalez-Navarro, H.; Collado, A.; Sanz, M.-J.; Piqueras, L. Vitamin D Receptor Activation Reduces Angiotensin-II–Induced Dissecting Abdominal Aortic Aneurysm in Apolipoprotein E–Knockout Mice. Arter. Thromb. Vasc. Biol. 2016, 36, 1587–1597. [Google Scholar] [CrossRef] [Green Version]
- Herrero-Cervera, A.; Vinué, Á.; Burks, D.J.; González-Navarro, H. Genetic inactivation of the LIGHT (TNFSF14) cytokine in mice restores glucose homeostasis and diminishes hepatic steatosis. Diabetologia 2019, 62, 2143–2157. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, A.; Rateri, D.; Hong, L.; Balakrishnan, A. Measuring Blood Pressure in Mice using Volume Pressure Recording, a Tail-cuff Method. J. Vis. Exp. 2009, 1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadi, T.; Boytard, L.; Silvestro, M.; Alebrahim, D.; Jacob, S.; Feinstein, J.; Barone, K.; Spiro, W.; Hutchison, S.; Simon, R.; et al. Macrophage-derived netrin-1 promotes abdominal aortic aneurysm formation by activating MMP3 in vascular smooth muscle cells. Nat. Commun. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Qin, Y.; Cao, X.; Guo, J.; Zhang, Y.; Pan, L.; Zhang, H.; Li, H.; Tang, C.; Du, J.; Shi, G.-P. Deficiency of cathepsin S attenuates angiotensin II-induced abdominal aortic aneurysm formation in apolipoprotein E-deficient mice. Cardiovasc. Res. 2012, 96, 401–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinué, Á.; Martínez-Hervás, S.; Herrero-Cervera, A.; Sánchez-García, V.; Andrés-Blasco, I.; Piqueras, L.; Sanz, M.J.; Real, J.T.; Ascaso, J.F.; Burks, D.J.; et al. Changes in CDKN2A/2B expression associate with T-cell phenotype modulation in atherosclerosis and type 2 diabetes mellitus. Transl. Res. 2019, 203, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Hervás, S.; Vinué, Á.; Núñez, L.; Andrés-Blasco, I.; Piqueras, L.; Real, J.T.; Ascaso, J.F.; Burks, D.J.; Sanz, M.J.; González-Navarro, H. Insulin resistance aggravates atherosclerosis by reducing vascular smooth muscle cell survival and increasing CX3CL1/CX3CR1 axis. Cardiovasc. Res. 2014, 103, 324–336. [Google Scholar] [CrossRef] [Green Version]
- Wolf, M.J.; Adili, A.; Piotrowitz, K.; Abdullah, Z.; Boege, Y.; Stemmer, K.; Ringelhan, M.; Simonavicius, N.; Egger, M.; Wohlleber, D.; et al. Metabolic Activation of Intrahepatic CD8+ T Cells and NKT Cells Causes Nonalcoholic Steatohepatitis and Liver Cancer via Cross-Talk with Hepatocytes. Cancer Cell 2014, 26, 549–564. [Google Scholar] [CrossRef] [Green Version]
- Saunders, B.M.; Rudnicka, C.; Filipovska, A.; Davies, S.; Ward, N.; Hricova, J.; Schlaich, M.P.; Matthews, V.B. Shining LIGHT on the metabolic role of the cytokine TNFSF14 and the implications on hepatic IL-6 production. Immunol. Cell Biol. 2018, 96, 41–53. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Gomez, D. Smooth Muscle Cell Phenotypic Diversity. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1715–1723. [Google Scholar] [CrossRef] [PubMed]
- Herro, R.; Antunes, R.D.S.; Aguilera, A.R.; Tamada, K.; Croft, M. The Tumor Necrosis Factor Superfamily Molecule LIGHT Promotes Keratinocyte Activity and Skin Fibrosis. J. Investig. Dermatol. 2015, 135, 2109–2118. [Google Scholar] [CrossRef] [Green Version]
- Sibilano, R.; Gaudenzio, N.; DeGorter, M.K.; Reber, L.; Hernandez, J.D.; Starkl, P.; Zurek, O.W.; Tsai, M.; Zahner, S.; Montgomery, S.; et al. A TNFRSF14-FcɛRI-mast cell pathway contributes to development of multiple features of asthma pathology in mice. Nat. Commun. 2016, 7, 13696. [Google Scholar] [CrossRef]
- Augstein, A.; Mierke, J.B.; Poitz, D.M.; Strasser, R.H. Sox9 is increased in arterial plaque and stenosis, associated with synthetic phenotype of vascular smooth muscle cells and causes alterations in extracellular matrix and calcification. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2526–2537. [Google Scholar] [CrossRef]
- Naik, V.; Leaf, E.M.; Hu, J.H.; Yang, H.-Y.; Nguyen, N.B.; Giachelli, C.M.; Speer, M.Y. Sources of cells that contribute to atherosclerotic intimal calcification: An in vivo genetic fate mapping study. Cardiovasc. Res. 2012, 94, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Briot, A.; Jaroszewicz, A.; Warren, C.M.; Lu, J.; Touma, M.; Rudat, C.; Hofmann, J.J.; Airik, R.; Weinmaster, G.; Lyons, K.; et al. Repression of Sox9 by Jag1 Is Continuously Required to Suppress the Default Chondrogenic Fate of Vascular Smooth Muscle Cells. Dev. Cell 2014, 31, 707–721. [Google Scholar] [CrossRef] [Green Version]
- Sitcheran, R.; Cogswell, P.C.; Baldwin, J.A.S. NF-B mediates inhibition of mesenchymal cell differentiation through a posttranscriptional gene silencing mechanism. Genes Dev. 2003, 17, 2368–2373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, J.-S.; Sierra, O.L.; Cohen, R.; Mecham, R.P.; Kovacs, A.; Wang, J.; Distelhorst, K.; Behrmann, A.; Halstead, L.R.; Towler, D.A. Vascular calcification and aortic fibrosis: A bifunctional role for osteopontin in diabetic arteriosclerosis. Arter. Thromb. Vasc. Biol. 2011, 31, 1821–1833. [Google Scholar] [CrossRef] [Green Version]
- Shankman, L.S.; Gomez, D.; Cherepanova, O.A.; Salmon, M.; Alencar, G.F.; Haskins, R.M.; Swiatlowska, P.; Newman, A.A.C.; Greene, E.S.; Straub, A.C.; et al. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat. Med. 2015, 21, 628–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alencar, G.F.; Owsiany, K.M.; Karnewar, S.; Sukhavasi, K.; Mocci, G.; Nguyen, A.T.; Williams, C.M.; Shamsuzzaman, S.; Mokry, M.; Henderson, C.A.; et al. Stem Cell Pluripotency Genes Klf4 and Oct4 Regulate Complex SMC Phenotypic Changes Critical in Late-Stage Atherosclerotic Lesion Pathogenesis. Circulation 2020, 142, 2045–2059. [Google Scholar] [CrossRef]
- Hollenbeck, S.T.; Sakakibara, K.; Faries, P.L.; Workhu, B.; Liu, B.; Kent, K. Stem cell factor and c-kit are expressed by and may affect vascular SMCs through an autocrine pathway. J. Surg. Res. 2004, 120, 288–294. [Google Scholar] [CrossRef]
- Grewal, N.; Groot, A.C.G.-D.; DeRuiter, M.C.; Klautz, R.J.; Poelmann, R.E.; Duim, S.; Lindeman, J.H.; Koenraadt, W.M.; Jongbloed, M.R.; Mohamed, S.A.; et al. Bicuspid aortic valve: Phosphorylation of c-Kit and downstream targets are prognostic for future aortopathy. Eur. J. Cardio-Thoracic Surg. 2014, 46, 831–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryer, E.J.; Garvin, R.P.; Schworer, C.M.; Bernard-Eckroth, K.R.; Tromp, G.; Franklin, D.P.; Elmore, J.R.; Kuivaniemi, H. Proinflammatory role of stem cells in abdominal aortic aneurysms. J. Vasc. Surg. 2015, 62, 1303–1311.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skeate, J.G.; Otsmaa, M.E.; Prins, R.; Fernandez, D.J.; Da Silva, D.M.; Kast, W.M. TNFSF14: LIGHTing the Way for Effective Cancer Immunotherapy. Front. Immunol. 2020, 11, 922. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrero-Cervera, A.; Espinós-Estévez, C.; Martín-Vañó, S.; Taberner-Cortés, A.; Aguilar-Ballester, M.; Vinué, Á.; Piqueras, L.; Martínez-Hervás, S.; González-Navarro, H. Dissecting Abdominal Aortic Aneurysm Is Aggravated by Genetic Inactivation of LIGHT (TNFSF14). Biomedicines 2021, 9, 1518. https://doi.org/10.3390/biomedicines9111518
Herrero-Cervera A, Espinós-Estévez C, Martín-Vañó S, Taberner-Cortés A, Aguilar-Ballester M, Vinué Á, Piqueras L, Martínez-Hervás S, González-Navarro H. Dissecting Abdominal Aortic Aneurysm Is Aggravated by Genetic Inactivation of LIGHT (TNFSF14). Biomedicines. 2021; 9(11):1518. https://doi.org/10.3390/biomedicines9111518
Chicago/Turabian StyleHerrero-Cervera, Andrea, Carla Espinós-Estévez, Susana Martín-Vañó, Alida Taberner-Cortés, María Aguilar-Ballester, Ángela Vinué, Laura Piqueras, Sergio Martínez-Hervás, and Herminia González-Navarro. 2021. "Dissecting Abdominal Aortic Aneurysm Is Aggravated by Genetic Inactivation of LIGHT (TNFSF14)" Biomedicines 9, no. 11: 1518. https://doi.org/10.3390/biomedicines9111518
APA StyleHerrero-Cervera, A., Espinós-Estévez, C., Martín-Vañó, S., Taberner-Cortés, A., Aguilar-Ballester, M., Vinué, Á., Piqueras, L., Martínez-Hervás, S., & González-Navarro, H. (2021). Dissecting Abdominal Aortic Aneurysm Is Aggravated by Genetic Inactivation of LIGHT (TNFSF14). Biomedicines, 9(11), 1518. https://doi.org/10.3390/biomedicines9111518