Structure and Dynamics of Oxidized Lipoproteins In Vivo: Roles of High-Density Lipoprotein
Abstract
:1. Introduction
1.1. Atherosclerotic Lesion
1.2. Background on the oxLDL Hypothesis
2. Oxidative Modification of In Vitro oxLDL
3. Clinical Evidence of oxLDL in CVD
4. The Presence of oxHDL and Its Possible Function
5. Candidates for In Vivo oxLDL
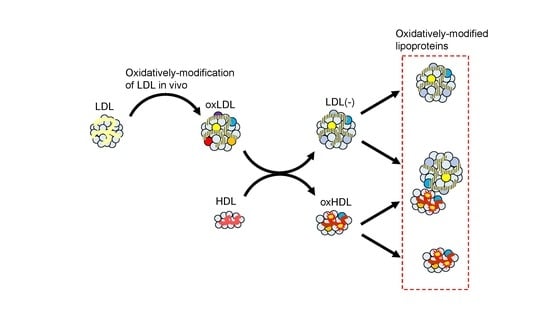
6. Involvement of oxHDL in In Vivo oxLDL Formation
7. oxPC Metabolism in the Presence of HDL
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMI | acute myocardial infarction |
| apoA1 | apolipoprotein A1 |
| apoB | apolipoprotein B-100 |
| apoM | apolipoprotein M |
| apo(a) | apolipoprotein small a |
| CVD | cardiovascular diseases |
| ELISA | enzyme-linked immunosorbent assay |
| FH | familial hypercholesterolemia |
| HDL | high-density lipoprotein |
| LCAT | lecithin-cholesterol acyltransferase |
| LC-MS/MS | liquid chromatography-tandem mass spectrometry |
| LDL | low-density lipoprotein |
| LDL(−) | electronegative low-density lipoprotein |
| LOX-1 | lectin-like oxidized low-density lipoprotein receptor-1 |
| Lp(a) | lipoprotein small a |
| Lp-PLA2 | lipoprotein associated phospholipase A2 |
| mAb | monoclonal antibody |
| MCP-1 | monocyte chemoattractant protein-1 |
| MM-LDL | minimally modified low-density lipoprotein |
| MPO | myeloperoxidase |
| NETs | neutrophils extracellular traps |
| NF-kB | nuclear factor-kappa binding protein |
| oxHDL | oxidized high-density lipoprotein |
| oxLDL | oxidized low-density lipoprotein |
| oxPC | oxidized phosphatidylcholine |
| PC | phosphatidylcholine |
| PON1 | paraoxonase-1 |
| PTCA | percutaneous transluminal coronary angioplasty |
| PUFA | polyunsaturated fatty acid |
| sdLDL | small dense low-density lipoprotein |
| SR-A | scavenger receptor type A |
| STEMI | ST-elevation myocardial infarction |
| TBARS | thiobarbituric acid reactive substances |
| TG | triacylglycerol |
| WHHL | Watanabe heritable hyperlipidemic |
References
- Hansson, G.K.; Libby, P.; Tabas, I. Inflammation and plaque vulnerability. J. Intern. Med. 2015, 278, 483–493. [Google Scholar] [CrossRef]
- Libby, P.; Pasterkamp, G.; Crea, F.; Jang, I.-K. Reassessing the mechanisms of acute coronary syndromes. Circ. Res. 2019, 124, 150–160. [Google Scholar] [CrossRef]
- Obama, T.; Itabe, H. Neutrophils as a novel target of modified low-density lipoproteins and an accelerator of cardiovascular diseases. Int. J. Mol. Sci. 2020, 21, 8312. [Google Scholar] [CrossRef] [PubMed]
- Mironov, A.A.; Sesorova, I.S.; Dimov, I.D.; Karelina, N.R.; Beznoussenko, G.V. Intracellular transports and atherogenesis. Front. Biosci. 2020, 25, 1230–1258. [Google Scholar] [CrossRef]
- Witztum, J.L.; Steinberg, D. Role of oxidized low density lipoprotein in atherogenesis. J. Clin. Investig. 1991, 86, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Mertens, A.; Holvoet, P. Oxidized LDL and HDL: Antagonists in atherothrombosis. FASEB J. 2001, 15, 2073–2084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamada, Y.; Doi, T.; Hamakubo, T.; Kodama, T. Scavenger receptor family proteins: Roles for atherosclerosis, host defense and disorders of the central nervous system. Cell Mol. Life Sci. 1998, 54, 628–640. [Google Scholar] [CrossRef]
- Greaves, D.R.; Gordon, S. Recent insights into the biology of macrophage scavenger receptors. J. Lipid Res. 2005, 46, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Ylä-Herttuala, S.; Palinski, W.; Butler, S.; Picard, S.; Steinberg, D.; Witztum, J.L. Rabbit and human atherosclerotic lesions contain IgG that recognizes epitopes of oxidized LDL. Arterioscler. Thromb. 1994, 14, 32–40. [Google Scholar] [CrossRef] [Green Version]
- Ehara, S.; Ueda, M.; Naruko, T.; Haze, K.; Itoh, A.; Otsuka, M.; Komatsu, R.; Matsuo, T.; Itabe, H.; Takano, T.; et al. Elevated levels of oxidized low density lipoprotein show a positive relationship with the severity of acute coronary syndromes. Circulation 2001, 103, 1955–1960. [Google Scholar] [CrossRef]
- Nishi, K.; Itabe, H.; Uno, M.; Kitazato, K.T.; Horiguchi, H.; Shinno, K.; Nagahiro, S. Oxidized LDL in carotid plaques and plasma associates with plaque instability. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1649–1654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, M.S.; Goldstein, J.L. Scavenger receptor shared. Nature 1985, 316, 680–681. [Google Scholar] [CrossRef] [PubMed]
- Hazen, S.L. Oxidized phospholipids as endogenous pattern recognition ligands in innate immunity. J. Biol. Chem. 2008, 283, 15527–15531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiolino, G.; Rossitto, G.; Caielli, P.; Bisogni, V.; Rossi, G.P.; Calò, L.A. The role of oxidized low-density lipoproteins in atherosclerosis: The myths and the facts. Mediat. Inflamm. 2013, 2013, 714653. [Google Scholar] [CrossRef] [Green Version]
- Bochkov, V.N.; Oskolkova, O.V.; Birukov, K.G.; Levonen, A.-L.; Binder, C.J.; Stöckl, J. Generation and biological activities of oxidized phospholipids. Antioxid. Redox Signal. 2010, 12, 1009–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itabe, H. Oxidative modification of LDL: Its pathological role in atherosclerosis. Clin. Rev. Allery Immunol. 2009, 37, 4–11. [Google Scholar] [CrossRef]
- Berliner, J. Lipid oxidation products and atherosclerosis. Vasc. Pharmacol. 2002, 38, 187–191. [Google Scholar] [CrossRef]
- Watson, A.D.; Leitinger, N.; Navab, M.; Faull, K.F.; Hörkkö, S.; Witztum, J.L.; Palinski, W.; Schwenke, D.; Salomon, R.G.; Sha, W.; et al. Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J. Biol. Chem. 1997, 272, 13597–13607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podrez, E.; Poliakov, E.; Shen, Z.; Zhang, R.; Deng, Y.; Sun, M.; Finton, P.J.; Shan, L.; Febbraio, M.; Hajjar, D.P.; et al. A novel family of atherogenic oxidized phospholipids promotes macrophage foam cell formation via the scavenger receptor CD36 and is enriched in atherosclerotic lesions. J. Biol. Chem. 2002, 277, 38517–38523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Birukov, K.G.; Romanoski, C.E.; Springstead, J.R.; Lusis, A.J.; Berliner, J.A. Role of phospholipid oxidation products in atherosclerosis. Circ. Res. 2012, 111, 778–799. [Google Scholar] [CrossRef] [PubMed]
- Salomon, R.G. Structural identification and cardiovascular activities of oxidized phospholipids. Circ. Res. 2012, 111, 930–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaut, J.P.; Byun, J.; Tran, H.D.; Lauber, W.M.; Carroll, J.A.; Hotchkiss, R.S.; Belaaouaj, A.; Heinecke, J.W. Myeloperoxidase produces nitrating oxidants in vivo. J. Clin. Investig. 2002, 109, 10311–11319. [Google Scholar] [CrossRef]
- Obama, T.; Kato, R.; Masuda, Y.; Takahashi, K.; Aiuchi, T.; Itabe, H. Analysis of modified apolipoprotein B-100 structures formed in oxidized low-density lipoprotein using LC-MS/MS. Proteomics 2007, 7, 2132–2141. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Kanematsu, M.; Morimitsu, Y.; Osawa, T.; Noguchi, N.; Niki, E. Acrolein is a product of lipid peroxidation reaction. Formation of free acrolein and its conjugate with lysine residues in oxidized low density lipoproteins. J. Biol. Chem. 1998, 273, 16058–16066. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; DiDonato, J.A.; Levison, B.S.; Schmitt, D.; Li, L.; Wu, Y.; Buffa, J.; Kim, T.; Gerstenecker, G.S.; Gu, X.; et al. An abundant dysfunctional apolipoprotein A1 in human atheroma. Nat. Med. 2014, 20, 193–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraley, A.E.; Tsimikas, S. Clinical applications of circulating oxidized low-density lipoprotein biomarkers in cardiovascular disease. Curr. Opin. Lipidol. 2006, 17, 502–509. [Google Scholar] [CrossRef]
- Itabe, H.; Ueda, M. Measurement of plasma oxidized low-density lipoprotein and its clinical implications. J. Atheroscler. Thromb. 2007, 14, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itabe, H.; Kato, R.; Sawada, N.; Obama, T.; Yamamoto, M. Significance of oxidized low-density lipoprotein in body fluids as a marker related to diseased conditions. Curr. Med. Chem. 2019, 26, 1576–1593. [Google Scholar] [CrossRef]
- Sawamura, T.; Wakabayashi, I.; Okamura, T. LOX-1 in atherosclerotic disease. Clin. Chim. Acta 2015, 440, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Tsimikas, S.; Witztum, J.L.; Miller, E.R.; Sasiela, W.J.; Szarek, M.; Olsson, A.G.; Schwartz, G.G. Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) Study Investigators. High-dose atorvastatin reduces total plasma levels of oxidized phospholipids and immune complexes present on apolipoprotein B-100 in patients with acute coronary syndromes in the MIRACL trial. Circulation 2004, 110, 1406–1412. [Google Scholar] [CrossRef] [Green Version]
- Tsimikas, S.; Willeit, P.; Willeit, J.; Santer, P.; Mayr, M.; Xu, Q.; Mayr, A.; Witztum, J.L.; Kiechl, S. Oxidation-specific biomarkers, prospective 15-year cardiovascular and stroke outcomes, and net reclassification of cardiovascular events. J. Am. Coll. Cardiol. 2012, 60, 2218–2229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasabe, N.; Keyamura, Y.; Obama, T.; Inoue, N.; Masuko, Y.; Igarashi, Y.; Aiuchi, T.; Kato, R.; Yamaguchi, T.; Kuwata, H.; et al. Time course-changes in phosphatidylcholine profile during oxidative modification of low-density lipoprotein. Lipids Health Dis. 2014, 13, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmiena, A.A.; Barlow, C.K.; Ng, T.W.; Tull, D.; Meikle, P.J. High density lipoprotein efficiently accepts surface but not internal oxidised lipids from oxidised low density lipoprotein. Biochim. Biophys. Acta 2016, 1861, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.; Koster, G.; Douet, L.J.; Scigelova, M.; Woendin, G.; Ward, J.M.; Smith, A.; Humphries, J.; Burnand, K.G.; Macphee, C.H.; et al. Electrospray ionization mass spectrometry identifies substrates and products of lipoprotein-associated phospholipase A2 in oxidized human low density lipoprotein. J. Biol. Chem. 2008, 283, 6428–6437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.; Zhang, W.; Gu, X.; Chen, X.; Hong, L.; Laird, J.M.; Salomon, R.G. Lysophosphatidylcholine is generated by spontaneous diacylation of oxidized phospholipids. Chem. Res. Toxicol. 2011, 24, 111–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surendran, A.; Zhang, H.; Winter, T.; Edel, A.; Aukem, H.; Ravandi, A. Oxylipin profile of human low-density lipoprotein is dependent on its extent. Atherosclerosis 2019, 288, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Dean, R.T.; Jessup, W. Free and esterified oxysterol: Formation during copper-oxidation of low density lipoprotein and uptake by macrophages. J. Lipid Res. 1996, 37, 320–335. [Google Scholar] [CrossRef]
- Heinecke, J.W. Oxidants and antioxidants in the pathogenesis of atherosclerosis: Implications for the oxidized low density lipoprotein hypothesis. Atherosclerosis 1998, 141, 1–15. [Google Scholar] [CrossRef]
- Tertov, V.V.; Kaplun, S.N.; Dvoryantsev, S.N.; Orekhov, A.N. Apolipoprotein B-bound lipids as a marker for evaluation of low density lipoprotein oxidation in vivo. Biochem. Biophys. Res. Commun. 1995, 214, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Itabe, H.; Takeshima, E.; Iwasaki, H.; Kimura, J.; Yoshida, Y.; Imanaka, T.; Takano, T. A monoclonal antibody against oxidized lipoprotein recognizes foam cells in atherosclerotic lesions. Complex formation of oxidized phosphatidylcholines and polypeptides. J. Biol. Chem. 1994, 269, 15274–15279. [Google Scholar] [CrossRef]
- Gao, D.; Willard, B.; Podrez, E.A. Analysis of covalent modifications of proteins by oxidized phospholipids using a novel method of peptide enrichment. Anal. Chem. 2014, 86, 1254–1262. [Google Scholar] [CrossRef] [Green Version]
- Szapacs, M.R.E.; Kim, H.-Y.H.; Porter, N.A.; Liebler, D.C. Identification of proteins adducted by lipid peroxidation products in plasma and modifications of apolipoprotein A1 with a novel biotinylated phospholipid probe. J. Proteome Res. 2008, 7, 4237–4246. [Google Scholar] [CrossRef] [Green Version]
- Gao, D.; Ashraf, M.Z.; Zhang, L.; Kar, N.; Byzona, T.V.; Podrez, E.A. Cross-linking modifications of HDL apoproteins by oxidized phospholipids: Structural characterization, in vivo detection and functional implications. J. Biol. Chem. 2020, 295, 1973–1984. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Podrez, E.A. Characterization of covalent modifications of HDL apoproteins by endogenous oxidized phospholipids. Free Radic. Biol. Med. 2018, 115, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Liao, F.; Berliner, J.A.; Mehrabian, M.; Navab, M.; Demer, L.L.; Lusis, A.J.; Fogelman, A.M. Minimally modified low density lipoprotein is biologically active in vivo in mice. J. Clin. Invest. 1991, 87, 2253–2257. [Google Scholar] [CrossRef] [PubMed]
- Itabe, H.; Mori, M.; Fujimoto, Y.; Higashi, Y.; Takano, T. Minimally modified LDL is an oxidized LDL enriched with oxidized phosphatidylcholines. J. Biochem. 2003, 134, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Loidl, A.; Sevcsik, E.; Riesenhuber, G.; Deigner, H.-P.; Hermetter, A. Oxidized phospholipids in minimally modified low density lipoprotein induce apoptotic signaling via activation of acid sphingomyelinase in arterial smooth muscle cells. J. Biol. Chem. 2003, 278, 32921–32928. [Google Scholar] [CrossRef] [Green Version]
- Reddy, S.; Hama, S.; Grijalva, V.; Hassan, K.; Mottahedeh, R.; Hough, G.; Wadleigh, D.J.; Navab, M.; Fogelman, A.M. Mitogen-activated protein kinase phosphatase 1 activity is necessary for oxidized phospholipids to induce monocyte chemotactic activity in human aortic endothelial cells. J. Biol. Chem. 2001, 276, 17030–17035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naruko, T.; Ueda, M.; Ehara, S.; Itoh, A.; Haze, K.; Shirai, N.; Ikura, Y.; Ohsawa, M.; Itabe, H.; Kobayashi, Y.; et al. Persistent high levels of plasma oxidized low-density lipoprotein after acute myocardial infarction predict stent restenosis. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 877–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsimikas, S.; Bergmark, C.; Beyer, R.W.; Patel, R.; Pattison, J.; Miller, E.; Juliano, J.; Witztum, J.L. Pattison, Temporal increases in plasma markers of oxidized low-density lipoprotein strongly reflect the presence of acute coronary syndromes. J. Am. Coll. Cardiol. 2003, 41, 360–370. [Google Scholar] [CrossRef] [Green Version]
- Imazu, M.; Ono, K.; Tadehara, F.; Kajiwara, K.; Yamamoto, H.; Sumii, K.; Tasaki, N.; Oiwa, J.; Shimohara, Y.; Gomyo, Y.; et al. Plasma levels of oxidized low density lipoprotein are associated with stable angina pectoris and modalities of acute coronary syndrome. Int. Heart J. 2008, 49, 515–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uno, M.; Kitazato, K.T.; Suzue, A.; Itabe, H.; Hao, L.; Nagahiro, S. Contribution of an imbalance between oxidant-antioxidant systems to plaque vulnerability in patients with carotid artery stenosis. J. Neurosurg. 2005, 103, 518–525. [Google Scholar] [CrossRef] [Green Version]
- Van Berkel, T.J.C.; De Rijke, Y.B.; Kruijt, J.K. Different fate in vivo of oxidatively modified low density lipoprotein and acetylated low density lipoprotein in rats. Recognition by various scavenger receptors on Kupffer and endothelial liver cells. J. Biol. Chem. 1991, 266, 2282–2289. [Google Scholar] [CrossRef]
- Tsimikas, S.; Aikawa, M.; Miller, F.J., Jr.; Miller, E.R.; Torzewski, M.; Lentz, S.R.; Bergmark, C.; Heistad, D.D.; Libby, P.; Witztum, J.L. Increased plasma oxidized phospholipid: Apolipoprotein B-100 ratio with concomitant depletion of oxidized phospholipids from atherosclerotic lesions after dietary lipid-lowering: A potential biomarker of early atherosclerosis regression. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Kato, R.; Mori, C.; Kitazato, K.; Arata, S.; Obama, T.; Mori, M.; Takahashi, K.; Aiuchi, T.; Takano, T.; Itabe, H. Transient increase in plasma oxidized LDL during the progression of atherosclerosis in apolipoprotein E knockout mice. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 33–39. [Google Scholar] [CrossRef]
- Keyamura, Y.; Nagano, C.; Kohashi, M.; Niimi, M.; Nozako, M.; Koyama, T.; Yasufuku, R.; Imaizumi, A.; Itabe, H.; Yoshikawa, T. Add-on effect of probucol in atherosclerotic, cholesterol-fed rabbits treated with atorvastatin. PLoS ONE 2014, 9, e96929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kontush, A.; Chapman, M.J. Antiatherogenic function of HDL particle subpopulations: Focus on antioxidative activities. Curr. Opin. Lipidol. 2010, 21, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Soran, H.; Schofield, J.D.; Durrington, P.N. Antioxidant properties of HDL. Front. Pharmacol. 2015, 6, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brites, F.; Martin, M.; Guillas, I.; Kontush, A. Antioxidative activity of high-density lipoprotein (HDL): Mechanistic insights into potential clinical benefit. BBA Clin. 2017, 8, 66–77. [Google Scholar] [CrossRef]
- Cukier, A.M.O.; Therond, P.; Didichenko, S.A.; Guillas, I.; Chapman, M.J.; Wright, S.D.; Kontush, A. Structure-function relationships in reconstituted HDL: Focus on antioxidative activity and cholesterol efflux capacity. Mol. Cell Biol. Lipids 2017, 1862, 890–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arkensteijn, B.W.C.; Berbée, J.F.P.; Rensen, P.C.N.; Nielsen, L.B.; Christoffersen, C. The apolipoprotein M–sphingosine-1-phosphate axis: Biological relevance in lipoprotein metabolism, lipid disorders and atherosclerosis. Int. J. Mol. Sci. 2013, 14, 4419–4431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurano, M.; Yatomi, Y. Sphingosine 1-phospahte and atherosclerosis. J. Atheroscler. Thromb. 2018, 25, 16–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, B. Site-specific oxidation of apolipoprotein A-I impairs cholesterol export by ABCA1, a key cardioprotective function of HDL. BBA Mol. Cell Biol. Lipids 2012, 1821, 490–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holzer, M.; Zangger, K.; El-Gamal, D.; Binder, V.; Curcic, S.; Konya, V.; Schuligoi, R.; Heinemann, A.; Marsche, G. Myeloperoxidase-derived chlorinating species induce protein carbamylation through decomposition of thiocyanate and urea: Novel pathways generating dysfunctional high-density lipoprotein. Antioxid. Redox Signal. 2012, 17, 1043–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chadwick, A.C.; Holme, R.L.; Chen, Y.; Thomas, M.J.; Sorci-Thomas, M.G.; Silverstein, R.L.; Pritchard, K.A., Jr.; Sahoo, D. Acrolein impairs the cholesterol transport functions of high density lipoproteins. PLoS ONE 2015, 10, e0123138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Arnal-Levron, M.; Hullin-Matsuda, F.; Knibbe, C.; Moulin, P.; Luquain-Costaz, C. In vitro oxidized HDL and HDL from type 2 diabetes patients have reduced ability to efflux oxysterols from THP-1 macrophages. Biochimie 2018, 153, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Pérez, L.; Vallejos, A.; Echeverria, C.; Varela, D.; Cabello-Verrugio, C.; Simon, F. OxHDL controls LOX-1 expression and plasma membrane localization through a mechanism dependent on NOX/ROS/NF-κB pathway on endothelial cells. Lab. Investig. 2019, 99, 421–437. [Google Scholar] [CrossRef]
- Schill, R.L.; Knaack, D.A.; Powers, H.R.; Chen, Y.; Yang, M.; Schill, D.J.; Silverstein, R.L.; Sahoo, D. Modification of HDL by reactive aldehydes alters select cardioprotective functions of HDL in macrophages. FEBS J. 2020, 287, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Suzuki, S.; Uchida, K.; Kikuchi, K.; Sugiyama, H.; Kohno, H.; Umeda, M.; Inoue, K. Natural autoantibody against apolipoprotein A-I, Detection and characterization of the monoclonal antibody established from normal unimmunized NALB/c mice. J. Immunol. 1994, 153, 2290–3201. [Google Scholar]
- Nakajima, T.; Sakagishi, Y.; Katahira, T.; Nagata, A.; Kuwae, T.; Nakamura, H.; Inoue, I.; Takahashi, K.; Katayama, S.; Komoda, T. Characterization of a specific monoclonal antibody 9F5-3a and the development of assay system for oxidized HDL. Biochem. Biophys. Res. Commun. 1995, 217, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Hayase, Y.; Mashiba, S. Establishment and evaluation of 2 monoclonal antibodies against oxidized apolipoprotein A-I (apoA-I) and its application to determine blood oxidized apoA-I levels. Clin. Chim. Acta 2007, 378, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Nagata, A. Immunochemical detection of circulating oxidized high-density lipoprotein with antioxidized apolipoprotein A-I monoclonal antibody. J. Lab. Clin. Med. 2003, 141, 378–384. [Google Scholar] [CrossRef]
- Honda, H.; Ueda, M.; Kojima, S.; Mashiba, S.; Michihata, T.; Takahashi, K.; Shishido, K.; Akizawa, T. Oxidized high-density lipoprotein as a risk factor for cardiovascular events in prevalent hemodialysis patients. Atherosclerosis 2012, 220, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Miyoshi, T.; Kotani, K.; Kohno, K.; Asonuma, H.; Sakuragi, S.; Koyama, Y.; Nakamura, K.; Ito, H. Decrease in oxidized high-density lipoprotein is associated with slowed progression of coronary artery calcification: Subanalysis of a prospective multicenter study. Atherosclerosis 2019, 283, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janac, J.M.; Zeljkovic, A.; Jelic-Ivanovic, Z.D.; Dimitrijevic-Sreckovic, V.S.; Vekic, J.; Mijkovic, M.M.; Stefanovic, A.; Kotur-Stevuljevic, J.M.; Ivanisevic, J.M.; Spasojevic-Kalimanovska, V.V. Increased oxidized high-density lipoprotein/high-density lipoprotein-cholesterol ratio as a potential indicator of disturbed metabolic health in overweight and obese individuals. Lab. Med. 2020, 51, 24–33. [Google Scholar] [CrossRef]
- Okada, T.; Sumida, M.; Ohama, T.; Katayama, Y.; Saga, A.; Inui, H.; Kanno, K.; Masuda, D.; Koseki, M.; Nishida, M.; et al. Development and clinical application of an enzyme-linked immunosorbent assay for oxidized high-density lipoprotein. J. Atheroscler. Thromb. 2020, 27. [Google Scholar] [CrossRef]
- Wang, X.S.; Shao, B.; Oda, M.N.; Heinecke, J.W.; Mahler, S.; Stocker, R. A sensitive and specific ELISA detects methionine sulfoxide-containing apolipoprotein A-I in HDL. J. Lipid Res. 2009, 50, 586–594. [Google Scholar] [CrossRef] [Green Version]
- Hoff, H.F.; O’Neil, J. Structural and functional changes in LDL after modification with both 4-hydroxynonenal and malondialdehyde. J. Lipid Res. 1993, 34, 1209–1217. [Google Scholar] [CrossRef]
- Kühn, H.; Belkner, J.; Suzuki, H.; Yamamoto, S. Oxidative modification of human lipoproteins by lipoxygenases of different positional specificities. J. Lipid Res. 1994, 35, 1749–1759. [Google Scholar] [CrossRef]
- Mabile, L.; Meihac, O.; Escargueil-Blanc, I.; Troly, M.; Pieraggi, M.-T.; Salvayre, R.; Nègre-Salvayre, A. Mitochondrial function is involved in LDL oxidation mediated by human cultured endothelial cells. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- Tertov, V.V.; Sobenin, Z.A.; Gabbasov, E.G.; Popov, E.G.; Jaakkola, O.; Solakivi, T.; Nikkari, T.; Smirnov, V.N.; Orekhov, A.N. Multiple-modified desialylated low density lipoproteins that cause intracellular lipid accumulation. Lab. Investig. 1992, 67, 665–675. [Google Scholar]
- Kotani, K.; Maekawa, M.; Kanno, T.; Kondo, A.; Toda, N.; Manabe, M. Distribution of immunoreactive malondialdehyde-modified low-density lipoprotein in human serum. Biochim. Biophys. Acta 1994, 1215, 121–125. [Google Scholar] [CrossRef]
- Hirano, T.; Ito, Y.; Koba, S.; Toyoda, M.; Ikejiri, A.; Saegusa, H.; Yamazaki, J.; Yoshino, G. Clinical significance of small dense low-density lipoprotein cholesterol levels determined by the simple precipitation method. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 558–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirano, T.; Ito, Y.; Saegusa, H.; Yoshino, G. A novel and simple method for quantification of small, dense LDL. J. Lipid Res. 2003, 44, 2193–2201. [Google Scholar] [CrossRef] [Green Version]
- Packerd, C.J. Small dense low-density lipoprotein and its role as an independent predictor of cardiovascular disease. Curr. Opin. Lipidol. 2006, 17, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Camejo, G.; Hurt-Camejo, E.; Wiklund, O.; Bondjers, G. Association of apo B lipoproteins with arterial proteoglycans: Pathological significance and molecular basis. Atherosclerosis 1998, 139, 205–222. [Google Scholar] [CrossRef]
- Jacob, R.F.; Walter, M.F.; Self-Medlin, Y.; Mason, R.P. Atorvastatin active metabolite inhibits oxidative modification of small dense low-density lipoprotein. J. Cardiovasc. Pharmacol. 2013, 62, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Noureen, A.; Kronenberg, F.; Utermann, G. Structure, function, and genetics of lipoprotein (a). J. Lipid Res. 2016, 56, 1339–1359. [Google Scholar] [CrossRef] [Green Version]
- Leibundgut, G.; Scipione, C.; Yin, H.; Schneider, M.; Boffa, M.B.; Green, S.; Yang, X.; Dennis, E.; Witztum, J.L.; Koschinsky, M.L.; et al. Determinants of binding of oxidaized phospholipids on apolipoprotein (a) and lipoprotein (a). J. Lipid Res. 2013, 54, 2815–2830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsimikas, S.; Mallat, Z.; Talmud, P.J.; Kastelein, J.J.P.; Wareham, N.J.; Sandhu, M.S.; Miller, E.R.; Benessiano, J.; Tedgui, A.; Witztum, J.L.; et al. Oxidation-specific biomarkers, lipoprotein(a), and risk of fatal and non-fatal coronary events. J. Am. Coll. Cardiol. 2010, 56, 946–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forbes, C.A.; Quek, R.G.W.; Deshpande, S.; Worthy, G.; Wolff, R.; Stirk, L.; Kleijnen, J.; Gandra, S.R.; Djedjos, S.; Wong, N.D. The relationship between Lp(a) and CVD outcomes: A systematic review. Lipids Health Dis. 2016, 15, 95. [Google Scholar] [CrossRef] [Green Version]
- Erqou, S.; Thompson, A.; Angelantonio, E.D.; Saleheen, D.; Kaptoge, S.; Marcovina, S.; Danesh, J. Apolipoprotein(a) isoforms and the risk of vascular disease: Systematic review of 40 studies involving 58,000 participants. J. Am. Coll. Cardiol. 2010, 55, 2160–2167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steffen, B.T.; Duprez, D.; Bertoni, A.G.; Guan, W.; Tsai, M.Y. Lp(a) [lipoprotein(a)]-related risk of heart failure is evident in whites but not in other racial/ethnic groups. The multi-ethnic study of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2498–2504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodis, H.N.; Kramsch, D.M.; Avogaro, P.; Bittolo-Bon, G.; Cazzolato, G.; Hwang, J.; Peterson, H.; Sevanian, A. Biochemical and cytotoxic characteristics of an in vivo circulating oxidized low density lipoprotein (LDL-). J. Lipid Res. 1994, 35, 669–677. [Google Scholar] [CrossRef]
- De Castellarnau, C.; Sánchez-Quesada, J.L.; Benítez, S.; Rosa, R.; Caveda, L.; Vila, L.; Ordóñez-Llanos, J. Electronegative LDL from normolipemic subjects induces IL-8 and monocyte chemotactic protein secretion by human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2281–2287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, H.-C.; Ke, L.-Y.; Chu, C.-S.; Lee, A.-S.; Shen, M.-Y.; Cruz, M.A.; Hsu, J.-F.; Cheng, K.-H.; Chan, H.-C.B.; Lu, J.; et al. Highly electronegative LDL from patients with ST-elevation myocardial infarction triggers platelet activation and aggregation. Blood 2013, 122, 3632–3641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, C.-S.; Chan, H.-C.; Tsai, M.-H.; Stancel, N.; Lee, H.-C.; Cheng, K.-H.; Tung, Y.-C.; Chan, H.-C.; Wang, C.-Y.; Shin, S.-J.; et al. Range of L5 LDL levels in healthy adults and L5′s predictive power in patients with hyperlipidemia or coronary artery disease. Sci. Rep. 2018, 8, 11866. [Google Scholar] [CrossRef]
- Estruch, M.; Rajamäki, K.; Sanchez-Quesada, J.L.; Kovanen, P.T.; Öörni, K.; Benitez, S.; Ordoñez-Llanos, J. Electronegative LDL induces priming and inflammasome activation leading to IL-1β release in human monocytes and macrophages. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2015, 1851, 1442–1449. [Google Scholar] [CrossRef]
- Benítez, S.; Camacho, M.; Arcelus, R.; Vila, L.; Bancells, C.; Ordóñez-Llanos, J.; Sánchez-Quesada, J.L. Increased lysophosphatidylcholine and non-esterified fatty acid content in LDL induces chemokine release in endothelial cells. Relationship with electronegative LDL. Atherosclerosis 2004, 177, 299–305. [Google Scholar] [CrossRef]
- Sánchez-Quesada, J.L.; Estruch, M.; Benítez, S.; Ordoñez-Llanos, J. Electronegative LDL: A useful biomarker of cardiovascular risk? Clin. Lipidol. 2012, 7, 345–359. [Google Scholar] [CrossRef]
- Sawada, N.; Obama, T.; Koba, S.; Takaki, T.; Iwamoto, S.; Aiuchi, T.; Kato, R.; Kikuchi, M.; Hirashima, U.; Itabe, H. Circulating oxidized LDL increased in patients with acute myocardial infarction is accompanied by heavily modified HDL. J. Lipid Res. 2020, 61, 816–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carnuta, M.G.; Stancu, C.S.; Toma, L.; Sanda, G.M.; Niculescu, L.S.; Deleanu, M.; Popescu, A.C.; Popescu, M.R.; Vlad, A.; Dimulescu, D.R.; et al. Dysfunctional high-density lipoproteins have distinct composition, diminished anti-inflammatory potential and discriminate acute coronary syndrome from stable coronary artery disease patients. Sci. Rep. 2017, 7, 7295. [Google Scholar] [CrossRef] [PubMed]
- Ru, D.; Zhiqing, H.; Lin, Z.; Feng, W.; Feng, Z.; Jiayou, Z.; Yusheng, R.; Min, F.; Chun, L.; Zonggui, W. Oxidized high-density lipoprotein accelerates atherosclerosis progression by inducing the imbalance between treg and teff in LDLR knockout mice. Acta Pathol. Microbiol. Immun. Scand. 2015, 123, 410–421. [Google Scholar] [CrossRef]
- DiDonato, J.A.; Huang, Y.; Aulak, K.S.; Even-Or, O.; Gerstenecker, G.; Gogonea, V.; Wu, Y.; Fox, P.L.; Tang, W.H.; Plow, E.F.; et al. Function and distribution of apolipoprotein A1 in the artery wall are markedly distinct from those in plasma. Circulation 2013, 128, 1644–1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.; Saku, K.; Zhang, B.; Hirata, K.; Shiomi, M.; Arakawa, K. In vivo kinetics of oxidatively modified HDL. Biochem. Med. Metab. Biol. 1993, 49, 392–397. [Google Scholar] [CrossRef]
- Sawada, N.; Obama, T.; Mizuno, M.; Fukuhara, K.; Iwamoto, S.; Aiuchi, T.; Makiyama, T.; Itabe, H. Transfer and enzyme-mediated metabolism of oxidized phosphatidylcholine and lysophosphatidylcholine between low- and high-density lipoproteins. Antioxidants 2020, 9, 1045. [Google Scholar] [CrossRef]
- Massey, J.B.; Bick, D.H.; Pownall, H.J. Spontaneous transfer of monoacyl amphiphiles between lipid and protein surfaces. Biophys. J. 1997, 72, 1732–1743. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Itabe, H.; Sawada, N.; Makiyama, T.; Obama, T. Structure and Dynamics of Oxidized Lipoproteins In Vivo: Roles of High-Density Lipoprotein. Biomedicines 2021, 9, 655. https://doi.org/10.3390/biomedicines9060655
Itabe H, Sawada N, Makiyama T, Obama T. Structure and Dynamics of Oxidized Lipoproteins In Vivo: Roles of High-Density Lipoprotein. Biomedicines. 2021; 9(6):655. https://doi.org/10.3390/biomedicines9060655
Chicago/Turabian StyleItabe, Hiroyuki, Naoko Sawada, Tomohiko Makiyama, and Takashi Obama. 2021. "Structure and Dynamics of Oxidized Lipoproteins In Vivo: Roles of High-Density Lipoprotein" Biomedicines 9, no. 6: 655. https://doi.org/10.3390/biomedicines9060655
APA StyleItabe, H., Sawada, N., Makiyama, T., & Obama, T. (2021). Structure and Dynamics of Oxidized Lipoproteins In Vivo: Roles of High-Density Lipoprotein. Biomedicines, 9(6), 655. https://doi.org/10.3390/biomedicines9060655