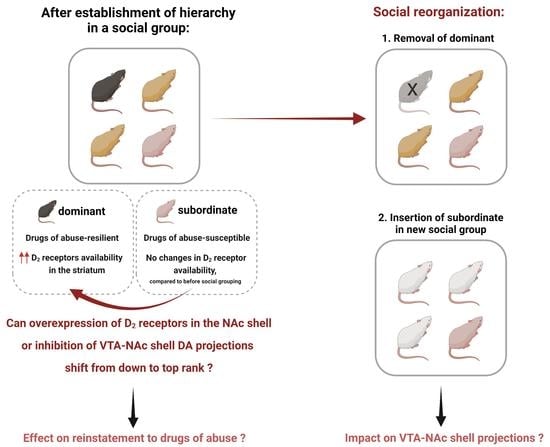
Is It Possible to Shift from Down to Top Rank? A Focus on the Mesolimbic Dopaminergic System and Cocaine Abuse
Abstract
:1. Introduction
2. Social Rank and Drugs of Abuse
3. Social Rank and the Mesolimbic Dopamine System
4. Social Rank and Sociability
5. Effects of Environmental Factors on Social Rank
6. Is It Possible to Shift from Down to Top?
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zernig, G.; Pinheiro, B.S. Dyadic social interaction inhibits cocaine-conditioned place preference and the associated activation of the accumbens corridor. Behav. Pharmacol. 2015, 26, 580–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Rawas, R.; Klement, S.; Kummer, K.K.; Fritz, M.; DeChant, G.; Saria, A.; Zernig, G. Brain regions associated with the acquisition of conditioned place preference for cocaine vs. social interaction. Front. Behav. Neurosci. 2012, 6, 63. [Google Scholar] [CrossRef] [Green Version]
- Tanner, C.J.; Jackson, A.L. Social structure emerges via the interaction between local ecology and individual behaviour. J. Anim. Ecol. 2011, 81, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Gilboa-Schechtman, E.; Shachar, I.; Helpman, L. Evolutionary Perspective on Social Anxiety. In Social Anxiety: Clinical, Developmental, and Social Perspectives, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 599–622. ISBN 9780123750969. [Google Scholar]
- Šabanović, M.; Liu, H.; Mlambo, V.; Aqel, H.; Chaudhury, D. What it takes to be at the top: The interrelationship between chronic social stress and social dominance. Brain Behav. 2020, 10, e01896. [Google Scholar] [CrossRef] [PubMed]
- Nader, M.A.; Czoty, P.W.; Nader, S.H.; Morgan, A. Nonhuman primate models of social behavior and cocaine abuse. Psychopharmacology 2012, 224, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Nader, M.A.; Czoty, P.W.; Gould, R.W.; Riddick, N.V. Positron emission tomography imaging studies of dopamine receptors in primate models of addiction. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 3223–3232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larrieu, T.; Sandi, C. Stress-Induced Depression: Is Social Rank a Predictive Risk Factor? BioEssays 2018, 40, e1800012. [Google Scholar] [CrossRef]
- Zhou, T.; Sandi, C.; Hu, H. Advances in understanding neural mechanisms of social dominance. Curr. Opin. Neurobiol. 2018, 49, 99–107. [Google Scholar] [CrossRef]
- Tarter, R.E.; Kirisci, L.; Kirillova, G.P.; Gavaler, J.; Giancola, P.; Vanyukov, M.M. Social dominance mediates the association of testosterone and neurobehavioral disinhibition with risk for substance use disorder. Psychol. Addict. Behav. 2007, 21, 462–468. [Google Scholar] [CrossRef]
- Covington, H.E.; Miczek, K.A. Repeated social-defeat stress, cocaine or morphine. Psychopharmacology 2001, 158, 388–398. [Google Scholar] [CrossRef]
- Covington, H.E.; Kikusui, T.; Goodhue, J.; Nikulina, E.M.; Hammer, R.P.; Miczek, K.A. Brief Social Defeat Stress: Long Lasting Effects on Cocaine Taking During a Binge and Zif268 mRNA Expression in the Amygdala and Prefrontal Cortex. Neuropsychopharmacology 2004, 30, 310–321. [Google Scholar] [CrossRef] [Green Version]
- Morgan, D.; Grant, K.A.; Gage, H.D.; Mach, R.H.; Kaplan, J.R.; Prioleau, O.; Nader, S.H.; Buchheimer, N.; Ehrenkaufer, R.L.; Nader, M.A. Social dominance in monkeys: Dopamine D2 receptors and cocaine self-administration. Nat. Neurosci. 2002, 5, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Jupp, B.; Murray, J.; Jordan, E.R.; Xia, J.; Fluharty, E.M.; Shrestha, S.; Robbins, T.; Dalley, J.W. Social dominance in rats: Effects on cocaine self-administration, novelty reactivity and dopamine receptor binding and content in the striatum. Psychopharmacology 2016, 233, 579–589. [Google Scholar] [CrossRef] [Green Version]
- EL Rawas, R.; Amaral, I.; Hofer, A. Social Interaction Reward: A Resilience Approach to Overcome Vulnerability to Drugs of Abuse. Eur. Neuropsychopharmacol. 2020, 1. [Google Scholar] [CrossRef] [PubMed]
- Covington, H.E.; Miczek, K.A. Intense cocaine self-administration after episodic social defeat stress, but not after aggressive behavior: Dissociation from corticosterone activation. Psychopharmacology 2005, 183, 331–340. [Google Scholar] [CrossRef]
- Tornatzky, W.; Miczek, K.A. Long-term impairment of autonomic circadian rhythms after brief intermittent social stress. Physiol. Behav. 1993, 53, 983–993. [Google Scholar] [CrossRef]
- Hollis, F.; Kabbaj, M. Social Defeat as an Animal Model for Depression. ILAR J. 2014, 55, 221–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czoty, P.W.; McCabe, C.; Nader, M.A. Assessment of the Relative Reinforcing Strength of Cocaine in Socially Housed Monkeys Using a Choice Procedure. J. Pharmacol. Exp. Ther. 2004, 312, 96–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Z.; Zhu, H.; Zhou, T.; Wang, S.; Wu, Y.; Hu, H. Using the tube test to measure social hierarchy in mice. Nat. Protoc. 2019, 14, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Battivelli, D.; Vernochet, C.; Nguyen, C.; Bhattacharya, S.; Zayed, A.; Meirsman, A.C.; Messaoudene, S.; Fieggen, A.; Tassin, J.P.; Marti, F.; et al. Social status influences normal and pathological behaviors in mice, a role for dopamine and stress signaling. bioRxiv 2019, 856781. [Google Scholar] [CrossRef] [Green Version]
- Yanovich, C.; Kirby, M.L.; Michaelevski, I.; Yadid, G.; Pinhasov, A. Social rank-associated stress vulnerability predisposes individuals to cocaine attraction. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calcagnetti, D.J.; Schechter, M.D. Place conditioning reveals the rewarding aspect of social interaction in juvenile rats. Physiol. Behav. 1992, 51, 667–672. [Google Scholar] [CrossRef]
- Fritz, M.; El Rawas, R.; Salti, A.; Klement, S.; Bardo, M.T.; Kemmler, G.; Dechant, G.; Saria, A.; Zernig, G. Reversal of cocaine-conditioned place preference and mesocorticolimbic Zif268 expression by social interaction in rats. Addict. Biol. 2011, 16, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Lemos, C.; Salti, A.; Amaral, I.M.; Fontebasso, V.; Singewald, N.; Dechant, G.; Hofer, A.; El Rawas, R. Social interaction reward in rats has anti-stress effects. Addict. Biol. 2021, 26, e12878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amaral, I.M.; Lemos, C.; Cera, I.; DeChant, G.; Hofer, A.; El Rawas, R. Involvement of cAMP-Dependent Protein Kinase in the Nucleus Accumbens in Cocaine Versus Social Interaction Reward. Int. J. Mol. Sci. 2020, 22, 345. [Google Scholar] [CrossRef] [PubMed]
- Fritz, M.; El Rawas, R.; Klement, S.; Kummer, K.; Mayr, M.J.; Eggart, V.; Salti, A.; Bardo, M.T.; Saria, A.; Zernig, G. Differential Effects of Accumbens Core vs. Shell Lesions in a Rat Concurrent Conditioned Place Preference Paradigm for Cocaine vs. Social Interaction. PLoS ONE 2011, 6, e26761. [Google Scholar] [CrossRef] [Green Version]
- Sampedro-Piquero, P.; Ávila-Gámiz, F.; Moreno-Fernandez, R.D.; Castilla-Ortega, E.; Santín, L.J. The presence of a social stimulus reduces cocaine-seeking in a place preference conditioning paradigm. J. Psychopharmacol. 2019, 33, 1501–1511. [Google Scholar] [CrossRef]
- Pinheiro, B.S.; Seidl, S.S.; Habazettl, E.; Gruber, B.E.; Bregolin, T.; Zernig, G. Dyadic social interaction of C57BL/6 mice versus interaction with a toy mouse. Behav. Pharmacol. 2016, 27, 279–288. [Google Scholar] [CrossRef]
- Bregolin, T.; Pinheiro, B.S.; El Rawas, R.; Zernig, G. Preventive Strength of Dyadic Social Interaction against Reacquisition/Reexpression of Cocaine Conditioned Place Preference. Front. Behav. Neurosci. 2017, 11, 225. [Google Scholar] [CrossRef] [Green Version]
- El Rawas, R.; Saria, A. The Two Faces of Social Interaction Reward in Animal Models of Drug Dependence. Neurochem. Res. 2016, 41, 492–499. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.A. Peer influences on drug self-administration: Social facilitation and social inhibition of cocaine intake in male rats. Psychopharmacology 2012, 224, 81–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dingess, P.M.; Deters, M.J.; Darling, R.A.; Yarborough, E.A.; Brown, T.E. A method for evaluating cocaine-induced social preference in rats. J. Biol. Methods 2017, 4, e66. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.A.; Pitts, E. Social preference and drug self-administration: A preclinical model of social choice within peer groups. Drug Alcohol Depend. 2014, 135, 140–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, M.A.; Strickland, J.; Bills, S.E.; Lacy, R.T. The effects of a shared history of drug exposure on social choice. Behav. Pharmacol. 2015, 26, 631–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiel, K.J.; Okun, A.C.; Neisewander, J.L. Social reward-conditioned place preference: A model revealing an interaction between cocaine and social context rewards in rats. Drug Alcohol Depend. 2008, 96, 202–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiel, K.J.; Sanabria, F.; Neisewander, J.L. Synergistic interaction between nicotine and social rewards in adolescent male rats. Psychopharmacology 2009, 204, 391–402. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, S. Drug–social interactions in the reinforcing property of methamphetamine in mice. Behav. Pharmacol. 2011, 22, 203–206. [Google Scholar] [CrossRef]
- Adinoff, B. Neurobiologic Processes in Drug Reward and Addiction. Harv. Rev. Psychiatry 2004, 12, 305–320. [Google Scholar] [CrossRef]
- Di Chiara, G.; Imperato, A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. USA 1988, 85, 5274–5278. [Google Scholar] [CrossRef] [Green Version]
- Chiara, G. Nucleus accumbens shell and core dopamine: Differential role in behavior and addiction. Behav. Brain Res. 2002, 137, 75–114. [Google Scholar] [CrossRef]
- Hyman, S.E.; Malenka, R.C.; Nestler, E.J. NEURAL MECHANISMS OF ADDICTION: The Role of Reward-Related Learning and Memory. Annu. Rev. Neurosci. 2006, 29, 565–598. [Google Scholar] [CrossRef] [Green Version]
- Arias-Carrión, O.; Stamelou, M.; Murillo-Rodríguez, E.; Menéndez-González, M.; Pöppel, E. Dopaminergic reward system: A short integrative review. Int. Arch. Med. 2010, 3, 24. [Google Scholar] [CrossRef] [Green Version]
- Missale, C.; Nash, S.R.; Robinson, S.W.; Jaber, M.; Caron, M.G. Dopamine Receptors: From Structure to Function. Physiol. Rev. 1998, 78, 189–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, D.; Orlowska, D.; Narendran, R.; Slifstein, M.; Liu, F.; Kumar, D.; Broft, A.; Van Heertum, R.; Kleber, H.D. Dopamine Type 2/3 Receptor Availability in the Striatum and Social Status in Human Volunteers. Biol. Psychiatry 2010, 67, 275–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowling, S.L.; Rowlett, J.K.; Bardo, M.T. The effect of environmental enrichment on amphetamine-stimulated locomotor activity, dopamine synthesis and dopamine release. Neuropharmacology 1993, 32, 885–893. [Google Scholar] [CrossRef]
- Solinas, M.; Thiriet, N.; El Rawas, R.; Lardeux, V.; Jaber, M. Environmental Enrichment During Early Stages of Life Reduces the Behavioral, Neurochemical, and Molecular Effects of Cocaine. Neuropsychopharmacology 2008, 34, 1102–1111. [Google Scholar] [CrossRef] [Green Version]
- El Rawas, R.; Thiriet, N.; Lardeux, V.; Jaber, M.; Solinas, M. Environmental enrichment decreases the rewarding but not the activating effects of heroin. Psychopharmacology 2009, 203, 561–570. [Google Scholar] [CrossRef]
- Czoty, P.W.; Gould, R.W.; Gage, H.D.; Nader, M.A. Effects of social reorganization on dopamine D2/D3 receptor availability and cocaine self-administration in male cynomolgus monkeys. Psychopharmacology 2017, 234, 2673–2682. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, M.R.; Bennett, E.L. Psychobiology of plasticity: Effects of training and experience on brain and behavior. Behav. Brain Res. 1996, 78, 57–65. [Google Scholar] [CrossRef]
- Solinas, M.; Thiriet, N.; Chauvet, C.; Jaber, M. Prevention and treatment of drug addiction by environmental enrichment. Prog. Neurobiol. 2010, 92, 572–592. [Google Scholar] [CrossRef]
- Laviola, G.; Hannan, A.; Macrì, S.; Solinas, M.; Jaber, M. Effects of enriched environment on animal models of neurodegenerative diseases and psychiatric disorders. Neurobiol. Dis. 2008, 31, 159–168. [Google Scholar] [CrossRef]
- Nader, M.A.; Nader, S.H.; Czoty, P.W.; Riddick, N.V.; Gage, H.D.; Gould, R.W.; Blaylock, B.L.; Kaplan, J.R.; Garg, P.K.; Davies, H.M.; et al. Social Dominance in Female Monkeys: Dopamine Receptor Function and Cocaine Reinforcement. Biol. Psychiatry 2012, 72, 414–421. [Google Scholar] [CrossRef] [Green Version]
- Bezard, E.; Dovero, S.; Belin, D.; Duconger, S.; Jackson-Lewis, V.; Przedborski, S.; Piazza, P.V.; Gross, C.E.; Jaber, M. Enriched Environment Confers Resistance to 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine and Cocaine: Involvement of Dopamine Transporter and Trophic Factors. J. Neurosci. 2003, 23, 10999–11007. [Google Scholar] [CrossRef] [Green Version]
- Grant, K.A.; Shively, C.A.; Nader, M.A.; Ehrenkaufer, R.L.; Line, S.W.; Morton, T.E.; Donald Gage, H.; Mach, R.H. Effect of social status on striatal dopamine D2 receptor binding characteristics in cynomolgus monkeys assessed with positron emission tomography. Synapse 1998, 29, 80–83. [Google Scholar] [CrossRef]
- Giros, B.; Jaber, M.; Jones, S.; Wightman, R.M.; Caron, M.G. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nat. Cell Biol. 1996, 379, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Rocha, B.A.; Fumagalli, F.; Gainetdinov, R.; Jones, S.R.; Ator, R.; Giros, B.; Miller, G.W.; Caron, M.G. Cocaine self-administration in dopamine-transporter knockout mice. Nat. Neurosci. 1998, 1, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Caine, S.B.; Negus, S.S.; Mello, N.K.; Patel, S.; Bristow, L.; Kulagowski, J.; Vallone, D.; Saiardi, A.; Borrelli, E. Role of Dopamine D2-like Receptors in Cocaine Self-Administration: Studies with D2 Receptor Mutant Mice and Novel D2 Receptor Antagonists. J. Neurosci. 2002, 22, 2977–2988. [Google Scholar] [CrossRef] [Green Version]
- Song, R.; Zhang, H.; Li, X.; Bi, G.-H.; Gardner, E.L.; Xi, Z.-X. Increased vulnerability to cocaine in mice lacking dopamine D3 receptors. Proc. Natl. Acad. Sci. USA 2012, 109, 17675–17680. [Google Scholar] [CrossRef] [Green Version]
- Dalley, J.W.; Fryer, T.D.; Brichard, L.; Robinson, E.; Theobald, D.E.H.; Lääne, K.; Peña, Y.; Murphy, E.R.; Shah, Y.; Probst, K.; et al. Nucleus Accumbens D2/3 Receptors Predict Trait Impulsivity and Cocaine Reinforcement. Science 2007, 315, 1267–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellés, L.; Dimiziani, A.; Tsartsalis, S.; Millet, P.; Herrmann, F.R.; Ginovart, N. Dopamine D2/3 Receptor Availabilities and Evoked Dopamine Release in Striatum Differentially Predict Impulsivity and Novelty Preference in Roman High- and Low-Avoidance Rats. Int. J. Neuropsychopharmacol. 2021, 24, 239–251. [Google Scholar] [CrossRef]
- London, E.D. Human Brain Imaging Links Dopaminergic Systems to Impulsivity. In Current Topics in Behavioral Neurosciences; Springer Science and Business Media Deutschland GmbH: Berlin/Heidelberg, Germany, 2020; Volume 47, pp. 53–71. [Google Scholar]
- Moreno, M.; Azocar, V.; Vergés, A.; Fuentealba, J.A. High impulsive choice is accompanied by an increase in dopamine release in rat dorsolateral striatum. Behav. Brain Res. 2021, 405, 113199. [Google Scholar] [CrossRef]
- Adriani, W.; Boyer, F.; Gioiosa, L.; Macrì, S.; Dreyer, J.-L.; Laviola, G. Increased impulsive behavior and risk proneness following lentivirus-mediated dopamine transporter over-expression in rats’ nucleus accumbens. Neuroscience 2009, 159, 47–58. [Google Scholar] [CrossRef] [Green Version]
- Riddick, N.V.; Czoty, P.W.; Gage, H.D.; Kaplan, J.R.; Nader, S.H.; Icenhower, M.; Pierre, P.J.; Bennett, A.; Garg, P.K.; Garg, S.; et al. Behavioral and neurobiological characteristics influencing social hierarchy formation in female cynomolgus monkeys. Neuroscience 2009, 158, 1257–1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czoty, P.W.; Gage, H.D.; Nader, M.A. Differences in D2 dopamine receptor availability and reaction to novelty in socially housed male monkeys during abstinence from cocaine. Psychopharmacology 2010, 208, 585–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalley, J.; Everitt, B.; Robbins, T. Impulsivity, Compulsivity, and Top-Down Cognitive Control. Neuron 2011, 69, 680–694. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, Y.; Lee, Y.-A.; Kato, A.; Jas, E.; Goto, Y. The Roles of Dopamine D2 Receptor in the Social Hierarchy of Rodents and Primates. Sci. Rep. 2017, 7, 43348. [Google Scholar] [CrossRef] [Green Version]
- Torquet, N.; Marti, F.; Campart, C.; Tolu, S.; Nguyen, C.; Oberto, V.; Benallaoua, M.; Naudé, J.; Didienne, S.; Debray, N.; et al. Social interactions impact on the dopaminergic system and drive individuality. Nat. Commun. 2018, 9, 3081. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.-J.; Fowler, J.S.; Thanos, P.; Logan, J.; Gatley, S.J.; Gifford, A.; Ding, Y.-S.; Wong, C.; Pappas, N. Brain DA D2 receptors predict reinforcing effects of stimulants in humans: Replication study. Synapse 2002, 46, 79–82. [Google Scholar] [CrossRef]
- Gjedde, A.; Kumakura, Y.; Cumming, P.; Linnet, J.; Møller, A. Inverted-U-shaped correlation between dopamine receptor availability in striatum and sensation seeking. Proc. Natl. Acad. Sci. USA 2010, 107, 3870–3875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Kooij, M.A.; Hollis, F.; Lozano, L.; Zalachoras, I.; Abad, S.; Zanoletti, O.; Grosse, J.; De Suduiraut, I.G.; Canto, C.; Sandi, C. Diazepam actions in the VTA enhance social dominance and mitochondrial function in the nucleus accumbens by activation of dopamine D1 receptors. Mol. Psychiatry 2017, 23, 569–578. [Google Scholar] [CrossRef] [Green Version]
- Lozano-Montes, L.; Astori, S.; Abad, S.; De Suduiraut, I.G.; Sandi, C.; Zalachoras, I. Latency to Reward Predicts Social Dominance in Rats: A Causal Role for the Dopaminergic Mesolimbic System. Front. Behav. Neurosci. 2019, 13, 69. [Google Scholar] [CrossRef] [Green Version]
- Plavén-Sigray, P.; Gustavsson, P.; Farde, L.; Borg, J.; Stenkrona, P.; Nyberg, L.; Bäckman, L.; Cervenka, S. Dopamine D1 receptor availability is related to social behavior: A positron emission tomography study. NeuroImage 2014, 102, 590–595. [Google Scholar] [CrossRef]
- Couppis, M.H.; Kennedy, C.H.; Stanwood, G.D. Differences in aggressive behavior and in the mesocorticolimbic DA system between A/J and BALB/cJ mice. Synapse 2008, 62, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Lee, Y.-A.; Kato, A.; Goto, Y. The Roles of Dopamine D1 Receptor on the Social Hierarchy of Rodents and Non-human Primates. Int. J. Neuropsychopharmacol. 2016, 20, 324–335. [Google Scholar] [CrossRef] [Green Version]
- Hollis, F.; van der Kooij, M.; Zanoletti, O.; Lozano, L.; Cantó, C.; Sandi, C. Mitochondrial function in the brain links anxiety with social subordination. Proc. Natl. Acad. Sci. USA 2015, 112, 15486–15491. [Google Scholar] [CrossRef] [Green Version]
- Larrieu, T.; Cherix, A.; Duque, A.; Rodrigues, J.; Lei, H.; Gruetter, R.; Sandi, C. Hierarchical Status Predicts Behavioral Vulnerability and Nucleus Accumbens Metabolic Profile Following Chronic Social Defeat Stress. Curr. Biol. 2017, 27, 2202–2210. [Google Scholar] [CrossRef] [Green Version]
- Kunkel, T.; Wang, H. Socially dominant mice in C57BL6 background show increased social motivation. Behav. Brain Res. 2018, 336, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Han, M.-H.; Graham, D.L.; Berton, O.; Renthal, W.; Russo, S.; LaPlant, Q.; Graham, A.; Lutter, M.; Lagace, D.C.; et al. Molecular Adaptations Underlying Susceptibility and Resistance to Social Defeat in Brain Reward Regions. Cell 2007, 131, 391–404. [Google Scholar] [CrossRef] [Green Version]
- Tansley, S.N.; Tuttle, A.H.; Wu, N.; Tohyama, S.; Dossett, K.; Gerstein, L.; Ham, B.; Austin, J.-S.; Sotocinal, S.G.; Mogil, J.S. Modulation of social behavior and dominance status by chronic pain in mice. Genes Brain Behav. 2018, 18, e12514. [Google Scholar] [CrossRef] [PubMed]
- Endo, N.; Makinodan, M.; Mannari-Sasagawa, T.; Horii-Hayashi, N.; Somayama, N.; Komori, T.; Kishimoto, T.; Nishi, M. The effects of maternal separation on behaviours under social-housing environments in adult male C57BL/6 mice. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-J.; Seo, B.A.; Lee, B.; Shin, H.-S.; Kang, M.-G. Stress-induced changes in social dominance are scaled by AMPA-type glutamate receptor phosphorylation in the medial prefrontal cortex. Sci. Rep. 2018, 8, 15008. [Google Scholar] [CrossRef]
- Isabel, M.; Cordero, M.I.; Sandi, C. Stress amplifies memory for social hierarchy. Front. Behav. Neurosci. 2007, 1, 175–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timmer, M.; Sandi, C. A role for glucocorticoids in the long-term establishment of a social hierarchy. Psychoneuroendocrinology 2010, 35, 1543–1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weger, M.; Sevelinges, Y.; Grosse, J.; de Suduiraut, I.G.; Zanoletti, O.; Sandi, C. Increased brain glucocorticoid actions following social defeat in rats facilitates the long-term establishment of social subordination. Physiol. Behav. 2018, 186, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zhu, H.; Fan, Z.; Wang, F.; Chen, Y.; Liang, H.; Yang, Z.; Zhang, L.; Lin, L.; Zhan, Y.; et al. History of winning remodels thalamo-PFC circuit to reinforce social dominance. Science 2017, 357, 162–168. [Google Scholar] [CrossRef] [Green Version]
- Ambroggi, F.; Turiault, M.; Milet, A.; Deroche-Gamonet, V.; Parnaudeau, S.; Balado, E.; Barik, J.; Van Der Veen, R.; Maroteaux, G.; Lemberger, T.; et al. Stress and addiction: Glucocorticoid receptor in dopaminoceptive neurons facilitates cocaine seeking. Nat. Neurosci. 2009, 12, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Červenka, S.; Gustavsson, J.P.; Halldin, C.; Farde, L. Association between striatal and extrastriatal dopamine D2-receptor binding and social desirability. NeuroImage 2010, 50, 323–328. [Google Scholar] [CrossRef]
- Bello, E.P.; Mateo, Y.; Gelman, D.M.; Noaín, D.; Shin, J.H.; Low, M.J.; Alvarez, V.; Lovinger, D.M.; Rubinstein, M. Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nat. Neurosci. 2011, 14, 1033–1038. [Google Scholar] [CrossRef] [Green Version]
- Mantsch, J.R.; Baker, D.; Funk, D.; Lê, A.D.; Shaham, Y. Stress-Induced Reinstatement of Drug Seeking: 20 Years of Progress. Neuropsychopharmacology 2016, 41, 335–356. [Google Scholar] [CrossRef]
- Sinha, R. How does stress increase risk of drug abuse and relapse? Psychopharmacology 2001, 158, 343–359. [Google Scholar] [CrossRef]
- Nader, J.; Claudia, C.; El Rawas, R.; Favot, L.; Jaber, M.; Thiriet, N.; Solinas, M. Loss of Environmental Enrichment Increases Vulnerability to Cocaine Addiction. Neuropsychopharmacology 2012, 37, 1579–1587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howell, L.L.; Cunningham, K.A. Serotonin 5-HT2 Receptor Interactions with Dopamine Function: Implications for Therapeutics in Cocaine Use Disorder. Pharmacol. Rev. 2015, 67, 176–197. [Google Scholar] [CrossRef] [Green Version]
- Ewatanabe, N.; Eyamamoto, M. Neural mechanisms of social dominance. Front. Neurosci. 2015, 9, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raleigh, M.J. Social and Environmental Influences on Blood Serotonin Concentrations in Monkeys. Arch. Gen. Psychiatry 1984, 41, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Raleigh, M.J.; McGuire, M.T.; Brammer, G.L.; Pollack, D.B.; Yuwiler, A. Serotonergic mechanisms promote dominance acquisition in adult male vervet monkeys. Brain Res. 1991, 559, 181–190. [Google Scholar] [CrossRef]
- Sapolsky, R.M. The Influence of Social Hierarchy on Primate Health. Science 2005, 308, 648–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kivimäki, M.; Batty, G.D.; Pentti, J.; Shipley, M.J.; Sipilä, P.; Nyberg, S.T.; Suominen, S.B.; Oksanen, T.; Stenholm, S.; Virtanen, M.; et al. Association between socioeconomic status and the development of mental and physical health conditions in adulthood: A multi-cohort study. Lancet Public Health 2020, 5, e140–e149. [Google Scholar] [CrossRef] [Green Version]
- Forkosh, O.; Karamihalev, S.; Roeh, S.; Alon, U.; Anpilov, S.; Touma, C.; Nussbaumer, M.; Flachskamm, C.; Kaplick, P.M.; Shemesh, Y.; et al. Identity domains capture individual differences from across the behavioral repertoire. Nat. Neurosci. 2019, 22, 2023–2028. [Google Scholar] [CrossRef] [PubMed]
- Karamihalev, S.; Brivio, E.; Flachskamm, C.; Stoffel, R.; Schmidt, M.V.; Chen, A. Social dominance mediates behavioral adaptation to chronic stress in a sex-specific manner. eLife 2020, 9, 1–18. [Google Scholar] [CrossRef]
- Shemesh, Y.; Sztainberg, Y.; Forkosh, O.; Shlapobersky, T.; Chen, A.; Schneidman, E. High-order social interactions in groups of mice. eLife 2013, 2, e00759. [Google Scholar] [CrossRef]
- Cantin, L.; Lenoir, M.; Augier, E.; Vanhille, N.; Dubreucq, S.; Serre, F.; Vouillac, C.; Ahmed, S.H. Cocaine Is Low on the Value Ladder of Rats: Possible Evidence for Resilience to Addiction. PLoS ONE 2010, 5, e11592. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaral, I.M.; Hofer, A.; El Rawas, R. Is It Possible to Shift from Down to Top Rank? A Focus on the Mesolimbic Dopaminergic System and Cocaine Abuse. Biomedicines 2021, 9, 877. https://doi.org/10.3390/biomedicines9080877
Amaral IM, Hofer A, El Rawas R. Is It Possible to Shift from Down to Top Rank? A Focus on the Mesolimbic Dopaminergic System and Cocaine Abuse. Biomedicines. 2021; 9(8):877. https://doi.org/10.3390/biomedicines9080877
Chicago/Turabian StyleAmaral, Inês M., Alex Hofer, and Rana El Rawas. 2021. "Is It Possible to Shift from Down to Top Rank? A Focus on the Mesolimbic Dopaminergic System and Cocaine Abuse" Biomedicines 9, no. 8: 877. https://doi.org/10.3390/biomedicines9080877
APA StyleAmaral, I. M., Hofer, A., & El Rawas, R. (2021). Is It Possible to Shift from Down to Top Rank? A Focus on the Mesolimbic Dopaminergic System and Cocaine Abuse. Biomedicines, 9(8), 877. https://doi.org/10.3390/biomedicines9080877