Transdermal Delivery of Lidocaine-Loaded Elastic Nano-Liposomes with Microneedle Array Pretreatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. LidH SLNs and ENLs Preparation
2.4. Determination of Entrapment Efficiency (EE)
2.5. Formulation Characterization
2.6. Cytotoxicity Test
2.7. Skin Insertion of MN
2.8. Transdermal Diffusion of LidH from ENLs In Vitro
2.9. In Vivo Thermal Hyperalgesia Study
2.10. Statistical Analysis
3. Results
3.1. Preparation and Optimization of LidH-Loaded ENLs
3.2. MN Pretreatment of the Skin
3.3. Transdermal Delivery of LidH from ENLs on MN-Pretreated Skin In Vitro
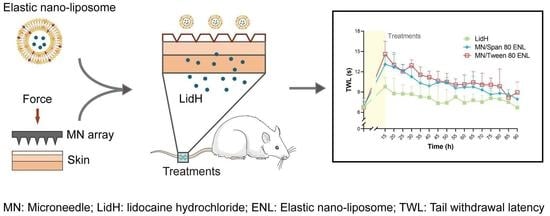
3.4. Pharmacodynamic Evaluation of LidH-Loaded ENLs In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviation
| CCK-8 | Cell Counting Kit-8 |
| DMEM | Dulbecco’s modified Eagle’s medium |
| EE | Encapsulation efficiency |
| ENL | Elastic nano-liposome |
| HLB | hydrophile-lipophile balance |
| LidH | Lidocaine hydrochloride |
| MN | Microneedle |
| PDI | Polydispersity index |
| PBS | Phosphate-buffered saline |
| SD | Standard deviation |
| SLN | Solid lipid nanoparticle |
| Span 80 | Sorbitan monooleate |
| SPC | Soybean phosphatidylcholine |
| Tween 80 | Polyoxyethylene sorbitan monooleate |
| TWL | Tail withdrawal latency |
References
- Oni, G.; Brown, S.A.; Kenkel, J.M. Can fractional lasers enhance transdermal absorption of topical lidocaine in an in vivo animal model? Lasers Surg. Med. 2012, 44, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Hermanns, H.; Hollmann, M.W.; Stevens, M.F.; Lirk, P.; Brandenburger, T.; Piegeler, T.; Werdehausen, R. Molecular mechanisms of action of systemic lidocaine in acute and chronic pain: A narrative review. Br. J. Anaesth. 2019, 123, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R. Engineering Microneedle Patches for Vaccination and Drug Delivery to Skin. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 177–200. [Google Scholar] [CrossRef] [PubMed]
- Hanaoka, K.; Arita, H.; Nagase, M.; Suzuki, T.; Ogawa, S. Transdermal Local Anesthetics. Masui 2015, 64, 1151–1159. [Google Scholar]
- Hellgren, U.; Kihamia, C.M.; Premji, Z.; Danielson, K. Local anaesthetic cream for the alleviation of pain during venepuncture in Tanzanian schoolchildren. Br. J. Clin. Pharmacol. 1989, 28, 205–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, J.R.; Kehl, L.J.; Beiraghi, S. Enhanced topical anesthesia of 4% lidocaine with microneedle pretreatment and iontophoresis. Northwest Dent. 2008, 87, 40–41. [Google Scholar]
- Nayak, A.; Babla, H.; Han, T.; Das, D.B. Lidocaine carboxymethylcellulose with gelatine co-polymer hydrogel delivery by combined microneedle and ultrasound. Drug Deliv. 2014, 23, 658–669. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.; Patel, N.; Shah, M.K.; Khatri, P.; Vora, N. Recent Advances in Lipid-Based Vesicles and Particulate Carriers for Topical and Transdermal Application. J. Pharm. Sci. 2017, 106, 423–445. [Google Scholar] [CrossRef]
- Cevc, G. Rational design of new product candidates: The next generation of highly deformable bilayer vesicles for noninvasive, targeted therapy. J. Control. Release 2012, 160, 135–146. [Google Scholar] [CrossRef]
- El Maghraby, G.; Williams, A.C.; Barry, B.W. Oestradiol skin delivery from ultradeformable liposomes: Refinement of surfactant concentration. Int. J. Pharm. 2000, 196, 63–74. [Google Scholar] [CrossRef]
- Knudsen, N.Ø.; Jorgensen, L.; Hansen, J.; Vermehren, C.; Frokjaer, S.; Foged, C. Targeting of liposome-associated calcipotriol to the skin: Effect of liposomal membrane fluidity and skin barrier integrity. Int. J. Pharm. 2011, 416, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Caddeo, C.; Valenti, D.; Nácher, A.; Manconi, M.; Fadda, A.M. Exploring the co-loading of lidocaine chemical forms in surfactant/phospholipid vesicles for improved skin delivery. J. Pharm. Pharmacol. 2015, 67, 909–917. [Google Scholar] [CrossRef]
- Prausnitz, M.R. Microneedles for transdermal drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Henry, S.; Allen, M.G.; Prausnitz, M.R. Microfabricated Microneedles: A Novel Approach to Transdermal Drug Delivery. J. Pharm. Sci. 1998, 87, 922–925. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Singh, R.R.T.; Woolfson, A.D. Microneedle-based drug delivery systems: Microfabrication, drug delivery, and safety. Drug Deliv. 2010, 17, 187–207. [Google Scholar] [CrossRef] [Green Version]
- Gill, H.S.; Denson, D.D.; Burris, B.A.; Prausnitz, M.R. Effect of Microneedle Design on Pain in Human Volunteers. Clin. J. Pain 2008, 24, 585–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Chen, G.; Bian, F.; Cai, L.; Zhao, Y. Encoded Microneedle Arrays for Detection of Skin Interstitial Fluid Biomarkers. Adv. Mater. 2019, 31, e1902825. [Google Scholar] [CrossRef]
- Ye, Y.; Yu, J.; Wen, D.; Kahkoska, A.R.; Gu, Z. Polymeric microneedles for transdermal protein delivery. Adv. Drug Deliv. Rev. 2018, 127, 106–118. [Google Scholar] [CrossRef]
- Yang, J.; Liu, X.; Fu, Y.; Song, Y. Recent advances of microneedles for biomedical applications: Drug delivery and beyond. Acta Pharm. Sin. B 2019, 9, 469–483. [Google Scholar] [CrossRef]
- Burton, S.A.; Ng, C.-Y.; Simmers, R.; Moeckly, C.; Brandwein, D.; Gilbert, T.; Johnson, N.; Brown, K.; Alston, T.; Prochnow, G.; et al. Rapid Intradermal Delivery of Liquid Formulations Using a Hollow Microstructured Array. Pharm. Res. 2011, 28, 31–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Maaden, K.; Jiskoot, W.; Bouwstra, J. Microneedle technologies for (trans)dermal drug and vaccine delivery. J. Control. Release 2012, 161, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Szoka, F.; Papahadjopoulos, D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc. Natl. Acad. Sci. USA 1978, 75, 4194–4198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Lu, W.-L.; Gu, W.; Lu, S.-S.; Chen, Z.-P.; Cai, B.-C. Skin permeation behavior of elastic liposomes: Role of formulation ingredients. Expert Opin. Drug Deliv. 2013, 10, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Pascual, C.; Lieu, C.; Oh, S.; Wang, J.; Zou, B.; Xie, J.; Li, Z.; Xie, J.; Yeomans, D.C.; et al. Analgesic Microneedle Patch for Neuropathic Pain Therapy. ACS Nano 2017, 11, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Gudin, J.; Nalamachu, S. Utility of lidocaine as a topical analgesic and improvements in patch delivery systems. Postgrad. Med. 2020, 132, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.; Gao, Y.; Hu, K.; Li, F. Enhancement of skin permeation of docetaxel: A novel approach combining microneedle and elastic liposomes. J. Control. Release 2008, 129, 144–150. [Google Scholar] [CrossRef]
- Abd-Elal, R.M.A.; Shamma, R.N.; Rashed, H.M.; Bendas, E.R. Trans-nasal zolmitriptan novasomes: In-vitro preparation, optimization and in-vivo evaluation of brain targeting efficiency. Drug Deliv. 2016, 23, 3374–3386. [Google Scholar] [CrossRef] [Green Version]
- Teaima, M.H.; El Mohamady, A.M.; El-Nabarawi, M.A.; Mohamed, A.I. Formulation and evaluation of niosomal vesicles containing ondansetron HCL for trans-mucosal nasal drug delivery. Drug Dev. Ind. Pharm. 2020, 46, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Maghraby, G.M.M.E.; Williams, A.C.; Barry, B.W. Skin hydration and possible shunt route penetration in controlled estradiol delivery from ultradeformable and standard liposomes. J. Pharm. Pharmacol. 2001, 53, 1311–1322. [Google Scholar] [CrossRef]
- Carafa, M.; Santucci, E.; Lucania, G. Lidocaine-loaded non-ionic surfactant vesicles: Characterization and in vitro permeation studies. Int. J. Pharm. 2002, 231, 21–32. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Wang, X.; Song, Y.; Wang, X.; Hao, J. Design and Development of Lidocaine Microemulsions for Transdermal Delivery. AAPS PharmSciTech 2019, 20, 63. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, D.; Ribeiro, L.; de Paula, E. Lipid-based carriers for the delivery of local anesthetics. Expert Opin. Drug Deliv. 2019, 16, 701–714. [Google Scholar] [CrossRef]
- Birchall, J.C.; Clemo, R.; Anstey, A.; John, D.N. Microneedles in Clinical Practice—An Exploratory Study into the Opinions of Healthcare Professionals and the Public. Pharm. Res. 2011, 28, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Duarah, S.; Sharma, M.; Wen, J. Recent advances in microneedle-based drug delivery: Special emphasis on its use in paediatric population. Eur. J. Pharm. Biopharm. 2019, 136, 48–69. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Kang, G.; Jang, M.; Um, D.J.; Shin, J.; Kim, H.; Hong, J.; Jung, H.; Ahn, H.; Gong, S.; et al. Development of Lidocaine-Loaded Dissolving Microneedle for Rapid and Efficient Local Anesthesia. Pharmaceutics 2020, 12, 1067. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Cheng, M.; Zhao, J.; Zhang, X.; Huang, Z.; Zang, Y.; Ding, Y.; Zhang, J.; Ding, Z. Transdermal Delivery of Lidocaine-Loaded Elastic Nano-Liposomes with Microneedle Array Pretreatment. Biomedicines 2021, 9, 592. https://doi.org/10.3390/biomedicines9060592
Liu Y, Cheng M, Zhao J, Zhang X, Huang Z, Zang Y, Ding Y, Zhang J, Ding Z. Transdermal Delivery of Lidocaine-Loaded Elastic Nano-Liposomes with Microneedle Array Pretreatment. Biomedicines. 2021; 9(6):592. https://doi.org/10.3390/biomedicines9060592
Chicago/Turabian StyleLiu, Yang, Maosen Cheng, Junqi Zhao, Xiaoying Zhang, Zhen Huang, Yuhui Zang, Ying Ding, Junfeng Zhang, and Zhi Ding. 2021. "Transdermal Delivery of Lidocaine-Loaded Elastic Nano-Liposomes with Microneedle Array Pretreatment" Biomedicines 9, no. 6: 592. https://doi.org/10.3390/biomedicines9060592
APA StyleLiu, Y., Cheng, M., Zhao, J., Zhang, X., Huang, Z., Zang, Y., Ding, Y., Zhang, J., & Ding, Z. (2021). Transdermal Delivery of Lidocaine-Loaded Elastic Nano-Liposomes with Microneedle Array Pretreatment. Biomedicines, 9(6), 592. https://doi.org/10.3390/biomedicines9060592