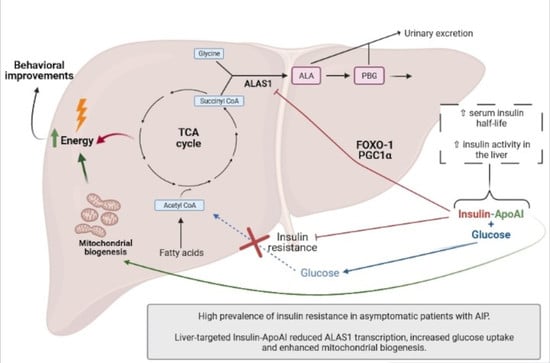
High Prevalence of Insulin Resistance in Asymptomatic Patients with Acute Intermittent Porphyria and Liver-Targeted Insulin as a Novel Therapeutic Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Participants and Study Design
2.3. Experimental Studies in a Murine Model of AIP
2.4. Statistics
3. Results
3.1. High Prevalence of a Pathological HOMA-IR Index in Patients with AIP
3.2. Ins-ApoAI Induced a Fast and Sustained Normalization in the Gene Transcription Involved in the Liver Regulation of Heme Synthesis, Gluconeogenesis, and Bile Acid Synthesis in Fasted WT and AIP Mice
3.3. The Relative Contribution of Insulin to Protect against alas1 Induction Modulated by Barbiturate Challenge
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elder, G.; Harper, P.; Badminton, M.; Sandberg, S.; Deybach, J.-C. The incidence of inherited porphyrias in Europe. J. Inherit. Metab. Dis. 2013, 36, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Puy, H.; Gouya, L.; Deybach, J.C. Porphyrias. Lancet 2010, 375, 924–937. [Google Scholar] [CrossRef]
- Anderson, K.; Sassa, S.; Bishop, D.; Desnick, R. Disorders of Heme Biosynthesis: X-Linked Sideroblastic Anemia and the Porphyrias. In The Metabolic and Molecular Bases of Inherited Disease, 8th ed.; Scriver, C.R.B.A., Sly, W.S., Valle, E., Eds.; McGraw Hill: New York, NY, USA, 2001; Volume 1, pp. 2991–3062. [Google Scholar]
- Chen, B.; Solis-Villa, C.; Hakenberg, J.; Qiao, W.; Srinivasan, R.R.; Yasuda, M.; Balwani, M.; Doheny, D.; Peter, I.; Chen, R.; et al. Acute Intermittent Porphyria: Predicted Pathogenicity of HMBS Variants Indicates Extremely Low Penetrance of the Autosomal Dominant Disease. Hum. Mutat. 2016, 37, 1215–1222. [Google Scholar] [CrossRef] [Green Version]
- Jaramillo-Calle, D.A.; Solano, J.M.; Rabinstein, A.A.; Bonkovsky, H.L. Porphyria-induced posterior reversible encephalopathy syndrome and central nervous system dysfunction. Mol. Genet. Metab. 2019, 128, 242–253. [Google Scholar] [CrossRef]
- Anderson, K.E.; Bloomer, J.R.; Bonkovsky, H.L.; Kushner, J.P.; Pierach, C.A.; Pimstone, N.R.; Desnick, R.J. Recommendations for the Diagnosis and Treatment of the Acute Porphyrias. Ann. Intern. Med. 2005, 142, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Stein, P.; Badminton, M.; Barth, J.; Rees, D.; Stewart, M.F. Best practice guidelines on clinical management of acute attacks of porphyria and their complications. Ann. Clin. Biochem. 2013, 50, 217–223. [Google Scholar] [CrossRef] [Green Version]
- Robert, T.L.; Varella, L.; Meguid, M.M. Nutrition management of acute intermittent porphyria. Nutrition 1994, 10, 551. [Google Scholar] [PubMed]
- Podvinec, M.; Handschin, C.; Looser, R.; Meyer, U.A. Identification of the xenosensors regulating human 5-aminolevulinate synthase. Proc. Natl. Acad. Sci. USA 2004, 101, 9127–9132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Handschin, C.; Lin, J.; Rhee, J.; Peyer, A.K.; Chin, S.; Wu, P.H.; Meyer, U.A.; Spiegelman, B.M. Nutritional regulation of hepatic heme biosynthesis and porphyria through PGC-1α. Cell 2005, 122, 505–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degenhardt, T.; Väisänen, S.; Rakhshandehroo, M.; Kersten, S.; Carlberg, C. Peroxisome proliferator-activated receptor alpha controls hepatic heme biosynthesis through ALAS1. J. Mol. Biol. 2009, 388, 225–238. [Google Scholar] [CrossRef]
- Duvigneau, J.C.; Esterbauer, H.; Kozlov, A.V. Role of Heme Oxygenase as a Modulator of Heme-Mediated Pathways. Antioxidants 2019, 8, 475. [Google Scholar] [CrossRef] [Green Version]
- Tian, Q.; Li, T.; Hou, W.; Zheng, J.; Schrum, L.W.; Bonkovsky, H.L. Lon peptidase 1 (LONP1)-dependent breakdown of mitochondrial 5-aminolevulinic acid synthase protein by heme in human liver cells. J. Biol. Chem. 2011, 286, 26424–26430. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, C.; Lenglet, H.; Yu, A.; Delaby, C.; Benecke, A.; Lefebvre, T.; Letteron, P.; Paradis, V.; Wahlin, S.; Sandberg, S.; et al. Recurrent attacks of acute hepatic porphyria: Major role of the chronic inflammatory response in the liver. J. Intern. Med. 2018, 284, 78–91. [Google Scholar] [CrossRef] [Green Version]
- Storjord, E.; Dahl, J.A.; Landsem, A.; Ludviksen, J.K.; Karlsen, M.B.; Karlsen, B.O.; Brekke, O.-L. Lifestyle factors including diet and biochemical biomarkers in acute intermittent porphyria: Results from a case-control study in northern Norway. Mol. Genet. Metab. 2019, 128, 254–270. [Google Scholar] [CrossRef] [PubMed]
- Balwani, M.; Wang, B.; Anderson, K.E.; Bloomer, J.R.; Bissell, D.M.; Bonkovsky, H.L.; Phillips, J.D.; Desnick, R.J.; Porphyrias Consortium of the Rare Diseases Clinical Research Network. Acute hepatic porphyrias: Recommendations for evaluation and long-term management. Hepatology 2017, 66, 1314–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sixel-Dietrich, F.; Verspohl, F.; Doss, M. Hyperinsulinemia in Acute Intermittent Porphyria. Horm. Metab. Res. 1985, 17, 375–376. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.A.; Tschudy, D.P. Acute Iintermittent Porphyria: A Clinical and Biochemical Study of 46 Patients. Medicine 1970, 49, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Collantes, M.; Serrano-Mendioroz, I.; Benito, M.; Molinet-Dronda, F.; Delgado, M.; Vinaixa, M.; Sampedro, A.; de Salamanca, R.E.; Prieto, E.; Pozo, M.A.; et al. Glucose metabolism during fasting is altered in experimental porphobilinogen deaminase deficiency. Hum. Mol. Genet. 2016, 25, 1318–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ardaiz, N.; Gomar, C.; Vasquez, M.; Tenesaca, S.; Fernandez-Sendin, M.; Di Trani, C.; Belsué, V.; Escalada, J.; Werner, U.; Tennagels, N.; et al. Insulin fused to Apolipoprotein AI reduces body weigth and steatosis in db/db mice. Front. Pharmacol. 2021. [Google Scholar] [CrossRef]
- Rajpal, G.; Liu, M.; Zhang, Y.; Arvan, P. Single-Chain Insulins as Receptor Agonists. Mol. Endocrinol. 2009, 23, 679–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Einhorn, D.; Reaven, G.M.; Cobin, R.H.; Ford, E.; Ganda, O.P.; Handelsman, Y.; Hellman, R.; Jellinger, P.S.; Kendall, D.; Krauss, R.M.; et al. American College of Endocrinology position statement on the insulin resistance syndrome. Endocr. Pr. 2003, 9, 237–252. [Google Scholar]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef]
- Hamburg, N.M.; McMackin, C.J.; Huang, A.L.; Shenouda, S.M.; Widlansky, M.E.; Schulz, E.; Gokce, N.; Ruderman, N.B.; Keaney, J.F., Jr.; Vita, J.A. Physical inactivity rapidly induces insulin resistance and microvascular dysfunction in healthy volunteers. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2650–2656. [Google Scholar] [CrossRef] [Green Version]
- Dunaif, A.; Segal, K.R.; Shelley, D.R.; Green, G.; Dobrjansky, A.; Licholai, T. Evidence for Distinctive and Intrinsic Defects in Insulin Action in Polycystic Ovary Syndrome. Diabetes 1992, 41, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Geer, E.B.; Islam, J.; Buettner, C. Mechanisms of glucocorticoid-induced insulin resistance: Focus on adipose tissue function and lipid metabolism. Endocrinol. Metab. Clin. N. Am. 2014, 43, 75–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadigan, C.; Kattakuzhy, S. Diabetes mellitus type 2 and abnormal glucose metabolism in the setting of human immunodeficiency virus. Endocrinol. Metab. Clin. N. Am. 2014, 43, 685–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semple, R.K.; Savage, D.B.; Cochran, E.K.; Gorden, P.; O’Rahilly, S. Genetic syndromes of severe insulin resistance. Endocr. Rev. 2011, 32, 498–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Gayoso-Diz, P.; Otero-González, A.; Rodriguez-Alvarez, M.X.; Gude, F.; García, F.; De Francisco, A.; Quintela, A.G. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: Effect of gender and age: EPIRCE cross-sectional study. BMC Endocr. Disord. 2013, 13, 47. [Google Scholar] [CrossRef] [Green Version]
- Lindberg, R.L.P.; Porcher, C.; Grandchamp, B.; Ledermann, B.; Bürki, K.; Brandner, S.; Aguzzi, A.; Meyer, U.A. Porphobilinogen deaminase deficiency in mice causes a neuropathy resembling that of human hepatic porphyria. Nat. Genet. 1996, 12, 195. [Google Scholar] [CrossRef]
- Jiang, L.; Berraondo, P.; Jericó, D.; Guey, L.T.; Sampedro, A.; Frassetto, A.; Benenato, K.E.; Burke, K.; Santamaría, E.; Alegre, M.; et al. Systemic messenger RNA as an etiological treatment for acute intermittent porphyria. Nat. Med. 2018, 1. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Ruifrok, A.; Johnston, D. Ruifrok AC, Johnston DA. Quantification of histochemical staining by color deconvolution. Anal. Quant. Cytol. Histol. 2001, 23, 291–299. [Google Scholar] [PubMed]
- Moreno-Aliaga, M.J.; Pérez-Echarri, N.; Marcos-Gómez, B.; Larequi, E.; Gil-Bea, F.J.; Viollet, B.; Gimenez, I.; Martínez, J.A.; Prieto, J.; Bustos, M. Cardiotrophin-1 Is a Key Regulator of Glucose and Lipid Metabolism. Cell Metab. 2011, 14, 242–253. [Google Scholar] [CrossRef] [Green Version]
- Prieto-Hontoria, P.L.; Pérez-Matute, P.; Fernández-Galilea, M.; Martínez, J.A.; Moreno-Aliaga, M.J. Lipoic acid inhibits leptin secretion and Sp1 activity in adipocytes. Mol. Nutr. Food Res. 2011, 55, 1059–1069. [Google Scholar] [CrossRef]
- Obesity: Preventing and Managing the Global Epidemic; World Health Organ Techical Report Series. 2000, pp. 1–253. Available online: https://www.who.int/nutrition/publications/obesity/WHO_TRS_894/en/ (accessed on 4 March 2021).
- Shin, D.-J.; Campos-Sandoval, J.; Gil, G.; Osborne, T. PGC-1 activates CYP7A1 and bile acid biosynthesis. J. Biol. Chem. 2004, 278, 50047–50052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, M.S.; Goldstein, J.L. Selective versus Total Insulin Resistance: A Pathogenic Paradox. Cell Metab. 2008, 7, 95–96. [Google Scholar] [CrossRef] [Green Version]
- Bódis, K.; Roden, M. Energy metabolism of white adipose tissue and insulin resistance in humans. Eur. J. Clin. Investig. 2018, 48, e13017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterling, K.; Silver, M.; Ricketts, H.T. Development of pophyria in Diabetes Mellitus: Report of Three Cases. Arch. Intern. Med. 1949, 84, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Andersson, C.; Bylesjö, I.; Lithner, F. Effects of diabetes mellitus on patients with acute intermittent porphyria. J. Intern. Med. 1999, 245, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Matkovic, L.B.; D’Andrea, F.; Fornes, D.; San Martín de Viale, L.C.; Mazzetti, M.B. How porphyrinogenic drugs modeling acute porphyria impair the hormonal status that regulates glucose metabolism. Their relevance in the onset of this disease. Toxicology 2011, 290, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Lelli, S.M.; De Viale, L.C.S.M.; Mazzetti, M.B. Response of glucose metabolism enzymes in an acute porphyria model: Role of reactive oxygen species. Toxicology 2005, 216, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, L.M.; Davio, C.; Batlle, A.M.d.C.; Gerez, E.N. ALAS1 gene expression is down-regulated by Akt-mediated phosphorylation and nuclear exclusion of FOXO1 by vanadate in diabetic mice. Biochem. J. 2012, 442, 303–310. [Google Scholar] [CrossRef] [Green Version]
- Song, P.; Kwon, Y.; Yea, K.; Moon, H.-Y.; Yoon, J.H.; Ghim, J.; Hyun, H.; Kim, D.; Koh, A.; Berggren, P.-O.; et al. Apolipoprotein a1 increases mitochondrial biogenesis through AMP-activated protein kinase. Cell. Signal. 2015, 27, 1873–1881. [Google Scholar] [CrossRef]
- Zhang, W.; Patil, S.; Chauhan, B.; Guo, S.; Powell, D.R.; Le, J.; Klotsas, A.; Matika, R.; Xiao, X.; Franks, R.; et al. FoxO1 Regulates Multiple Metabolic Pathways in the Liver: Effects on gluconeogenis, glycolytic, and lipogenic gene expression. J. Biol. Chem. 2006, 281, 10105–10117. [Google Scholar] [CrossRef] [Green Version]
- Honma, M.; Sawada, S.; Ueno, Y.; Murakami, K.; Yamada, T.; Gao, J.; Kodama, S.; Izumi, T.; Takahashi, K.; Tsukita, S.; et al. Selective insulin resistance with differential expressions of IRS-1 and IRS-2 in human NAFLD livers. Int. J. Obes. 2018, 42, 1544–1555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Homedan, C.; Laafi, J.; Schmitt, C.; Gueguen, N.; Lefebvre, T.; Karim, Z.; Desquiret-Dumas, V.; Wetterwald, C.; Deybach, J.C.; Gouya, L.; et al. Acute intermittent porphyria causes hepatic mitochondrial energetic failure in a mouse model. Int. J. Biochem. Cell Biol. 2014, 51, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, M.; Gan, L.; Chen, B.; Kadirvel, S.; Yu, C.; Phillips, J.D.; New, M.I.; Liebow, A.; Fitzgerald, K.; Querbes, W.; et al. RNAi-mediated silencing of hepatic Alas1 effectively prevents and treats the induced acute attacks in acute intermittent porphyria mice. Proc. Natl. Acad. Sci. USA 2014, 111, 7777–7782. [Google Scholar] [CrossRef] [Green Version]
- Unzu, C.; Sampedro, A.; Mauleón, I.; Alegre, M.; Beattie, S.G.; De Salamanca, R.E.; Snapper, J.; Twisk, J.; Petry, H.; González-Aseguinolaza, G.; et al. Sustained enzymatic correction by rAAV-mediated liver gene therapy protects against induced motor neuropathy in acute porphyria mice. Mol. Ther. 2011, 19, 243–250. [Google Scholar] [CrossRef]
- Ruan, X.; Li, Z.; Zhang, Y.; Yang, L.; Pan, Y.; Wang, Z.; Feng, G.-S.; Chen, Y. Apolipoprotein A-I possesses an anti-obesity effect associated with increase of energy expenditure and up-regulation of UCP1 in brown fat. J. Cell. Mol. Med. 2011, 15, 763–772. [Google Scholar] [CrossRef] [Green Version]





| CV (n=55) | AIP (n=44) | AIP-SD (n=18) | AIP-ASHE (n=18) | AIP-AD (n=8) | p. CV vs AIP | |
|---|---|---|---|---|---|---|
| Woman (n) (%) | 43 78.2% | 36 81.8% | 13 72.2% | 15 83.3% | 8 100% | 0.8 |
| Age (y) (range) | 39.2 ± 12.9 17–68 | 42 ± 14.1 17–68 | 44.3 ± 16.3 17–68 | 44.5 ± 11.2 22–63 | 33 ± 10.9 17–46 | 0.15 |
| Body weight (kg) (range) | 65.3 ± 11.7 40–92 | 63.6 ± 16.3 30–98 | 65.7 ± 17.3 43–97 | 66.8 ± 16.1 45–98 | 51.9 ± 9.6 30–59 | 0.58 |
| HOMA index (range) | 1.64 ± 0.74 0.56–3.53 | 2.22 ± 1.23 0.68–6.07 | 2.47 ± 1.56 0.88–6.07 | 2.16 ± 0.92 0.8–4.1 | 1.80 ± 0.82 0.68–2.93 | 0.008 |
| Serum glucose (mg/dl) (range) | 89.2 ± 8.57 68.0–111.5 | 93.7 ± 9.40 71.0–125.0 | 95.7 ± 9.95 71.0–113.0 | 92.8 ± 10.04 81.0–125.0 | 91.3 ± 6.16 83.0–99.0 | 0.015 |
| Serum insulin (µU/dl) (range) | 7.55 ± 3.23 2.63–14.9 | 9.48 ± 5.13 1.8–26.5 | 10.4 ± 7.03 1.8–26.5 | 9.27 ± 3.23 3.7–13.9 | 7.9 ± 3.38 3–11.5 | 0.032 |
| Metabolic Sd. (nº) (%) | 1/55 1.82% | 3/44 6.82% | 0/18 0% | 1/18 5.6% | 2/8 25% | 0.333 |
| Sedentary lifestyle (nº) (%) | 22 of 49 * 44.89% | 9/44 20.45% | 3/18 16.7% | 4/18 22.2% | 2/8 25% | 0.012 |
| BMI (kg/m2) (range) | 22.4 ± 3.87 16.7–32.98 | 24.2 ± 5.88 11.9–36.4 | 24.9 ± 5.70 16.2–36.4 | 25.8 ± 5.99 16.7–36.3 | 19.14 ± 3.27 11.9–22.5 | 0.99 |
| ALA (μg/mg creat.) (range) | 4.97 ± 0.24 3.2–5.0 | 9.54 ± 10 0.98–57 | 4.48 ± 1.17 1.3–5.0 | 9.63 ± 6.60 0.98–25 | 20.7 ± 17.3 3.3–57 | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solares, I.; Izquierdo-Sánchez, L.; Morales-Conejo, M.; Jericó, D.; Castelbón, F.J.; Córdoba, K.M.; Sampedro, A.; Lumbreras, C.; Moreno-Aliaga, M.J.; Enríquez de Salamanca, R.; et al. High Prevalence of Insulin Resistance in Asymptomatic Patients with Acute Intermittent Porphyria and Liver-Targeted Insulin as a Novel Therapeutic Approach. Biomedicines 2021, 9, 255. https://doi.org/10.3390/biomedicines9030255
Solares I, Izquierdo-Sánchez L, Morales-Conejo M, Jericó D, Castelbón FJ, Córdoba KM, Sampedro A, Lumbreras C, Moreno-Aliaga MJ, Enríquez de Salamanca R, et al. High Prevalence of Insulin Resistance in Asymptomatic Patients with Acute Intermittent Porphyria and Liver-Targeted Insulin as a Novel Therapeutic Approach. Biomedicines. 2021; 9(3):255. https://doi.org/10.3390/biomedicines9030255
Chicago/Turabian StyleSolares, Isabel, Laura Izquierdo-Sánchez, Montserrat Morales-Conejo, Daniel Jericó, Francisco Javier Castelbón, Karol Marcela Córdoba, Ana Sampedro, Carlos Lumbreras, María Jesús Moreno-Aliaga, Rafael Enríquez de Salamanca, and et al. 2021. "High Prevalence of Insulin Resistance in Asymptomatic Patients with Acute Intermittent Porphyria and Liver-Targeted Insulin as a Novel Therapeutic Approach" Biomedicines 9, no. 3: 255. https://doi.org/10.3390/biomedicines9030255
APA StyleSolares, I., Izquierdo-Sánchez, L., Morales-Conejo, M., Jericó, D., Castelbón, F. J., Córdoba, K. M., Sampedro, A., Lumbreras, C., Moreno-Aliaga, M. J., Enríquez de Salamanca, R., Berraondo, P., & Fontanellas, A. (2021). High Prevalence of Insulin Resistance in Asymptomatic Patients with Acute Intermittent Porphyria and Liver-Targeted Insulin as a Novel Therapeutic Approach. Biomedicines, 9(3), 255. https://doi.org/10.3390/biomedicines9030255