In Vitro Evaluation of the Anti-Inflammatory Effect of KMUP-1 and In Vivo Analysis of Its Therapeutic Potential in Osteoarthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Cell Culture and Lipopolysaccharide-Induced Inflammation
2.3. Cell Viability Assay
2.4. Measurement of Nitrite Oxide
2.5. Measurement of TNF-α, IL-6, MMP-2, and MMP-9
2.6. Western Blot Analysis
2.7. Microarray
2.8. Intracellular Reactive Oxygen Species (ROS) Measurement
2.9. Animals
2.10. OA Induction in Rats
2.11. Hindpaw Mechanical Hyperalgesia in Rats
2.12. Macroscopic and Histopathologic Observations of Cartilage
2.13. Serum Levels of TNF-α and IL-6
2.14. Statistical Analyses
3. Results
3.1. KMUP-1 Effects on LPS-Induced Cytotoxicity and NO Production

3.2. KMUP-1 Inhibits LPS-Induced Inflammatory Cytokines Production
3.3. KMUP-1 Prevents LPS-Induced Inflammatory Protein Expressions
3.4. KMUP-1 Downregulates TNF and COX Family Gene Expressions in LPS-Induced Cells
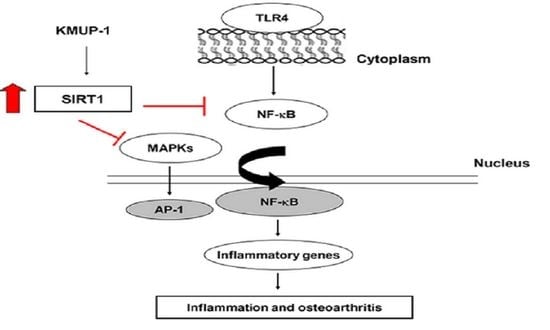
3.5. KMUP-1 Inhibits MAPK and NF-κB Signaling Pathways in LPS-Induced Cells
3.6. KMUP-1 Suppresses LPS-Induced ROS Production and Restores SIRT1 Level
3.7. KMUP-1 Alleviated Mechanical Hyperalgesia and Serum Inflammatory Cytokines Levels in MIA-Induced OA Rats
3.8. Protective Effects of KMUP-1 on Articular Cartilage Erosion in MIA-Induced OA Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldring, M.B.; Otero, M. Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.-P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Batt, M. An update on the pathophysiology of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 333–339. [Google Scholar] [CrossRef]
- Castrogiovanni, P.; Di Rosa, M.; Ravalli, S.; Castorina, A.; Guglielmino, C.; Imbesi, R.; Vecchio, M.; Drago, F.; Szychlinska, M.A.; Musumeci, G. Moderate physical activity as a prevention method for knee osteoarthritis and the role of synoviocytes as biological key. Int. J. Mol. Sci. 2019, 20, 511. [Google Scholar] [CrossRef] [Green Version]
- Szychlinska, M.A.; Castrogiovanni, P.; Nsir, H.; Di Rosa, M.; Guglielmino, C.; Parenti, R.; Calabrese, G.; Pricoco, E.; Salvatorelli, L.; Magro, G. Engineered cartilage regeneration from adipose tissue derived-mesenchymal stem cells: A morphomolecular study on osteoblast, chondrocyte and apoptosis evaluation. Exp. Cell Res. 2017, 357, 222–235. [Google Scholar] [CrossRef]
- Guarente, L. Sirtuins, aging, and medicine. N. Engl. J. Med. 2011, 364, 2235–2244. [Google Scholar] [CrossRef]
- Morris, B.J. Seven sirtuins for seven deadly diseases of aging. Free Radic. Biol. Med. 2013, 56, 133–171. [Google Scholar] [CrossRef]
- Dvir-Ginzberg, M.; Mobasheri, A.; Kumar, A. The role of sirtuins in cartilage homeostasis and osteoarthritis. Curr. Rheumatol. Rep. 2016, 18, 43. [Google Scholar] [CrossRef]
- Kauppinen, A.; Suuronen, T.; Ojala, J.; Kaarniranta, K.; Salminen, A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal. 2013, 25, 1939–1948. [Google Scholar] [CrossRef]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef] [Green Version]
- Wu, B.-N.; Chen, C.-W.; Liou, S.-F.; Yeh, J.-L.; Chung, H.-H.; Chen, J. Inhibition of proinflammatory tumor necrosis factor-α-induced inducible nitric-oxide synthase by xanthine-based 7-[2-[4-(2-chlorobenzene) piperazinyl] ethyl]-1, 3-dimethylxanthine (KMUP-1) and 7-[2-[4-(4-nitrobenzene) piperazinyl] ethyl]-1, 3-dimethylxanthine (KMUP-3) in rat trachea: The involvement of soluble guanylate cyclase and protein kinase G. Mol. Pharmacol. 2006, 70, 977–985. [Google Scholar]
- Yeh, J.L.; Hsu, J.H.; Wu, P.J.; Liou, S.F.; Liu, C.P.; Chen, I.J.; Wu, B.N.; Dai, Z.K.; Wu, J.R. KMUP-1 attenuates isoprenaline-induced cardiac hypertrophy in rats through NO/cGMP/PKG and ERK1/2/calcineurin A pathways. Br. J. Pharmacol. 2010, 159, 1151–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, Y.-Y.; Liu, C.-M.; Tsai, H.-H.; Jong, Y.-J.; Chen, J.; Lo, Y.-C. KMUP-1 attenuates serum deprivation-induced neurotoxicity in SH-SY5Y cells: Roles of PKG, PI3K/Akt and Bcl-2/Bax pathways. Toxicology 2010, 268, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Liou, S.-F.; Hsu, J.-H.; Lin, I.-L.; Ho, M.-L.; Hsu, P.-C.; Chen, L.-W.; Chen, J.; Yeh, J.-L. KMUP-1 suppresses RANKL-induced osteoclastogenesis and prevents ovariectomy-induced bone loss: Roles of MAPKs, Akt, NF-κB and calcium/calcineurin/NFATc1 pathways. PLoS ONE 2013, 8, e69468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liou, S.F.; Hsu, J.H.; Chu, H.C.; Lin, H.H.; Chen, I.J.; Yeh, J.L. KMUP-1 promotes osteoblast differentiation through cAMP and cGMP pathways and signaling of BMP-2/Smad1/5/8 and Wnt/β-catenin. J. Cell. Physiol. 2015, 230, 2038–2048. [Google Scholar] [CrossRef]
- Liu, H.; Ding, J.; Wang, J.; Wang, Y.; Yang, M.; Zhang, Y.; Chang, F.; Chen, X. Remission of collagen-induced arthritis through combination therapy of microfracture and transplantation of thermogel-encapsulated bone marrow mesenchymal stem cells. PLoS ONE 2015, 10, e0120596. [Google Scholar]
- Pester, J.K.; Stumpfe, S.; Steinert, S.; Marintschev, I.; Aurich, M.; Hofmann, G.O. BMP-2 shows characteristic extracellular patterns in osteoarthritic cartilage: A preliminary report. GMS Interdiscip. Plast. Reconstr. Surg. DGPW 2013, 2. [Google Scholar] [CrossRef]
- Waldstein, W.; Perino, G.; Gilbert, S.L.; Maher, S.A.; Windhager, R.; Boettner, F. OARSI osteoarthritis cartilage histopathology assessment system: A biomechanical evaluation in the human knee. J. Orthop. Res. 2016, 34, 135–140. [Google Scholar] [CrossRef]
- Cui, X.; Churchill, G.A. Statistical tests for differential expression in cDNA microarray experiments. Genome Biol. 2003, 4, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, J.-H.; Moon, S.-J.; Jhun, J.-Y.; Yang, E.-J.; Cho, M.-L.; Min, J.-K. Eupatilin exerts antinociceptive and chondroprotective properties in a rat model of osteoarthritis by downregulating oxidative damage and catabolic activity in chondrocytes. PLoS ONE 2015, 10, e0130882. [Google Scholar]
- Lee, M.-L.; Sulistyowati, E.; Hsu, J.-H.; Huang, B.-Y.; Dai, Z.-K.; Wu, B.-N.; Chao, Y.-Y.; Yeh, J.-L. KMUP-1 ameliorates ischemia-induced cardiomyocyte apoptosis through the NO–cGMP–MAPK signaling pathways. Molecules 2019, 24, 1376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, Y.C.; Tseng, Y.T.; Liu, C.M.; Wu, B.N.; Wu, S.N. Actions of KMUP-1, a xanthine and piperazine derivative, on voltage-gated Na+ and Ca2+-activated K+ currents in GH3 pituitary tumour cells. Br. J. Pharmacol. 2015, 172, 5110–5122. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-P.; Chau, P.-C.; Chang, C.-T.; An, L.-M.; Yeh, J.-L.; Chen, I.-J.; Wu, B.-N. KMUP-1, a GPCR modulator, attenuates triglyceride accumulation involved MAPKs/Akt/PPARγ and PKA/PKG/HSL signaling in 3T3-L1 preadipocytes. Molecules 2018, 23, 2433. [Google Scholar] [CrossRef] [Green Version]
- Barreto, G.; Manninen, M.; Eklund, K.K. Osteoarthritis and Toll-like receptors: When innate immunity meets chondrocyte apoptosis. Biology 2020, 9, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohtake, P.J.; Lee, A.C.; Scott, J.C.; Hinman, R.S.; Ali, N.A.; Hinkson, C.R.; Needham, D.M.; Shutter, L.; Smith-Gabai, H.; Spires, M.C. Physical impairments associated with post–intensive care syndrome: Systematic review based on the world health organization’s international classification of functioning, disability and health framework. Phys. Ther. 2018, 98, 631–645. [Google Scholar] [CrossRef] [Green Version]
- Philp, A.M.; Davis, E.T.; Jones, S.W. Developing anti-inflammatory therapeutics for patients with osteoarthritis. Rheumatology 2017, 56, 869–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu-Bryan, R. Inflammation and intracellular metabolism: New targets in OA. Osteoarthr. Cartil. 2015, 23, 1835–1842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, Z.-K.; Lin, T.-C.; Liou, J.-C.; Cheng, K.-I.; Chen, J.-Y.; Chu, L.-W.; Chen, I.-J.; Wu, B.-N. Xanthine derivative KMUP-1 reduces inflammation and hyperalgesia in a bilateral chronic constriction injury model by suppressing MAPK and NFκB activation. Mol. Pharm 2014, 11, 1621–1631. [Google Scholar] [CrossRef]
- Kong, L.; Smith, W.; Hao, D. Overview of RAW264. 7 for osteoclastogensis study: Phenotype and stimuli. J. Cell Mol. Med. 2019, 23, 3077–3087. [Google Scholar] [CrossRef] [Green Version]
- Feng, Z.; Li, X.; Lin, J.; Zheng, W.; Hu, Z.; Xuan, J.; Ni, W.; Pan, X. Oleuropein inhibits the IL-1β-induced expression of inflammatory mediators by suppressing the activation of NF-κB and MAPKs in human osteoarthritis chondrocytes. Food Funct. 2017, 8, 3737–3744. [Google Scholar] [CrossRef]
- Nakamura, H.; Tanaka, M.; Masuko-Hongo, K.; Yudoh, K.; Kato, T.; Beppu, M.; Nishioka, K. Enhanced production of MMP-1, MMP-3, MMP-13, and RANTES by interaction of chondrocytes with autologous T cells. Rheumatol. Int. 2006, 26, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Klatt, A.R.; Paul-Klausch, B.; Klinger, G.; Kühn, G.; Renno, J.H.; Banerjee, M.; Malchau, G.; Wielckens, K. A critical role for collagen II in cartilage matrix degradation: Collagen II induces pro-inflammatory cytokines and MMPs in primary human chondrocytes. J. Orthop. Res. 2009, 27, 65–70. [Google Scholar] [CrossRef]
- Bau, B.; Gebhard, P.M.; Haag, J.; Knorr, T.; Bartnik, E.; Aigner, T. Relative messenger RNA expression profiling of collagenases and aggrecanases in human articular chondrocytes in vivo and in vitro. Arthritis Rheum. 2002, 46, 2648–2657. [Google Scholar] [CrossRef]
- Lipari, L.; Gerbino, A. Expression of gelatinases (MMP-2, MMP-9) in human articular cartilage. Int. J. Immunopathol. Pharmacol. 2013, 26, 817–823. [Google Scholar] [CrossRef] [Green Version]
- Zeng, G.; Chen, A.; Li, W.; Song, J.; Gao, C. High MMP-1, MMP-2, and MMP-9 protein levels in osteoarthritis. Genet. Mol. Res. 2015, 14, 14811–14822. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, T.; Sasaki, H.; Takayama, K.; Ishida, K.; Matsumoto, T.; Kubo, S.; Matsuzaki, T.; Nishida, K.; Kurosaka, M.; Kuroda, R. The overexpression of SIRT1 inhibited osteoarthritic gene expression changes induced by interleukin-1β in human chondrocytes. J. Orthop. Res. 2013, 31, 531–537. [Google Scholar] [CrossRef]
- Tanaka, Y.; Nakayamada, S.; Okada, Y. Osteoblasts and osteoclasts in bone remodeling and inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Thalhamer, T.; McGrath, M.; Harnett, M. MAPKs and their relevance to arthritis and inflammation. Rheumatology 2008, 47, 409–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Z.; Zheng, W.; Li, X.; Lin, J.; Xie, C.; Li, H.; Cheng, L.; Wu, A.; Ni, W. Cryptotanshinone protects against IL-1β-induced inflammation in human osteoarthritis chondrocytes and ameliorates the progression of osteoarthritis in mice. Int. Immunopharmacol. 2017, 50, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Piao, T.; Wang, Y.; Liu, J. Astragalin inhibits IL-1β-induced inflammatory mediators production in human osteoarthritis chondrocyte by inhibiting NF-κB and MAPK activation. Int. Immunopharmacol. 2015, 25, 83–87. [Google Scholar] [CrossRef]
- Olivotto, E.; Otero, M.; Marcu, K.B.; Goldring, M.B. Pathophysiology of osteoarthritis: Canonical NF-κB/IKKβ-dependent and kinase-independent effects of IKKα in cartilage degradation and chondrocyte differentiation. RMD Open 2015, 1, e000061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.-C.; Yeh, W.-C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.-C.; Jo, J.; Park, J.; Kang, H.K.; Park, Y. NF-κB signaling pathways in osteoarthritic cartilage destruction. Cells 2019, 8, 734. [Google Scholar] [CrossRef] [Green Version]
- Rigoglou, S.; Papavassiliou, A.G. The NF-κB signalling pathway in osteoarthritis. Int. J. Biochem. Cell Biol. 2013, 45, 2580–2584. [Google Scholar] [CrossRef] [PubMed]
- Pulai, J.I.; Chen, H.; Im, H.-J.; Kumar, S.; Hanning, C.; Hegde, P.S.; Loeser, R.F. NF-κB mediates the stimulation of cytokine and chemokine expression by human articular chondrocytes in response to fibronectin fragments. J. Immunol. 2005, 174, 5781–5788. [Google Scholar] [CrossRef] [PubMed] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.-E.; Sulistyowati, E.; Chao, Y.-Y.; Wu, B.-N.; Dai, Z.-K.; Hsu, J.-H.; Yeh, J.-L. In Vitro Evaluation of the Anti-Inflammatory Effect of KMUP-1 and In Vivo Analysis of Its Therapeutic Potential in Osteoarthritis. Biomedicines 2021, 9, 615. https://doi.org/10.3390/biomedicines9060615
Huang S-E, Sulistyowati E, Chao Y-Y, Wu B-N, Dai Z-K, Hsu J-H, Yeh J-L. In Vitro Evaluation of the Anti-Inflammatory Effect of KMUP-1 and In Vivo Analysis of Its Therapeutic Potential in Osteoarthritis. Biomedicines. 2021; 9(6):615. https://doi.org/10.3390/biomedicines9060615
Chicago/Turabian StyleHuang, Shang-En, Erna Sulistyowati, Yu-Ying Chao, Bin-Nan Wu, Zen-Kong Dai, Jong-Hau Hsu, and Jwu-Lai Yeh. 2021. "In Vitro Evaluation of the Anti-Inflammatory Effect of KMUP-1 and In Vivo Analysis of Its Therapeutic Potential in Osteoarthritis" Biomedicines 9, no. 6: 615. https://doi.org/10.3390/biomedicines9060615
APA StyleHuang, S.-E., Sulistyowati, E., Chao, Y.-Y., Wu, B.-N., Dai, Z.-K., Hsu, J.-H., & Yeh, J.-L. (2021). In Vitro Evaluation of the Anti-Inflammatory Effect of KMUP-1 and In Vivo Analysis of Its Therapeutic Potential in Osteoarthritis. Biomedicines, 9(6), 615. https://doi.org/10.3390/biomedicines9060615