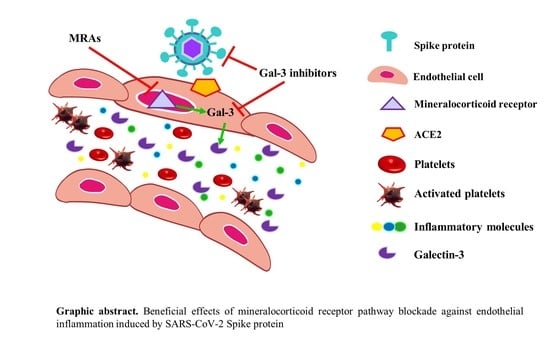
Beneficial Effects of Mineralocorticoid Receptor Pathway Blockade against Endothelial Inflammation Induced by SARS-CoV-2 Spike Protein
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatments
2.2. Western Blot Analysis
2.3. Cytokine Array
2.4. Enzyme-Linked Immuno Sorbent Assay (ELISA)
2.5. Statistical Analyses
3. Results
3.1. SARS-CoV-2 Spike Protein Effects on HAECs
3.2. Preventive MR/Gal-3 Pathway Inhibition on SARS-CoV-2 Spike Protein-Mediated Inflammatory Effects in HAECs
3.3. Inhibition of the MR/Gal-3 Pathway on SARS-CoV-2 Spike Protein-Mediated Inflammatory Effects in HAECs
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glowacka, I.; Bertram, S.; Müller, M.A.; Allen, P.D.; Soilleux, E.J.; Pfefferle, S.; Steffen, I.; Tsegaye, T.S.; He, Y.; Gnirss, K.; et al. Evidence that TMPRSS2 Activates the Severe Acute Respiratory Syndrome Coronavirus Spike Protein for Membrane Fusion and Reduces Viral Control by the Humoral Immune Response. J. Virol. 2011, 85, 4122–4134. [Google Scholar] [CrossRef] [Green Version]
- Ridker, P.M. From C-Reactive Protein to Interleukin-6 to Interleukin-1 Moving Upstream to Identify Novel Targets for Atheroprotection. Circ. Res. 2016, 118, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Libby, P.; Lüscher, T. COVID-19 is, in the end, an endothelial disease. Eur. Hear. J. 2020, 41, 3038–3044. [Google Scholar] [CrossRef]
- Oxford, A.E.; Halla, F.; Robertson, E.B.; Morrison, B.E. Endothelial Cell Contributions to COVID-19. Pathogens 2020, 9, 785. [Google Scholar] [CrossRef]
- Cadegiani, F.A.; Wambier, C.G.; Goren, A. Spironolactone: An Anti-androgenic and Anti-hypertensive Drug That May Provide Protection Against the Novel Coronavirus (SARS-CoV-2) Induced Acute Respiratory Distress Syndrome (ARDS) in COVID-19. Front. Med. 2020, 7, 453. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Ji, W.; Yang, H.; Chen, S.; Zhang, W.; Duan, G. Endothelial activation and dysfunction in COVID-19: From basic mechanisms to potential therapeutic approaches. Signal Transduct. Target. Ther. 2020, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kotfis, K.; Lechowicz, K.; Drożdżal, S.; Niedźwiedzka-Rystwej, P.; Wojdacz, T.; Grywalska, E.; Biernawska, J.; Wiśniewska, M.; Parczewski, M. COVID-19—The Potential Beneficial Therapeutic Effects of Spironolactone during SARS-CoV-2 Infection. Pharmaceuticals 2021, 14, 71. [Google Scholar] [CrossRef]
- Cadegiani, F.A.; Goren, A.; Wambier, C.G. Spironolactone may provide protection from SARS-CoV-2: Targeting androgens, angiotensin converting enzyme 2 (ACE2), and renin-angiotensin-aldosterone system (RAAS). Med. Hypotheses 2020, 143, 110112. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Angioni, R.; Sánchez-Rodríguez, R.; Munari, F.; Bertoldi, N.; Arcidiacono, D.; Cavinato, S.; Marturano, D.; Zaramella, A.; Realdon, S.; Cattelan, A.; et al. Age-severity matched cytokine profiling reveals specific signatures in Covid-19 patients. Cell Death Dis. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Zuo, Y.; Warnock, M.; Harbaugh, A.; Yalavarthi, S.; Gockman, K.; Zuo, M.; Madison, J.A.; Knight, J.S.; Kanthi, Y.; Lawrence, D.A. Plasma tissue plasminogen activator and plasminogen activator inhibitor-1 in hospitalized COVID-19 patients. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- De Biasi, S.; Meschiari, M.; Gibellini, L.; Bellinazzi, C.; Borella, R.; Fidanza, L.; Gozzi, L.; Iannone, A.; Tartaro, D.L.; Mattioli, M.; et al. Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat. Commun. 2020, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Caprio, M.; Newfell, B.G.; La Sala, A.; Baur, W.; Fabbri, A.; Rosano, G.; Mendelsohn, M.E.; Jaffe, I.Z. Functional Mineralocorticoid Receptors in Human Vascular Endothelial Cells Regulate Intercellular Adhesion Molecule-1 Expression and Promote Leukocyte Adhesion. Circ. Res. 2008, 102, 1359–1367. [Google Scholar] [CrossRef] [Green Version]
- Amador, C.; Barrientos, V.; Peña, J.; Herrada, A.A.; González, M.; Valdés, S.; Carrasco, L.; Alzamora, R.; Figueroa, F.; Kalergis, A.M.; et al. Spironolactone Decreases DOCA–Salt–Induced Organ Damage by Blocking the Activation of T Helper 17 and the Downregulation of Regulatory T Lymphocytes. Hypertension 2014, 63, 797–803. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Martínez, E.; Calvier, L.; Fernández-Celis, A.; Rousseau, E.; López, R.J.; Rossoni, L.V.; Jaisser, F.; Zannad, F.; Rossignol, P.; Cachofeiro, V.; et al. Galectin-3 Blockade Inhibits Cardiac Inflammation and Fibrosis in Experimental Hyperaldosteronism and Hypertension. Hypertension 2015, 66, 767–775. [Google Scholar] [CrossRef] [Green Version]
- Henderson, N.C.; Sethi, T. The regulation of inflammation by galectin-3. Immunol. Rev. 2009, 230, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Calvier, L.; Miana, M.; Reboul, P.; Cachofeiro, V.; Martinez-Martinez, E.; De Boer, R.A.; Poirier, F.; Lacolley, P.; Zannad, F.; Rossignol, P.; et al. Galectin-3 Mediates Aldosterone-Induced Vascular Fibrosis. Arter. Thromb. Vasc. Biol. 2013, 33, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Martínez, E.; López-Ándres, N.; López, R.J.; Rousseau, E.; Bartolomé, M.V.; Fernández-Celis, A.; Rossignol, P.; Islas, F.; Antequera, A.; Prieto, S.; et al. Galectin-3 Participates in Cardiovascular Remodeling Associated With Obesity. Hypertension 2015, 66, 961–969. [Google Scholar] [CrossRef]
- Martinez-Martinez, E.; Ibarrola, J.F.; Calvier, L.; Fernandez-Celis, A.; Leroy, C.; Cachofeiro, V.; Rossignol, P.; López-Ándres, N. Galectin-3 Blockade Reduces Renal Fibrosis in Two Normotensive Experimental Models of Renal Damage. PLoS ONE 2016, 11, e0166272. [Google Scholar] [CrossRef]
- DeRoo, E.P.; Wrobleski, S.K.; Shea, E.M.; Al-Khalil, R.K.; Hawley, A.E.; Henke, P.K.; Myers, D.D.J.; Wakefield, T.W.; Diaz, J.A. The role of galectin-3 and galectin-3–binding protein in venous thrombosis. Blood 2015, 125, 1813–1821. [Google Scholar] [CrossRef] [Green Version]
- Stowell, S.R.; Arthur, C.M.; Dias-Baruffi, M.; Rodrigues, L.C.; Gourdine, J.-P.; Heimburg-Molinaro, J.; Ju, T.; Molinaro, R.J.; Rivera-Marrero, C.; Xia, B.; et al. Innate immune lectins kill bacteria expressing blood group antigen. Nat. Med. 2010, 16, 295–301. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.-C.; Arthur, C.M.; Wang, J.; Verkerke, H.; Josephson, C.D.; Kalman, D.; Roback, J.D.; Cummings, R.D.; Stowell, S.R. The SARS-CoV-2 receptor-binding domain preferentially recognizes blood group A. Blood Adv. 2021, 5, 1305–1309. [Google Scholar] [CrossRef]
- Libby, P. Inflammation in Atherosclerosis—No Longer a Theory. Clin. Chem. 2021, 67, 131–142. [Google Scholar] [CrossRef]
- Glowacka, I.; Bertram, S.; Herzog, P.; Pfefferle, S.; Steffen, I.; Muench, M.O.; Simmons, G.; Hofmann, H.; Kuri, T.; Weber, F.; et al. Differential Downregulation of ACE2 by the Spike Proteins of Severe Acute Respiratory Syndrome Coronavirus and Human Coronavirus NL63. J. Virol. 2009, 84, 1198–1205. [Google Scholar] [CrossRef] [Green Version]
- Oudit, G.Y.; Kassiri, Z.; Jiang, C.; Liu, P.P.; Poutanen, S.M.; Penninger, J.M.; Butany, J. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur. J. Clin. Investig. 2009, 39, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Zhang, J.; Schiavon, C.R.; He, M.; Chen, L.; Shen, H.; Zhang, Y.; Yin, Q.; Cho, Y.; Andrade, L.; et al. SARS-CoV-2 Spike Protein Impairs Endothelial Function via Downregulation of ACE 2. Circ. Res. 2021, 128, 1323–1326. [Google Scholar] [CrossRef]
- Piva, F.; Sabanovic, B.; Cecati, M.; Giulietti, M. Expression and co-expression analyses of TMPRSS2, a key element in COVID-19. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 451–455. [Google Scholar] [CrossRef]
- Montopoli, M.; Zumerle, S.; Vettor, R.; Rugge, M.; Zorzi, M.; Catapano, C.; Carbone, G.; Cavalli, A.; Pagano, F.; Ragazzi, E.; et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: A population-based study (N = 4532). Ann. Oncol. 2020, 31, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Zannad, F.; Stough, W.G.; Rossignol, P.; Bauersachs, J.; McMurray, J.J.; Swedberg, K.; Struthers, A.D.; Voors, A.A.; Ruilope, L.M.; Bakris, G.L.; et al. Mineralocorticoid receptor antagonists for heart failure with reduced ejection fraction: Integrating evidence into clinical practice. Eur. Hear. J. 2012, 33, 2782–2795. [Google Scholar] [CrossRef] [Green Version]
- Miura, R.; Nakamura, K.; Miura, D.; Miura, A.; Hisamatsu, K.; Kajiya, M.; Nagase, S.; Morita, H.; Kusano, K.F.; Ohe, T.; et al. Anti-inflammatory Effect of Spironolactone on Human Peripheral Blood Mononuclear Cells. J. Pharmacol. Sci. 2006, 101, 256–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bendtzen, K.; Hansen, P.R.; Rieneck, K.; The Spironolactone/Arthritis Study Group. Spironolactone inhibits production of proinflammatory cytokines, including tumour necrosis factor-α and interferon-γ, and has potential in the treatment of arthritis. Clin. Exp. Immunol. 2003, 134, 151–158. [Google Scholar] [CrossRef]
- Hansen, P.R.; Rieneck, K.; Bendtzen, K. Spironolactone inhibits production of proinflammatory cytokines by human mononuclear cells. Immunol. Lett. 2004, 91, 87–91. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, Z.; Wang, R.; Zheng, Y.; Li, H.; Yang, L. Galectin-3 Is a Potential Mediator for Atherosclerosis. J. Immunol. Res. 2020, 2020, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Revilla, J.; Deierborg, T.; Venero, J.L.; Boza-Serrano, A. Hyperinflammation and Fibrosis in Severe COVID-19 Patients: Galectin-3, a Target Molecule to Consider. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Liu, Y.; Yuan, J.; Wen, Y.; Xu, G.; Zhao, J.; Cheng, L.; Li, J.; Wang, X.; Wang, F.; et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020, 26, 842–844. [Google Scholar] [CrossRef] [PubMed]
- Behloul, N.; Baha, S.; Shi, R.; Meng, J. Role of the GTNGTKR motif in the N-terminal receptor-binding domain of the SARS-CoV-2 spike protein. Virus Res. 2020, 286, 198058. [Google Scholar] [CrossRef] [PubMed]
- Caniglia, J.L.; Asuthkar, S.; Tsung, A.J.; Guda, M.R.; Velpula, K.K. Immunopathology of galectin-3: An increasingly promising target in COVID-19. F1000Research 2020, 9, 1078. [Google Scholar] [CrossRef] [PubMed]
- Yip, P.K.; Jiménez, A.C.; King, P.; Vilalta, A.; Nomura, K.; Chau, C.C.; Egerton, A.M.S.; Liu, Z.-H.; Shetty, A.J.; Tremoleda, J.L.; et al. Galectin-3 released in response to traumatic brain injury acts as an alarmin orchestrating brain immune response and promoting neurodegeneration. Sci. Rep. 2017, 7, srep41689. [Google Scholar] [CrossRef] [Green Version]
- Ren, Z.; Liang, W.; Sheng, J.; Xun, C.; Xu, T.; Cao, R.; Sheng, W. Gal-3 is a potential biomarker for spinal cord injury and Gal-3 deficiency attenuates neuroinflammation through ROS/TXNIP/NLRP3 signaling pathway. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, P.M.; Wells, A.U.; Jenkins, R.G. Pulmonary fibrosis and COVID-19: The potential role for antifibrotic therapy. Lancet Respir. Med. 2020, 8, 807–815. [Google Scholar] [CrossRef]





| Target | Vendor | Catalog # | Working Conc. | Application |
|---|---|---|---|---|
| ACE2 | Abcam | ab15348 | 20 μg/mL | WB |
| TMPRSS2 | Santa Cruz | sc-515727 | 2 μg/mL | WB |
| β-Actin | Santa Cruz | sc-47778 | 2 μg/mL | WB |
| IL-6 | R&D Systems | DY206 | As recommended 1 | ELISA |
| CCL-2 | R&D Systems | DY279 | As recommended 1 | ELISA |
| IL-18 | R&D Systems | DY318-05 | As recommended 1 | ELISA |
| IL-27 | R&D Systems | DY2526 | As recommended 1 | ELISA |
| IFN-γ | R&D Systems | DY285B | As recommended 1 | ELISA |
| PAI-1 | R&D Systems | DY1786 | As recommended 1 | ELISA |
| Gal-3 | R&D Systems | DY1154 | As recommended 1 | ELISA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jover, E.; Matilla, L.; Garaikoetxea, M.; Fernández-Celis, A.; Muntendam, P.; Jaisser, F.; Rossignol, P.; López-Andrés, N. Beneficial Effects of Mineralocorticoid Receptor Pathway Blockade against Endothelial Inflammation Induced by SARS-CoV-2 Spike Protein. Biomedicines 2021, 9, 639. https://doi.org/10.3390/biomedicines9060639
Jover E, Matilla L, Garaikoetxea M, Fernández-Celis A, Muntendam P, Jaisser F, Rossignol P, López-Andrés N. Beneficial Effects of Mineralocorticoid Receptor Pathway Blockade against Endothelial Inflammation Induced by SARS-CoV-2 Spike Protein. Biomedicines. 2021; 9(6):639. https://doi.org/10.3390/biomedicines9060639
Chicago/Turabian StyleJover, Eva, Lara Matilla, Mattie Garaikoetxea, Amaya Fernández-Celis, Pieter Muntendam, Frédéric Jaisser, Patrick Rossignol, and Natalia López-Andrés. 2021. "Beneficial Effects of Mineralocorticoid Receptor Pathway Blockade against Endothelial Inflammation Induced by SARS-CoV-2 Spike Protein" Biomedicines 9, no. 6: 639. https://doi.org/10.3390/biomedicines9060639
APA StyleJover, E., Matilla, L., Garaikoetxea, M., Fernández-Celis, A., Muntendam, P., Jaisser, F., Rossignol, P., & López-Andrés, N. (2021). Beneficial Effects of Mineralocorticoid Receptor Pathway Blockade against Endothelial Inflammation Induced by SARS-CoV-2 Spike Protein. Biomedicines, 9(6), 639. https://doi.org/10.3390/biomedicines9060639