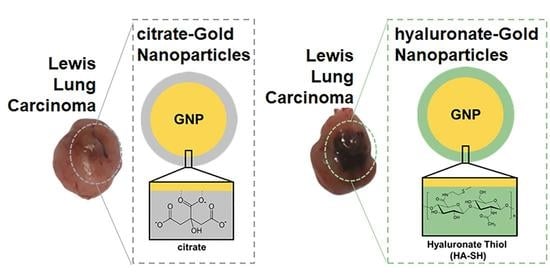
Hyaluronate-Thiol Passivation Enhances Gold Nanoparticle Peritumoral Distribution When Administered Intratumorally in Lung Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Gold Nanoparticles Synthesis
2.2. Surface Passivation of the Gold Nanoparticles with Hyaluronate-Thiol
2.3. GNP Characterization
2.3.1. Ultraviolet-Visible (UV-VIS) Spectroscopy
2.3.2. Dynamic Light Scattering (DLS) and ζ-Potential
2.4. In Vitro Assessment of Cytotoxicity and GNP Uptake
2.4.1. Cell Line and Passaging
2.4.2. MTT Assay for Cell Viability Due to Metabolic Activity
2.4.3. Trypan Blue Assay for Cell Viability
2.4.4. Elemental Analysis to Quantify Intracellular GNP Uptake
2.5. In Vivo Biodistribution Analysis
2.5.1. Animal Model of Lung Cancer
2.5.2. Experimental Timeline
2.5.3. Site-Specific Intratumoral Distribution of GNP: Elemental Analysis
2.6. Statistical Analysis
3. Results
3.1. GNP Characterization
3.2. In Vitro Cytotoxicity Assessments
3.3. In Vivo Biodistribution in a Murine Lung Cancer Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| DLS | dynamic light scattering |
| FBS | fetal bovine serum |
| GNP | gold nanoparticle |
| HA-SH | hyaluronate-thiol |
| IACUC | Institutional Animal Care and Use Committee |
| ICP-OES | Inductively Coupled Plasma-Optical Emission Spectrometry |
| IFP | interstitial fluid pressure |
| IT | intra-tumoral |
| LLC | Lewis lung carcinoma |
| MTT | (3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide) |
| NSCLC | non-small cell lung cancer |
| PBS | phosphate buffered saline |
| PDI | polydispersity index |
| s.e.m. | standard error of the mean |
| SPR | surface plasmon resonance |
| SEM | scanning electron microscopy |
| TME | tumor microenvironment |
References
- Heinrich, A.-K.; Lucas, H.; Schindler, L.; Chytil, P.; Etrych, T.; Mäder, K.; Mueller, T. Improved Tumor-Specific Drug Accumulation by Polymer Therapeutics with PH-Sensitive Drug Release Overcomes Chemotherapy Resistance. Mol. Cancer Ther. 2016, 15, 998–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chua, C.Y.X.; Ho, J.; Demaria, S.; Ferrari, M.; Grattoni, A. Emerging Technologies for Local Cancer Treatment. Adv. Ther. 2020, 3, 2000027. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Susnjar, A.; Rhudy, J.; Sizovs, A.; Lolli, G.; Pino, R.; Chua, C.Y.X.; Butler, E.B.; Demaria, S.; Grattoni, A. Localizing Radioimmunotherapy via Nanochannel Device for Sustained Intratumoral Drug Delivery for Solid Tumor Treatment. JCO 2019, 37, 37. [Google Scholar] [CrossRef]
- Park, C.G.; Hartl, C.A.; Schmid, D.; Carmona, E.M.; Kim, H.-J.; Goldberg, M.S. Extended Release of Perioperative Immunotherapy Prevents Tumor Recurrence and Eliminates Metastases. Sci. Transl. Med. 2018, 10, eaar1916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Momin, N.; Mehta, N.K.; Bennett, N.R.; Ma, L.; Palmeri, J.R.; Chinn, M.M.; Lutz, E.A.; Kang, B.; Irvine, D.J.; Spranger, S.; et al. Anchoring of Intratumorally Administered Cytokines to Collagen Safely Potentiates Systemic Cancer Immunotherapy. Sci. Transl. Med. 2019, 11, eaaw2614. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-C.; Viswanath, D.I.; Pesaresi, F.; Xu, Y.; Zhang, L.; Trani, N.D.; Paez-Mayorga, J.; Hernandez, N.; Wang, Y.; Erm, D.R.; et al. Potentiating Antitumor Efficacy Through Radiation and Sustained Intratumoral Delivery of Anti-CD40 and Anti-PDL1. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of Nanoparticle Design for Overcoming Biological Barriers to Drug Delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Böckelmann, L.C.; Schumacher, U. Targeting Tumor Interstitial Fluid Pressure: Will It Yield Novel Successful Therapies for Solid Tumors? Expert Opin. Ther. Targets 2019, 23, 1005–1014. [Google Scholar] [CrossRef]
- Maulhardt, H.A.; Hylle, L.; Frost, M.V.; Tornio, A.; Dafoe, S.; Drummond, L.; Quinn, D.I.; Kamat, A.M.; diZerega, G.S. Local Injection of Submicron Particle Docetaxel Is Associated with Tumor Eradication, Reduced Systemic Toxicity and an Immunologic Response in Uro-Oncologic Xenografts. Cancers 2019, 11, 577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernauer, C.; Man, Y.K.S.; Chisholm, J.C.; Lepicard, E.Y.; Robinson, S.P.; Shipley, J.M. Hypoxia and Its Therapeutic Possibilities in Paediatric Cancers. Br. J. Cancer 2021, 124, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Torok, S.; Rezeli, M.; Kelemen, O.; Vegvari, A.; Watanabe, K.; Sugihara, Y.; Tisza, A.; Marton, T.; Kovacs, I.; Tovari, J.; et al. Limited Tumor Tissue Drug Penetration Contributes to Primary Resistance against Angiogenesis Inhibitors. Theranostics 2017, 7, 400–412. [Google Scholar] [CrossRef] [Green Version]
- Bae, Y.H.; Park, K. Targeted Drug Delivery to Tumors: Myths, Reality and Possibility. J. Control Release 2011, 153, 198–205. [Google Scholar] [CrossRef] [Green Version]
- Farooq, M.U.; Novosad, V.; Rozhkova, E.A.; Wali, H.; Ali, A.; Fateh, A.A.; Neogi, P.B.; Neogi, A.; Wang, Z. Gold Nanoparticles-Enabled Efficient Dual Delivery of Anticancer Therapeutics to HeLa Cells. Sci. Rep. 2018, 8, 2907. [Google Scholar] [CrossRef] [Green Version]
- Yafout, M.; Ousaid, A.; Khayati, Y.; El Otmani, I.S. Gold Nanoparticles as a Drug Delivery System for Standard Chemotherapeutics: A New Lead for Targeted Pharmacological Cancer Treatments. Sci. Afr. 2021, 11, e00685. [Google Scholar] [CrossRef]
- Lai, S.-F.; Ko, B.-H.; Chien, C.-C.; Chang, C.-J.; Yang, S.-M.; Chen, H.-H.; Petibois, C.; Hueng, D.-Y.; Ka, S.-M.; Chen, A.; et al. Gold Nanoparticles as Multimodality Imaging Agents for Brain Gliomas. J. Nanobiotechnol. 2015, 13, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, V.P.; Qian, W.; Li, Y.; Liu, B.; Aaberg, M.; Henry, J.; Zhang, W.; Wang, X.; Paulus, Y.M. Chain-like Gold Nanoparticle Clusters for Multimodal Photoacoustic Microscopy and Optical Coherence Tomography Enhanced Molecular Imaging. Nat. Commun. 2021, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Bishnoi, S.W.; Rozell, C.J.; Levin, C.S.; Gheith, M.K.; Johnson, B.R.; Johnson, D.H.; Halas, N.J. All-Optical Nanoscale PH Meter. Nano. Lett. 2006, 6, 1687–1692. [Google Scholar] [CrossRef]
- Cheheltani, R.; Ezzibdeh, R.M.; Chhour, P.; Pulaparthi, K.; Kim, J.; Jurcova, M.; Hsu, J.C.; Blundell, C.; Litt, H.I.; Ferrari, V.A.; et al. Tunable, Biodegradable Gold Nanoparticles as Contrast Agents for Computed Tomography and Photoacoustic Imaging. Biomaterials 2016, 102, 87–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levin, C.S.; Janesko, B.G.; Bardhan, R.; Scuseria, G.E.; Hartgerink, J.D.; Halas, N.J. Chain-Length-Dependent Vibrational Resonances in Alkanethiol Self-Assembled Monolayers Observed on Plasmonic Nanoparticle Substrates. Nano Lett. 2006, 6, 2617–2621. [Google Scholar] [CrossRef]
- Kimm, M.A.; Shevtsov, M.; Werner, C.; Sievert, W.; Zhiyuan, W.; Schoppe, O.; Menze, B.H.; Rummeny, E.J.; Proksa, R.; Bystrova, O.; et al. Gold Nanoparticle Mediated Multi-Modal CT Imaging of Hsp70 Membrane-Positive Tumors. Cancers 2020, 12, 1331. [Google Scholar] [CrossRef]
- Su, W.; Chen, C.; Wang, T.; Li, X.; Liu, Y.; Wang, H.; Zhao, S.; Zuo, C.; Sun, G.; Bu, W. Radionuclide-Labeled Gold Nanoparticles for Nuclei-Targeting Internal Radio-Immunity Therapy. Mater. Horiz. 2020, 7, 1115–1125. [Google Scholar] [CrossRef]
- Bartelink, I.H.; Jones, E.F.; Shahidi-Latham, S.K.; Lee, P.R.E.; Zheng, Y.; Vicini, P.; van ‘t Veer, L.; Wolf, D.; Iagaru, A.; Kroetz, D.L.; et al. Tumor Drug Penetration Measurements Could Be the Neglected Piece of the Personalized Cancer Treatment Puzzle. Clin. Pharmacol. Ther. 2019, 106, 148–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, A.; Vighetto, V.; Di Marzio, N.; Ferraro, F.; Hirsch, M.; Ferrante, N.; Mitra, S.; Grattoni, A.; Filgueira, C.S. Gold Nanoparticles Radio-Sensitize and Reduce Cell Survival in Lewis Lung Carcinoma. Nanomaterials 2020, 10, 1717. [Google Scholar] [CrossRef] [PubMed]
- Terracciano, R.; Zhang, A.; Simeral, M.L.; Demarchi, D.; Hafner, J.H.; Filgueira, C.S. Improvements in Gold Nanorod Biocompatibility with Sodium Dodecyl Sulfate Stabilization. J. Nanotheranostics 2021, 2, 157–173. [Google Scholar] [CrossRef]
- Terracciano, R.; Zhang, A.; Butler, E.B.; Demarchi, D.; Hafner, J.H.; Grattoni, A.; Filgueira, C.S. Effects of Surface Protein Adsorption on the Distribution and Retention of Intratumorally Administered Gold Nanoparticles. Pharmaceutics 2021, 13, 216. [Google Scholar] [CrossRef]
- Terracciano, R.; Sprouse, M.L.; Wang, D.; Ricchetti, S.; Hirsch, M.; Ferrante, N.; Butler, E.B.; Demarchi, D.; Grattoni, A.; Filgueira, C.S. Intratumoral Gold Nanoparticle-Enhanced CT Imaging: An in Vivo Investigation of Biodistribution and Retention. In Proceedings of the 2020 IEEE 20th International Conference on Nanotechnology (IEEE-NANO), Virtual, 29–31 July 2020; pp. 349–353. [Google Scholar]
- Lichtenberg, J.Y.; Ling, Y.; Kim, S. Non-Specific Adsorption Reduction Methods in Biosensing. Sensors 2019, 19, 2488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, J.; Park, C.; Yi, G.; Lee, D.; Koo, H. Active Targeting Strategies Using Biological Ligands for Nanoparticle Drug Delivery Systems. Cancers 2019, 11, 640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levin, C.S.; Bishnoi, S.W.; Grady, N.K.; Halas, N.J. Determining the Conformation of Thiolated Poly(Ethylene Glycol) on Au Nanoshells by Surface-Enhanced Raman Scattering Spectroscopic Assay. Anal. Chem. 2006, 78, 3277–3281. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, Y.; Fleischer, C.C.; Huang, J.; Lin, R.; Yang, L.; Mao, H. Impact of Anti-Biofouling Surface Coatings on the Properties of Nanomaterials and Their Biomedical Applications. J. Mater. Chem. B 2018, 6, 9–24. [Google Scholar] [CrossRef]
- Sanchez-Cano, C.; Carril, M. Recent Developments in the Design of Non-Biofouling Coatings for Nanoparticles and Surfaces. Int. J. Mol. Sci. 2020, 21, 1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, W.; Ma, G.; Kampf, N.; Yuan, Z.; Chen, S. Development of Long-Circulating Zwitterionic Cross-Linked Micelles for Active-Targeted Drug Delivery. Biomacromolecules 2016, 17, 2010–2018. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Tang, M.; Cao, W.; Zhang, Z.; Li, X. Hierarchically Targetable Fiber Rods Decorated with Dual Targeting Ligands and Detachable Zwitterionic Coronas. Acta Biomater. 2020, 110, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.A.; Werfel, T.A.; Curvino, E.J.; Yu, F.; Kavanaugh, T.E.; Sarett, S.M.; Dockery, M.D.; Kilchrist, K.V.; Jackson, A.N.; Giorgio, T.D.; et al. Zwitterionic Nanocarrier Surface Chemistry Improves SiRNA Tumor Delivery and Silencing Activity Relative to Polyethylene Glycol. ACS Nano. 2017, 11, 5680–5696. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Huang, H. Application of Hyaluronic Acid as Carriers in Drug Delivery. Drug Deliv. 2018, 25, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Callahan, L.A.S.; Ganios, A.M.; McBurney, D.L.; Dilisio, M.F.; Weiner, S.D.; Horton, W.E.; Becker, M.L. ECM Production of Primary Human and Bovine Chondrocytes in Hybrid PEG Hydrogels Containing Type I Collagen and Hyaluronic Acid. Biomacromolecules 2012, 13, 1625–1631. [Google Scholar] [CrossRef] [Green Version]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic Acid: A Key Molecule in Skin Aging. Derm.-Endocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef] [Green Version]
- Holmes, M.W.; Bayliss, M.T.; Muir, H. Hyaluronic Acid in Human Articular Cartilage. Age-Related Changes in Content and Size. Biochem. J. 1988, 250, 435–441. [Google Scholar] [CrossRef] [Green Version]
- Hegewald, A.A.; Ringe, J.; Bartel, J.; Krüger, I.; Notter, M.; Barnewitz, D.; Kaps, C.; Sittinger, M. Hyaluronic Acid and Autologous Synovial Fluid Induce Chondrogenic Differentiation of Equine Mesenchymal Stem Cells: A Preliminary Study. Tissue Cell 2004, 36, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Schmaus, A.; Klusmeier, S.; Rothley, M.; Dimmler, A.; Sipos, B.; Faller, G.; Thiele, W.; Allgayer, H.; Hohenberger, P.; Post, S.; et al. Accumulation of Small Hyaluronan Oligosaccharides in Tumour Interstitial Fluid Correlates with Lymphatic Invasion and Lymph Node Metastasis. Br. J. Cancer 2014, 111, 559–567. [Google Scholar] [CrossRef] [Green Version]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic Acid Hydrogels for Biomedical Applications. Adv. Mater 2011, 23, H41–H56. [Google Scholar] [CrossRef]
- Collins, M.N.; Birkinshaw, C. Hyaluronic Acid Based Scaffolds for Tissue Engineering—A Review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Fakhari, A.; Berkland, C. Applications and Emerging Trends of Hyaluronic Acid in Tissue Engineering, as a Dermal Filler and in Osteoarthritis Treatment. Acta. Biomater. 2013, 9, 7081–7092. [Google Scholar] [CrossRef] [Green Version]
- Jordan, A.R.; Racine, R.R.; Hennig, M.J.P.; Lokeshwar, V.B. The Role of CD44 in Disease Pathophysiology and Targeted Treatment. Front. Immunol. 2015, 6, 182. [Google Scholar] [CrossRef]
- Leung, E.L.-H.; Fiscus, R.R.; Tung, J.W.; Tin, V.P.-C.; Cheng, L.C.; Sihoe, A.D.-L.; Fink, L.M.; Ma, Y.; Wong, M.P. Non-Small Cell Lung Cancer Cells Expressing CD44 Are Enriched for Stem Cell-like Properties. PLoS ONE 2010, 5, e14062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almalik, A.; Benabdelkamel, H.; Masood, A.; Alanazi, I.O.; Alradwan, I.; Majrashi, M.A.; Alfadda, A.A.; Alghamdi, W.M.; Alrabiah, H.; Tirelli, N.; et al. Hyaluronic Acid Coated Chitosan Nanoparticles Reduced the Immunogenicity of the Formed Protein Corona. Sci. Rep. 2017, 7, 10542. [Google Scholar] [CrossRef]
- Kim, H.; Shin, M.; Han, S.; Kwon, W.; Hahn, S.K. Hyaluronic Acid Derivatives for Translational Medicines. Biomacromolecules 2019, 20, 2889–2903. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Pang, L.; Feng, H.; Dong, H.; Wang, S.; Cong, H.; Shen, Y.; Bing, Y. Recent Advantage of Hyaluronic Acid for Anti-Cancer Application: A Review of “3S” Transition Approach. Carbohydr. Polym. 2020, 238, 116204. [Google Scholar] [CrossRef]
- Chiang, M.-T.; Wang, H.-L.; Han, T.-Y.; Hsieh, Y.-K.; Wang, J.; Tsai, D.-H. Assembly and Detachment of Hyaluronic Acid on a Protein-Conjugated Gold Nanoparticle. Langmuir 2020, 36, 14782–14792. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Atyabi, F.; Varnamkhasti, B.S.; Hosseinzadeh, R.; Ostad, S.N.; Ghahremani, M.H.; Dinarvand, R. SN38 Conjugated Hyaluronic Acid Gold Nanoparticles as a Novel System against Metastatic Colon Cancer Cells. Int. J. Pharm. 2017, 526, 339–352. [Google Scholar] [CrossRef]
- Liu, X.; Gao, C.; Gu, J.; Jiang, Y.; Yang, X.; Li, S.; Gao, W.; An, T.; Duan, H.; Fu, J.; et al. Hyaluronic Acid Stabilized Iodine-Containing Nanoparticles with Au Nanoshell Coating for X-Ray CT Imaging and Photothermal Therapy of Tumors. ACS Appl. Mater. Interfaces 2016, 8, 27622–27631. [Google Scholar] [CrossRef]
- Li, L.-S.; Ren, B.; Yang, X.; Cai, Z.-C.; Zhao, X.-J.; Zhao, M.-X. Hyaluronic Acid-Modified and Doxorubicin-Loaded Gold Nanoparticles and Evaluation of Their Bioactivity. Pharmaceuticals 2021, 14, 101. [Google Scholar] [CrossRef]
- Gotov, O.; Battogtokh, G.; Ko, Y.T. Docetaxel-Loaded Hyaluronic Acid–Cathepsin B-Cleavable-Peptide–Gold Nanoparticles for the Treatment of Cancer. Mol. Pharm. 2018, 15, 4668–4676. [Google Scholar] [CrossRef] [PubMed]
- Gotov, O.; Battogtokh, G.; Shin, D.; Ko, Y.T. Hyaluronic Acid-Coated Cisplatin Conjugated Gold Nanoparticles for Combined Cancer Treatment. J. Ind. Eng. Chem. J. 2018, 65, 236–243. [Google Scholar] [CrossRef]
- Apaolaza, P.S.; Busch, M.; Asin-Prieto, E.; Peynshaert, K.; Rathod, R.; Remaut, K.; Dünker, N.; Göpferich, A. Hyaluronic Acid Coating of Gold Nanoparticles for Intraocular Drug Delivery: Evaluation of the Surface Properties and Effect on Their Distribution. Exp. Eye Res. 2020, 198, 108151. [Google Scholar] [CrossRef]
- Sonntag, T.; Froemel, F.; Stamer, W.D.; Ohlmann, A.; Fuchshofer, R.; Breunig, M. Distribution of Gold Nanoparticles in the Anterior Chamber of the Eye after Intracameral Injection for Glaucoma Therapy. Pharmaceutics 2021, 13, 901. [Google Scholar] [CrossRef]
- Mendes, C.; dos Santos Haupenthal, D.P.; Zaccaron, R.P.; de Bem Silveira, G.; Corrêa, M.E.A.B.; de Roch Casagrande, L.; de Sousa Mariano, S.; de Souza Silva, J.I.; de Andrade, T.A.M.; Feuser, P.E.; et al. Effects of the Association between Photobiomodulation and Hyaluronic Acid Linked Gold Nanoparticles in Wound Healing. ACS Biomater. Sci. Eng. 2020, 6, 5132–5144. [Google Scholar] [CrossRef] [PubMed]
- Egeblad, M.; Nakasone, E.S.; Werb, Z. Tumors as Organs: Complex Tissues That Interface with the Entire Organism. Dev. Cell 2010, 18, 884–901. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Lee, M.-Y.; Bhang, S.H.; Kim, B.-S.; Kim, Y.S.; Ju, J.H.; Kim, K.S.; Hahn, S.K. Hyaluronate–Gold Nanoparticle/Tocilizumab Complex for the Treatment of Rheumatoid Arthritis. ACS Nano. 2014, 8, 4790–4798. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.L.; Urban, D.A.; Rodriguez-Lorenzo, L.; Milosevic, A.; Crippa, F.; Spuch-Calvar, M.; Balog, S.; Rothen-Rutishauser, B.; Lattuada, M.; Petri-Fink, A. Nanoparticle Administration Method in Cell Culture Alters Particle-Cell Interaction. Sci. Rep. 2019, 9, 900. [Google Scholar] [CrossRef]
- Card, J.W.; Carey, M.A.; Bradbury, J.A.; DeGraff, L.M.; Morgan, D.L.; Moorman, M.P.; Flake, G.P.; Zeldin, D.C. Gender Differences in Murine Airway Responsiveness and Lipopolysaccharide-Induced Inflammation. J. Immunol. 2006, 177, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.G.; Peiffer, D.S.; Larson, M.; Navarro, F.; Watkins, S.K. FOXO3, Estrogen Receptor Alpha, and Androgen Receptor Impact Tumor Growth Rate and Infiltration of Dendritic Cell Subsets Differentially between Male and Female Mice. Cancer Immunol. Immunother. 2017, 66, 615–625. [Google Scholar] [CrossRef]
- MacRosty, C.R.; Rivera, M.P. Lung Cancer in Women: A Modern Epidemic. Clin. Chest Med. 2020, 41, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Kellar, A.; Egan, C.; Morris, D. Preclinical Murine Models for Lung Cancer: Clinical Trial Applications. Available online: https://www.hindawi.com/journals/bmri/2015/621324/ (accessed on 27 June 2019).
- Terracciano, R.; Ricchetti, S.; Butler, E.B.; Demarchi, D.; Grattoni, A.; Filgueira, C.S. Abstract PO-058: Intratumoral Distribution and Retention of Gold Nanoparticles Characterized by Computed Tomography in a Non-Small Cell Lung Cancer Model. Cancer Res 2020, 80, PO-PO-058. [Google Scholar] [CrossRef]
- Terracciano, R.; Butler, B.E.; Demarchi, D.; Grattoni, A.; Filgueira, C.S. Abstract 2800: The Effect of Surface Protein Adsorption on Gold Nanoparticle-Intratumoral Distribution and Retention in a Pre-Clinical Model of Non-Small Cell Lung Cancer. Cancer Res. 2021, 81, 2800. [Google Scholar] [CrossRef]
- Levin, C.S.; Kundu, J.; Barhoumi, A.; Halas, N.J. Nanoshell-Based Substrates for Surface Enhanced Spectroscopic Detection of Biomolecules. Analyst 2009, 134, 1745–1750. [Google Scholar] [CrossRef] [PubMed]
- Brinson, B.E.; Lassiter, J.B.; Levin, C.S.; Bardhan, R.; Mirin, N.; Halas, N.J. Nanoshells Made Easy: Improving Au Layer Growth on Nanoparticle Surfaces. Langmuir 2008, 24, 14166–14171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lal, S.; Grady, N.K.; Kundu, J.; Levin, C.S.; Lassiter, J.B.; Halas, N.J. Tailoring Plasmonic Substrates for Surface Enhanced Spectroscopies. Chem. Soc. Rev. 2008, 37, 898–911. [Google Scholar] [CrossRef] [PubMed]
- Levin, C.S.; Kundu, J.; Janesko, B.G.; Scuseria, G.E.; Raphael, R.M.; Halas, N.J. Interactions of Ibuprofen with Hybrid Lipid Bilayers Probed by Complementary Surface-Enhanced Vibrational Spectroscopies. J. Phys. Chem. B 2008, 112, 14168–14175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Levin, C.S.; Halas, N.J. Nanosphere Arrays with Controlled Sub-10-Nm Gaps as Surface-Enhanced Raman Spectroscopy Substrates. J. Am. Chem. Soc. 2005, 127, 14992–14993. [Google Scholar] [CrossRef]
- Souza, G.R.; Levin, C.S.; Hajitou, A.; Pasqualini, R.; Arap, W.; Miller, J.H. In Vivo Detection of Gold−Imidazole Self-Assembly Complexes: NIR-SERS Signal Reporters. Anal. Chem. 2006, 78, 6232–6237. [Google Scholar] [CrossRef]
- Dissociation Constants of Organic Acids and Bases. Available online: http://www.zirchrom.com/organic.htm (accessed on 24 September 2021).
- Zheng, T.; Bott, S.; Huo, Q. Techniques for Accurate Sizing of Gold Nanoparticles Using Dynamic Light Scattering with Particular Application to Chemical and Biological Sensing Based on Aggregate Formation. ACS Appl. Mater. Interfaces 2016, 8, 21585–21594. [Google Scholar] [CrossRef]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef]
- Karakocak, B.B.; Liang, J.; Biswas, P.; Ravi, N. Hyaluronate Coating Enhances the Delivery and Biocompatibility of Gold Nanoparticles. Carbohydr. Polym. 2018, 186, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, S.; Allen, C.; Pintilie, M.; Jaffray, D.A. Tumor Perfusion Imaging Predicts the Intra-Tumoral Accumulation of Liposomes. J. Control. Release 2013, 172, 351–357. [Google Scholar] [CrossRef]
- Stapleton, S.; Milosevic, M.; Tannock, I.F.; Allen, C.; Jaffray, D.A. The Intra-Tumoral Relationship between Microcirculation, Interstitial Fluid Pressure and Liposome Accumulation. J. Control. Release 2015, 211, 163–170. [Google Scholar] [CrossRef]
- Wang, W.; Li, D.; Zhang, Y.; Zhang, W.; Ma, P.; Wang, X.; Song, D.; Sun, Y. One-Pot Synthesis of Hyaluronic Acid–Coated Gold Nanoparticles as SERS Substrate for the Determination of Hyaluronidase Activity. Microchim. Acta 2020, 187, 604. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Cai, R.; Yang, L.; Zhang, L.; Jiang, Y.; Yang, Y.; Cui, C.; Wan, S.; Chu, X.; Tan, W. Core–Shell HA-AuNPs@SiNPs Nanoprobe for Sensitive Fluorescence Hyaluronidase Detection and Cell Imaging. ACS Sustain. Chem. Eng. 2018, 6, 16555–16562. [Google Scholar] [CrossRef]
- Kim, K.; Choi, H.; Choi, E.S.; Park, M.-H.; Ryu, J.-H. Hyaluronic Acid-Coated Nanomedicine for Targeted Cancer Therapy. Pharmaceutics 2019, 11, 301. [Google Scholar] [CrossRef] [Green Version]
- Kumar, C.S.; Raja, M.D.; Sundar, D.S.; Gover Antoniraj, M.; Ruckmani, K. Hyaluronic Acid Co-Functionalized Gold Nanoparticle Complex for the Targeted Delivery of Metformin in the Treatment of Liver Cancer (HepG2 Cells). Carbohydr. Polym. 2015, 128, 63–74. [Google Scholar] [CrossRef]
- Xu, W.; Qian, J.; Hou, G.; Wang, Y.; Wang, J.; Sun, T.; Ji, L.; Suo, A.; Yao, Y. A Dual-Targeted Hyaluronic Acid-Gold Nanorod Platform with Triple-Stimuli Responsiveness for Photodynamic/Photothermal Therapy of Breast Cancer. Acta Biomater. 2019, 83, 400–413. [Google Scholar] [CrossRef]
- Melero, I.; Castanon, E.; Alvarez, M.; Champiat, S.; Marabelle, A. Intratumoural Administration and Tumour Tissue Targeting of Cancer Immunotherapies. Nat. Rev. Clin. Oncol. 2021, 18, 558–576. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.; Shim, W.Y.; Kim, J.H. Metabolic Engineering of Pichia Pastoris for Production of Hyaluronic Acid with High Molecular Weight. J. Biotechnol. 2014, 185, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Kuehl, C.; Zhang, T.; Kaminskas, L.M.; Porter, C.J.H.; Davies, N.M.; Forrest, L.; Berkland, C. Hyaluronic Acid Molecular Weight Determines Lung Clearance and Biodistribution after Instillation. Mol. Pharm. 2016, 13, 1904–1914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guelfi, G.; Stefanetti, V.; Zampini, D.; Oommen, O.P.; Brecchia, G.; Dall’Aglio, C.; Arcelli, R.; Cochetti, G.; Boni, A.; Mearini, E. Gold Nanoparticles Approach to Detect Chondroitin Sulphate and Hyaluronic Acid Urothelial Coating. Sci. Rep. 2017, 7, 10355. [Google Scholar] [CrossRef]
- Shen, H.; Shi, S.; Zhang, Z.; Gong, T.; Sun, X. Coating Solid Lipid Nanoparticles with Hyaluronic Acid Enhances Antitumor Activity against Melanoma Stem-like Cells. Theranostics 2015, 5, 755–771. [Google Scholar] [CrossRef]
- Chiesa, E.; Riva, F.; Dorati, R.; Greco, A.; Ricci, S.; Pisani, S.; Patrini, M.; Modena, T.; Conti, B.; Genta, I. On-Chip Synthesis of Hyaluronic Acid-Based Nanoparticles for Selective Inhibition of CD44+ Human Mesenchymal Stem Cell Proliferation. Pharmaceutics 2020, 12, 260. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Zeng, J.; Luo, L.; Yang, J.; Chen, J.; Li, B.; Shen, K. Identification of a Cancer Stem Cell-like Side Population in the HeLa Human Cervical Carcinoma Cell Line. Oncol. Lett. 2013, 6, 1673–1680. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terracciano, R.; Carcamo-Bahena, Y.; Butler, E.B.; Demarchi, D.; Grattoni, A.; Filgueira, C.S. Hyaluronate-Thiol Passivation Enhances Gold Nanoparticle Peritumoral Distribution When Administered Intratumorally in Lung Cancer. Biomedicines 2021, 9, 1561. https://doi.org/10.3390/biomedicines9111561
Terracciano R, Carcamo-Bahena Y, Butler EB, Demarchi D, Grattoni A, Filgueira CS. Hyaluronate-Thiol Passivation Enhances Gold Nanoparticle Peritumoral Distribution When Administered Intratumorally in Lung Cancer. Biomedicines. 2021; 9(11):1561. https://doi.org/10.3390/biomedicines9111561
Chicago/Turabian StyleTerracciano, Rossana, Yareli Carcamo-Bahena, E. Brian Butler, Danilo Demarchi, Alessandro Grattoni, and Carly S. Filgueira. 2021. "Hyaluronate-Thiol Passivation Enhances Gold Nanoparticle Peritumoral Distribution When Administered Intratumorally in Lung Cancer" Biomedicines 9, no. 11: 1561. https://doi.org/10.3390/biomedicines9111561
APA StyleTerracciano, R., Carcamo-Bahena, Y., Butler, E. B., Demarchi, D., Grattoni, A., & Filgueira, C. S. (2021). Hyaluronate-Thiol Passivation Enhances Gold Nanoparticle Peritumoral Distribution When Administered Intratumorally in Lung Cancer. Biomedicines, 9(11), 1561. https://doi.org/10.3390/biomedicines9111561