Analysis of Adverse Drug Reactions in Pediatric Patients with Epilepsy: An Intensive Pharmacovigilance Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Settings, and Ethical Considerations
2.2. Participants
2.3. General Procedure
2.4. Data Collection and Processing
2.5. Instruments
2.6. Data Analysis
3. Results
3.1. Characteristics of Seizures; the Presence of ASM-ADRs and EEGs of Patients with Epilepsy
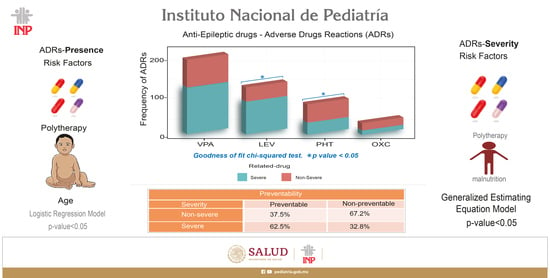
3.2. Risk Factors Associated with the Presence of ADRs
3.3. Frequency and Incidence of ASM-ADRs
3.4. Type, Frequency, and Distribution of the Main ASM-ADRs by Drug
3.5. Organs and Systems Affected by Drugs
3.6. Causality Assessment of ASM-ADRs
3.7. Severity Assessment of ASM-ADRs
3.8. Severity Assessment of ASM-ADRs According to NOM-220-SSA1-2016
3.9. Seriousness Assessment of ASM-ADRs According to NOM-220-SSA1-2016
3.10. Risk Factors Associated with the Seriousness of ADRs
3.11. Preventability Assessment of ASM-ADRs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kotsopoulos, I.A.; van Merode, T.; Kessels, F.G.; de Krom, M.C.; Knottnerus, J.A. Systematic review and meta-analysis of incidence studies of epilepsy and unprovoked seizures. Epilepsia 2002, 43, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Cruz, M.D.R.; Gallardo-Elías, J.; Paredes-Solís, S.; Legorreta-Soberanis, J.; Flores-Moreno, M.; Andersson, N. Factores asociados a epilepsia en niños en Mexico: Un estudio caso-control [Factors associated with epilepsy in children in Mexico: A case-control study]. Bol. Med. Hosp. Infant Mex. 2017, 74, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, A.Y. Crisis convulsivas. Concepto, clasificación y etiología. Emergencias 2005, 17, 568–573. [Google Scholar]
- Stafstrom, C.E. Pathophysiological mechanisms of seizures and epilepsy: A primer. In Epilepsy: Mechanisms, Models, and Translational Perspectives, 1st ed.; Rho, J.M., Sankar, R., Stafstrom, C.E., Eds.; CRC Press: New York, NY, USA, 2010; pp. 3–19. [Google Scholar]
- WHO, World Health Organization. Epilepsy. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/epilepsy (accessed on 5 July 2023).
- GBD 2016 Epilepsy Collaborators. Global, regional, and national burden of epilepsy, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 357–375. [Google Scholar] [CrossRef] [PubMed]
- Hauser, W.A.; Beghi, E. First seizure definitions and worldwide incidence and mortality. Epilepsia 2008, 49 (Suppl 1), 8–12. [Google Scholar] [CrossRef]
- McHugh, J.C.; Delanty, N. Epidemiology and classification of epilepsy: Gender comparisons. Int. Rev. Neurobiol. 2008, 83, 11–26. [Google Scholar] [CrossRef]
- Rubio, F.; Vanegas, M.A. Epilepsy Priority Program in Mexico. In Epilepsy in Latin America; Technical document based on presentations at the international workshop held in Santiago, Chile. August 2013, Plus Subsequent Contributions, Santiago, Chile, August 2013; Kestel, D., Acevedo, C., Medina, M.T., Mesa, T., Rodríguez, J., Eds.; Pan American Health Organization, World Health Organization: Washington, DC, USA, 2013; pp. 51–56. Available online: https://www.ilae.org/files/dmfile/PAHO-report2016-English1.pdf (accessed on 3 July 2023).
- López Hernández, N.J. Reacciones Adversas a Medicamentos Antiepilépticos en Pacientes Pediátricos Hospitalizados en el INP con Diagnóstico de Epilepsia Informe Preliminar; Universidad Nacional Autónoma de Mexico: Mexico City, Mexico, 2014; Available online: http://repositorio.pediatria.gob.mx:8180/bitstream/20.500.12103/866/1/tesis2014_12.pdf (accessed on 28 June 2023).
- Crepeau, A.Z.; Treiman, D.M. Levetiracetam: A comprehensive review. Expert Rev. Neurother. 2010, 10, 159–171. [Google Scholar] [CrossRef]
- Tirado, P.; Alba, M. Epilepsia en la infancia y en la adolescencia. Pediatría Integral 2015, 19, 609–621. [Google Scholar]
- Schmidt, D.; Gram, L. Monotherapy versus polytherapy in epilepsy: A reappraisal. CNS Drugs 1995, 3, 194–208. [Google Scholar] [CrossRef]
- Schneiderman, J.H. Monotherapy versus polytherapy in epilepsy: A framework for patient management. Can. J. Neurol. Sci. 1998, 25, S9–S13. [Google Scholar] [CrossRef]
- Anderson, M.; Egunsola, O.; Cherrill, J.; Millward, C.; Fakis, A.; Choonara, I. A prospective study of adverse drug reactions to antiepileptic drugs in children. BMJ Open 2015, 5, e008298. [Google Scholar] [CrossRef]
- Lobo, M.G.; Pinheiro, S.M.; Castro, J.G.; Momenté, V.G.; Pranchevicius, M.C. Adverse drug reaction monitoring: Support for pharmacovigilance at a tertiary care hospital in Northern Brazil. BMC Pharmacol. Toxicol. 2013, 14, 5. [Google Scholar] [CrossRef]
- Vázquez-Alvarez, A.O.; Brennan-Bourdon, L.M.; Rincón-Sánchez, A.R.; Islas-Carbajal, M.C.; Huerta-Olvera, S.G. Improved drug safety through intensive pharmacovigilance in hospitalized pediatric patients. BMC Pharmacol. Toxicol. 2017, 18, 79. [Google Scholar] [CrossRef] [PubMed]
- Impicciatore, P.; Choonara, I.; Clarkson, A.; Provasi, D.; Pandolfini, C.; Bonati, M. Incidence of adverse drug reactions in pASMiatric in/out-patients: A systematic review and meta-analysis of prospective studies. Br. J. Clin. Pharmacol. 2001, 52, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Clavenna, A.; Bonati, M. Adverse drug reactions in childhood: A review of prospective studies and safety alerts. Arch. Dis. Child. 2009, 94, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Slawomirski, L.; Auraaen, A.; Klazinga, N.S. The Economics of Patient Safety: Strengthening a Value-Based Approach to Reducing Patient Harm at National Level; OECD: Paris, France, 2017; pp. 6–65. Available online: https://www.oecd.org/els/health-systems/The-economics-of-patient-safety-March-2017.pdf (accessed on 27 June 2023).
- Bansal, D.; Azad, C.; Kaur, M.; Rudroju, N.; Vepa, P.; Guglani, V. Adverse effects of antiepileptic drugs in North Indian pediatric outpatients. Clin. Neuropharmacol. 2013, 36, 107–113. [Google Scholar] [CrossRef]
- Choonara, I. Anti-Epileptic Drug Toxicity in Children. Children 2018, 5, 57. [Google Scholar] [CrossRef]
- Clarkson, A.; Choonara, I. Surveillance for fatal suspected adverse drug reactions in the UK. Arch. Dis. Child. 2002, 87, 462–466. [Google Scholar] [CrossRef]
- Le, J.; Nguyen, T.; Law, A.V.; Hodding, J. Adverse drug reactions among children over a 10-year period. Pediatrics 2006, 118, 555–562. [Google Scholar] [CrossRef]
- NOM-220-SSA1-2002; Instalación y Operación de la Farmacovigilancia. NORMA Oficial Mexicana: Mexico City, Mexico, 2002. Available online: https://dof.gob.mx/nota_detalle.php?codigo=684600&fecha=15/11/2004#gsc.tab=0 (accessed on 5 July 2023).
- Härmark, L.; van Grootheest, A.C. Pharmacovigilance: Methods, recent developments and future perspectives. Eur. J. Clin. Pharmacol. 2008, 64, 743–752. [Google Scholar] [CrossRef]
- Balda, M.V.; Daray, F.M. Farmacovigilancia intensiva de clozapina en Argentina [Intensive pharmacovigilance of clozapine in Argentina]. Vertex 2015, 26, 292–301. [Google Scholar] [PubMed]
- Fisher, R.S.; Cross, J.H.; French, J.A.; Higurashi, N.; Hirsch, E.; Jansen, F.E.; Lagae, L.; Moshé, S.L.; Peltola, J.; Roulet Perez, E.; et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 522–530. [Google Scholar] [CrossRef] [PubMed]
- NOM-008-SSA2-1993; Control de la Nutrición, Crecimiento y Desarrollo del niño y del Adolescente. Criterios y Procedimientos Para la Prestación del Servicio. NORMA Oficial Mexicana: Mexico City, Mexico, 1993. Available online: https://www.ucol.mx/content/cms/13/file/NOM/NOM_008_SSA2.pdf (accessed on 5 July 2023).
- Naranjo, C.A.; Busto, U.; Sellers, E.M.; Sandor, P.; Ruiz, I.; Roberts, E.A.; Janecek, E.; Domecq, C.; Greenblatt, D.J. A method for estimating the probability of adverse drug reactions. Clin. Pharmacol. Ther. 1981, 30, 239–245. [Google Scholar] [CrossRef] [PubMed]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012. Adverse Drug Reaction Probability Scale (Naranjo) in Drug Induced Liver Injury. [Updated 2019 May 4]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548069/ (accessed on 5 July 2023).
- Hartwig, S.C.; Siegel, J.; Schneider, P.J. Preventability and severity assessment in reporting adverse drug reactions. Am. J. Hosp. Pharm. 1992, 49, 2229–2232. [Google Scholar] [CrossRef] [PubMed]
- Petrova, G.; Stoimenova, A.; Dimitrova, M.; Kamusheva, M.; Petrova, D.; Georgiev, O. Assessment of the expectancy, seriousness and severity of adverse drug reactions reported for chronic obstructive pulmonary disease therapy. SAGE Open Med. 2017, 5, 2050312117690404. [Google Scholar] [CrossRef]
- NOM-220-SSA1-2016; Instalación y Operación de la Farmacovigilancia. NORMA Oficial Mexicana: Mexico City, Mexico, 2016. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5490830&fecha=19/07/2017#gsc.tab=0 (accessed on 5 July 2023).
- Schumock, G.T.; Thornton, J.P. Focusing on the preventability of adverse drug reactions. Hosp. Pharm. 1992, 27, 538. [Google Scholar] [PubMed]
- Iftikhar, S.; Sarwar, M.R.; Saqib, A.; Sarfraz, M. Causality and preventability assessment of adverse drug reactions and adverse drug events of antibiotics among hospitalized patients: A multicenter, cross-sectional study in Lahore, Pakistan. PLoS ONE 2018, 13, e0199456. [Google Scholar] [CrossRef]
- Agresti, A. An Introduction to Categorical Data Analysis, 2nd ed.; John Willey and Sons: New York, NY, USA, 2007; ISBN 10470114746. [Google Scholar]
- McCullagh, P.; Nelder, J.A. Generalized Linear Models, 2nd ed.; Chapman and Hall: London, UK, 1989. [Google Scholar]
- Liang, K.Y.; Zeger, S.L. Longitudinal Data Analysis Using Generalized Linear Models. Biometrika 1986, 73, 13–22. Available online: https://www.jstor.org/stable/2336267 (accessed on 17 May 2023). [CrossRef]
- Ziegler, A. Generalized Estimating Equations, 1st ed.; Springer Science & Business Media: New York, NY, USA, 2011. [Google Scholar]
- Wickham, H. Elegant Graphics for Data Analysis (ggplot2). Applied Spatial Data Analysis R, 784, 785. 2009. Available online: https://ggplot2.tidyverse.org (accessed on 19 May 2023).
- Højsgaard, S.; Halekoh, U.; Yan, J. The R Package geepack for Generalized Estimating Equations. J. Stat. Softw. 2005, 15, 1–11. [Google Scholar] [CrossRef]
- Merative-Micromedex. Available online: https://www.merative.com/clinical-decision-support (accessed on 5 July 2023).
- Lee, J. Antiepileptic Drugs in Children: Current Concept. J. Korean Neurosurg. Soc. 2019, 62, 296–301. [Google Scholar] [CrossRef]
- Kaushik, S.; Chopra, D.; Sharma, S.; Aneja, S. Adverse Drug Reactions of Anti-Epileptic Drugs in Children with Epilepsy: A Cross-Sectional Study. Curr. Drug Saf. 2019, 14, 217–224. [Google Scholar] [CrossRef]
- George, J.; Kulkarni, C.; Sarma, G.R.K. Antiepileptic Drugs and Quality of Life in Patients with Epilepsy: A Tertiary Care Hospital-Based Study. Value Health Reg. Issues 2015, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Priyadharsini, R.; Surendiran, A.; Adithan, C.; Sreenivasan, S.; Sahoo, F.K. A study of adverse drug reactions in pediatric patients. J. Pharmacol. Pharmacother. 2011, 2, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Safdar, A.; Ismail, F. A comprehensive review on pharmacological applications and drug-induced toxicity of valproic acid. Saudi Pharm. J. 2023, 31, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Herranz, J.L.; Arteaga, R.; Armijo, J.A. Side effects of sodium valproate in monotherapy controlled by plasma levels: A study in 88 pediatric patients. Epilepsia 1982, 23, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Star, K.; Edwards, I.R.; Choonara, I. Valproic acid and fatalities in children: A review of individual case safety reports in VigiBase. PLoS ONE 2014, 9, e108970. [Google Scholar] [CrossRef]
- Chicharro, A.V.; de Marinis, A.J.; Kanner, A.M. The measurement of ammonia blood levels in patients taking valproic acid: Looking for problems where they do not exist. Epilepsy Behav. 2007, 11, 361–366. [Google Scholar] [CrossRef]
- Schmitt, B.; Martin, F.; Critelli, H.; Molinari, L.; Jenni, O.G. Effects of valproic acid on sleep in children with epilepsy. Epilepsia 2009, 50, 1860–1867. [Google Scholar] [CrossRef]
- Kurahashi, H.; Takami, A.; Murotani, K.; Numoto, S.; Okumura, A. Decreased platelet count in children with epilepsy treated with valproate and its relationship to the immature platelet fraction. Int. J. Hematol. 2018, 107, 105–111. [Google Scholar] [CrossRef]
- Delgado, M.R.; Riela, A.R.; Mills, J.; Browne, R.; Roach, E.S. Thrombocytopenia secondary to high valproate levels in children with epilepsy. J. Child Neurol. 1994, 9, 311–314. [Google Scholar] [CrossRef]
- Kumar, R.; Vidaurre, J.; Gedela, S. Valproic Acid-Induced Coagulopathy. Pediatr. Neurol. 2019, 98, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Gerstner, T.; Teich, M.; Bell, N.; Longin, E.; Dempfle, C.E.; Brand, J.; König, S. Valproate-associated coagulopathies are frequent and variable in children. Epilepsia 2006, 47, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.T.; Wang, Z.; Hunsberger, J.G.; Chuang, D.M. Therapeutic potential of mood stabilizers lithium and valproic acid: Beyond bipolar disorder. Pharmacol. Rev. 2013, 65, 105–142. [Google Scholar] [CrossRef] [PubMed]
- Contreras-García, I.J.; Cárdenas-Rodríguez, N.; Romo-Mancillas, A.; Bandala, C.; Zamudio, S.R.; Gómez-Manzo, S.; Hernández-Ochoa, B.; Mendoza-Torreblanca, J.G.; Pichardo-Macías, L.A. Levetiracetam Mechanisms of Action: From Molecules to Systems. Pharmaceuticals 2022, 15, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Egunsola, O.; Choonara, I.; Sammons, H.M. Safety of Levetiracetam in PASMiatrics: A Systematic Review. PLoS ONE 2016, 11, e0149686. [Google Scholar] [CrossRef]
- Tekgül, H.; Gencpinar, P.; Çavuşoğlu, D.; Dündar, N.O. The efficacy, tolerability and safety of levetiracetam therapy in a pediatric population. Seizure 2016, 36, 16–21. [Google Scholar] [CrossRef]
- Halma, E.; de Louw, A.J.; Klinkenberg, S.; Aldenkamp, A.P.; IJff, D.M.; Majoie, M. Behavioral side-effects of levetiracetam in children with epilepsy: A systematic review. Seizure 2014, 23, 685–691. [Google Scholar] [CrossRef]
- Tekin, U.; Tekin, E.; Uçar, H.N. Irritability and its relationship with psychosocial symptoms and quality of life in adolescents with epilepsy receiving levetiracetam therapy: A case-control study. Epilepsy Behav. 2022, 135, 108877. [Google Scholar] [CrossRef]
- Hansen, C.C.; Ljung, H.; Brodtkorb, E.; Reimers, A. Mechanisms Underlying Aggressive Behavior Induced by Antiepileptic Drugs: Focus on Topiramate, Levetiracetam, and Perampanel. Behav. Neurol. 2018, 2018, 2064027. [Google Scholar] [CrossRef]
- Verrotti, A.; Prezioso, G.; Di Sabatino, F.; Franco, V.; Chiarelli, F.; Zaccara, G. The adverse event profile of levetiracetam: A meta-analysis on children and adults. Seizure 2015, 31, 49–55. [Google Scholar] [CrossRef]
- Pichardo Macías, L.A.; Ramírez Mendiola, B.A.; Contreras García, I.J.; Zamudio Hernández, S.R.; Chávez Pacheco, J.L.; Sánchez Huerta, K.B.; Mendoza Torreblanca, J.G. Effect of levetiracetam on extracellular amino acid levels in the dorsal hippocampus of rats with temporal lobe epilepsy. Epilepsy Res. 2018, 140, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, H.; Ulkevan, T.; Ustundag, M.F.; Yucel, A. Levetiracetam-induced acute mania. Clin. Psychopharmacol. 2015, 25, 319–320. [Google Scholar] [CrossRef]
- Glauser, T.; Shinnar, S.; Gloss, D.; Alldredge, B.; Arya, R.; Bainbridge, J.; Bare, M.; Bleck, T.; Dodson, W.E.; Garrity, L.; et al. Evidence-Based Guideline: Treatment of Convulsive Status Epilepticus in Children and Adults: Report of the Guideline Committee of the American Epilepsy Society. Epilepsy Curr. 2016, 16, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Dalziel, S.R.; Borland, M.L.; Furyk, J.; Bonisch, M.; Neutze, J.; Donath, S.; Francis, K.L.; Sharpe, C.; Harvey, A.S.; Davidson, A.; et al. Levetiracetam versus phenytoin for second-line treatment of convulsive status epilepticus in children (ConSEPT): An open-label, multicentre, randomised controlled trial. Lancet 2019, 393, 2135–2145. [Google Scholar] [CrossRef]
- Appleton, R.E.; Gill, A. Adverse events associated with intravenous phenytoin in children: A prospective study. Seizure 2003, 12, 369–372. [Google Scholar] [CrossRef]
- Lai, M.L.; Huang, J.D. Dual effect of valproic acid on the pharmacokinetics of phenytoin. Biopharm. Drug Dispos. 1993, 14, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Perucca, E.; Hebdige, S.; Frigo, G.M.; Gatti, G.; Lecchini, S.; Crema, A. Interaction between phenytoin and valproic acid: Plasma protein binding and metabolic effects. Clin. Pharmacol. Ther. 1980, 28, 779–789. [Google Scholar] [CrossRef]
- Pitton Rissardo, J.; Fornari Caprara, A.L.; Casares, M.; Skinner, H.J.; Hamid, U. Antiseizure Medication-Induced Alopecia: A Literature Review. Medicines 2023, 10, 35. [Google Scholar] [CrossRef]
- Missori, P.; Currà, A. Reversible subacute hair loss induced by levetiracetam. Neurol Sci. 2023, 44, 2207–2208. [Google Scholar] [CrossRef]
- Zou, X.; Hong, Z.; Zhou, D. Hair loss with levetiracetam in five patients with epilepsy. Seizure 2014, 23, 158–160. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Tasdemir, H.A.; Paksu, M.S. The influence of valproic acid treatment on hair and serum zinc levels and serum biotinidase activity. Eur. J. PASMiatr. Neurol. 2009, 13, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Rigo, J.M.; Hans, G.; Nguyen, L.; Rocher, V.; Belachew, S.; Malgrange, B.; Leprince, P.; Moonen, G.; Selak, I.; Matagne, A.; et al. The anti-epileptic drug levetiracetam reverses the inhibition by negative allosteric modulators of neuronal GABA- and glycine-gated currents. Br. J. Pharmacol. 2002, 136, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Crepin, S.; Godet, B.; Chassain, B.; Preux, P.M.; Desport, J.C. Malnutrition and epilepsy: A two-way relationship. Clin. Nutr. 2009, 28, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Karimzadeh, P.; Bakrani, V. Antiepileptic drug-related adverse reactions and factors influencing these reactions. Iran. J. Child Neurol. 2013, 7, 25–29. [Google Scholar] [PubMed]
- Krishnaswamy, K. Drug metabolism and pharmacokinetics in malutrition. Clin. Pharmacokinet. 1978, 3, 216–240. [Google Scholar] [CrossRef] [PubMed]
- Daniels, Z.S.; Nick, T.G.; Liu, C.; Cassedy, A.; Glauser, T.A. Obesity is a common comorbidity for pediatric patients with untreated, newly diagnosed epilepsy. Neurology 2009, 73, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Wang, M.; Zhao, J.; Ruan, Y.; Yang, J.; Chai, S.; Dai, X.; Yang, B.; Cai, Y.; Zhou, Y.; et al. Study on the relationship between obesity and complications of Pediatric Epilepsy surgery. BMC Pediatr. 2023, 23, 142. [Google Scholar] [CrossRef]
- Mistry, R.A.; Solanki, K.C.; Prajapati, H.K.; Doshi, T.M.; Trivedi, H.R. Drug utilization pattern of antiseizure drugs and their adverse effects in the pediatric population, in a tertiary care hospital attached to a medical college. IJBCP 2014, 3, 336–342. [Google Scholar] [CrossRef]
- Manterola, C.; Otzen, T. Estudios observacionales: Los diseños utilizados con mayor frecuencia en investigación clínica. Int. J. Morphol. 2014, 32, 634–645. [Google Scholar] [CrossRef]




| Seizure Type (ILAE 2017) | ASM-ADRs | EEG | ||||
|---|---|---|---|---|---|---|
| Total | Present | Absent | Abnormal | Normal | Not Performed | |
| Focal onset | 39 | 30 | 9 | 30 | 2 | 7 |
| Focal to bilateral tonic—clonic | 14 | 10 | 4 | 13 | 0 | 1 |
| Focal onset impaired awareness | 36 | 26 | 10 | 31 | 0 | 5 |
| Focal onset aware | 4 | 4 | 0 | 2 | 1 | 1 |
| Focal onset motor | 25 | 17 | 8 | 20 | 2 | 3 |
| Focal motor with impaired awareness | 1 | 1 | 0 | 1 | 0 | 0 |
| Focal onset nonmotor | 3 | 2 | 1 | 1 | 0 | 2 |
| Generalized onset | 10 | 7 | 3 | 9 | 0 | 1 |
| Generalized onset motor | 137 | 96 | 41 | 104 | 6 | 27 |
| Generalized onset nonmotor | 6 | 2 | 4 | 4 | 0 | 2 |
| Unclassified | 40 | 16 | 24 | 13 | 6 | 21 |
| Total | 315 | 211 | 104 | 228 | 17 | 70 |
| Variable | Adverse Drug Reactions | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|---|
| Categories | Presence n = 211 | Absence n = 104 | OR (95% CI) | p Value | OR (95% CI) | p Value |
| Sex | ||||||
| Ref: 2 = Boys | 116 (65.9%) | 60 (34.1%) | 1 | 1 | ||
| 1 = Girls | 95 (68.3%) | 44 (31.7%) | 1.12 (0.70, 1.80) | 0.648 | 1.21 (0.72, 2.05) | 0.463 |
| Age | ||||||
| Infant (1 m and <1 yr) | 61 (74.4%) | 21 (25.6%) | 2.90 (1.47, 5.85) | 0.002 * | 2.80 (1.33, 6.03) | 0.007 * |
| Older infant (1 yr and <2 yr) | 28 (65.1%) | 15 (34.9%) | 1.87 (0.86, 4.17) | 0.120 | 1.91 (0.82, 4.55) | 0.137 |
| Preschool (2–4 yr 11 m) | 48 (73.8%) | 17 (26.2%) | 2.82 (1.38, 5.95) | 0.005 * | 2.69 (1.25, 5.93) | 0.013 * |
| School-aged (5–9 yr 11 m) | 40 (70.2%) | 17 (29.8%) | 2.35 (1.13, 5.01) | 0.023 * | 2.28 (1.05, 5.10) | 0.040 * |
| Ref: Adolescent (10–18 yr) | 34 (50.0%) | 34 (50.0%) | 1 | 1 | ||
| Seizure type | ||||||
| Ref: Generalized | 105 (68.6%) | 48 (31.4%) | 1 | 1 | ||
| Focal | 90 (73.8%) | 32 (26.2%) | 1.29 (0.76, 2.19) | 0.351 | 1.29 (0.74, 2.27) | 0.378 |
| Unclassified | 16 (40.0%) | 24 (60.0%) | 0.30 (0.15, 0.62) | 0.001 * | 0.41 (0.19, 0.89) | 0.024 * |
| Therapy | ||||||
| Ref: Monotherapy | 103 (58.5%) | 73 (41.5%) | 1 | 1 | ||
| Polytherapy | 108 (77.7%) | 31 (22.3%) | 2.47 (1.51, 4.11) | 0.001 * | 2.08 (1.22, 3.58) | 0.007 * |
| Nutritional status | ||||||
| 1 = Severe malnutrition | 72 (61.5%) | 45 (38.5%) | 0.60 (0.35, 1.03) | 0.067 | 0.58 (0.32, 1.05) | 0.073 |
| 2 = Mild malnutrition | 40 (71.4%) | 16 (28.6%) | 0.94 (0.47, 1.93) | 0.858 | 0.79 (0.37, 1.74) | 0.553 |
| Ref: 3 = Normal weight | 88 (72.7%) | 33 (27.3%) | 1 | 1 | ||
| 4 = Obesity | 11 (52.4%) | 10 (47.6%) | 0.41 (0.16, 1.08) | 0.066 | 0.46 (0.16, 1.32) | 0.144 |
| ADR | VPA | LEV | PHT | OXC | Others * | Total | % |
|---|---|---|---|---|---|---|---|
| Drowsiness | 36 | 33 | 6 | 9 | 12 | 96 | 17.5 |
| Irritability | 21 | 23 | 1 | 2 | 2 | 49 | 8.9 |
| Thrombocytopenia | 35 | 4 | 8 | 0 | 2 | 49 | 8.9 |
| Alopecia | 21 | 13 | 1 | 1 | 1 | 37 | 6.8 |
| Low VPA levels | 14 | 3 | 12 | 0 | 1 | 30 | 5.5 |
| Hyperammonemia | 24 | 0 | 1 | 0 | 0 | 25 | 4.6 |
| Erythema | 9 | 6 | 8 | 2 | 0 | 25 | 4.6 |
| Constipation | 11 | 4 | 6 | 1 | 2 | 24 | 4.4 |
| Low PHT levels | 3 | 0 | 17 | 0 | 0 | 20 | 3.6 |
| Supratherapeutic VPA levels | 13 | 2 | 2 | 0 | 1 | 18 | 3.3 |
| Neutropenia | 5 | 2 | 2 | 2 | 2 | 13 | 2.4 |
| Uncontrolled seizure | 5 | 4 | 1 | 0 | 0 | 10 | 1.8 |
| Elevated liver enzymes | 9 | 0 | 1 | 0 | 0 | 10 | 1.8 |
| Metabolic acidosis | 2 | 0 | 0 | 0 | 7 | 9 | 1.6 |
| Rash | 2 | 3 | 2 | 2 | 0 | 9 | 1.6 |
| Supratherapeutic PHT levels | 2 | 0 | 4 | 1 | 0 | 7 | 1.3 |
| Edema | 0 | 3 | 2 | 1 | 0 | 6 | 1.1 |
| Liver damage | 2 | 2 | 2 | 0 | 0 | 6 | 1.1 |
| Others | 39 | 29 | 20 | 6 | 11 | 105 | 19.2 |
| Total | 253 | 131 | 96 | 27 | 41 | 548 | 100% |
| Organs and Systems | VPA | LEV | PHT | OXC | TPM | CZP | CBZ | CLB | GBP | Total | % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hematologic | 113 | 15 | 49 | 5 | 4 | 0 | 3 | 0 | 0 | 189 | 34.5 |
| Nervous | 71 | 66 | 14 | 13 | 4 | 10 | 2 | 2 | 1 | 183 | 33.4 |
| Dermatologic | 41 | 32 | 22 | 8 | 1 | 0 | 0 | 0 | 1 | 105 | 19.2 |
| Gastrointestinal | 25 | 12 | 10 | 1 | 1 | 1 | 4 | 0 | 0 | 54 | 9.8 |
| Endocrine-metabolic | 2 | 0 | 0 | 0 | 7 | 0 | 0 | 0 | 0 | 9 | 1.6 |
| Cardiovascular | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0.5 |
| Immunological | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.4 |
| Respiratory | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.4 |
| Musculoskeletal | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.2 |
| Total | 253 | 131 | 96 | 27 | 17 | 11 | 9 | 2 | 2 | 548 | 100% |
| Level | Characteristics | ADR | % |
|---|---|---|---|
| 1 | No treatment change required | 298 | 54.4 |
| 2 | Treatment was suspended, but no other medication or antidote was needed, nor was the length of hospital stay increased | 87 | 15.9 |
| 3 | The treatment was suspended or applied differently, and another medication or antidote was needed, but there was no increase in the length of hospital stay | 115 | 21.0 |
| 4.1 | The treatment was suspended or applied differently, another medication or antidote was needed, and the length of hospital stay increased by at least one day | 11 | 2.0 |
| 4.2 | Was the reason for hospital admission | 28 | 5.1 |
| 5 | Met some of the level 4 conditions and needed intensive care unit admission | 0 | 0 |
| 6 | Permanent harm was caused | 8 | 1.4 |
| 7 | Was directly or indirectly related to the death of the patient | 1 | 0.2 |
| Total | 548 | 100 |
| Variable | Adverse Drug Reactions | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|---|
| Categories | Severe n = 264 | Nonsevere n = 284 | OR (95% CI) | p Value | OR (95% CI) | p Value |
| Sex | ||||||
| Ref: 2 = Boys | 148 (46.5%) | 170 (53.5%) | 1 | 1 | ||
| 1 = Girls | 116 (50.4%) | 114 (49.6%) | 1.17 (0.75, 1.82) | 0.482 | 0.99 (0.65, 1.51) | 0.965 |
| Age | ||||||
| Infant (1 m and <1 yr) | 73 (44.2%) | 92 (55.8%) | 1.02 (0.53, 1.99) | 0.940 | 0.96 (0.50, 1.85) | 0.893 |
| Older infant (1 yr and <2 yr) | 30 (49.2%) | 31 (50.8%) | 1.25 (0.63, 2.48) | 0.520 | 1.03 (0.54, 1.94) | 0.939 |
| Preschool (2–4 yr 11 m) | 64 (50.0%) | 64 (50.0%) | 1.29 (0.65, 2.54) | 0.450 | 1.66 (0.88, 3.12) | 0.108 |
| Scholar (5–9 yr 11 m) | 59 (55.1%) | 48 (44.9%) | 1.59 (0.72, 3.47) | 0.240 | 1.54 (0.75, 3.18) | 0.228 |
| Ref: Adolescent (10–18 yr) | 38 (43.7%) | 49 (56.3%) | 1 | 1 | ||
| Seizure type | ||||||
| Ref: Generalized | 124 (49.0%) | 129 (51.0%) | 1 | 1 | ||
| Focal | 118 (46.5%) | 136 (53.5%) | 0.90 (0.57, 1.42) | 0.650 | 0.86 (0.56, 1.33) | 0.496 |
| Unclassified | 22 (53.7%) | 19 (46.3%) | 1.20 (0.57, 2.56) | 0.620 | 1.46 (0.66, 3.21) | 0.337 |
| Therapy | ||||||
| Ref: Monotherapy | 83 (39.0%) | 130 (61.0%) | 1 | 1 | ||
| Polytherapy | 181 (54.0%) | 154 (46.0%) | 1.84 (1.21, 2.81) | 0.004 * | 2.11 (1.36,3.30) | 0.001 * |
| Nutritional status | ||||||
| 1 = Severe malnutrition | 88 (53.3%) | 77 (46.7%) | 1.56 (0.99, 2.46) | 0.053 | 1.67 (1.04, 2.67) | 0.029 * |
| 2 = Mild malnutrition | 49 (57.6%) | 36 (42.4%) | 1.86 (0.90, 3.82) | 0.087 | 2.06 (1.00, 4.25) | 0.046 * |
| Ref: 3 = Normal weight | 110 (42.3%) | 150 (57.7%) | 1 | 1 | ||
| 4 = Obesity | 17 (44.7%) | 21 (55.3%) | 1.10 (0.51, 2.38) | 0.797 | 1.00 (0.57, 1.75) | 0.992 |
| Preventability | ||||
|---|---|---|---|---|
| Severity | Preventable | Probably Preventable | Nonpreventable | Total |
| Mild | 53 (36.8%) | 187 (55.0%) | 40 (62.5%) | 280 |
| Moderate | 19 (13.2%) | 37 (10.9%) | 10 (15.6%) | 66 |
| Severe | 72 (50.0%) | 116 (34.1%) | 14 (21.9%) | 202 |
| Total | 144 | 340 | 64 | 548 |
| Preventability | ||||
|---|---|---|---|---|
| Gravity | Preventable | Probably Preventable | Nonpreventable | Total |
| Nonsevere | 54 (37.5%) | 187 (55.0%) | 43 (67.2%) | 284 |
| Severe | 90 (62.5%) | 153 (45%) | 21 (32.8%) | 264 |
| Total | 144 | 340 | 64 | 548 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández García, E.; Naranjo, L.; Pichardo-Macías, L.A.; Bernad Bernad, M.J.; Castro-Pastrana, L.I.; Ruíz García, M.; García Bernal, T.A.; Mendoza Solís, J.L.; Calderón Guzmán, D.; Díaz-García, L.; et al. Analysis of Adverse Drug Reactions in Pediatric Patients with Epilepsy: An Intensive Pharmacovigilance Study. Children 2023, 10, 1775. https://doi.org/10.3390/children10111775
Hernández García E, Naranjo L, Pichardo-Macías LA, Bernad Bernad MJ, Castro-Pastrana LI, Ruíz García M, García Bernal TA, Mendoza Solís JL, Calderón Guzmán D, Díaz-García L, et al. Analysis of Adverse Drug Reactions in Pediatric Patients with Epilepsy: An Intensive Pharmacovigilance Study. Children. 2023; 10(11):1775. https://doi.org/10.3390/children10111775
Chicago/Turabian StyleHernández García, Ernestina, Lizbeth Naranjo, Luz Adriana Pichardo-Macías, María Josefa Bernad Bernad, Lucila Isabel Castro-Pastrana, Matilde Ruíz García, Tanya Alejandra García Bernal, Jessica Lizbeth Mendoza Solís, David Calderón Guzmán, Luisa Díaz-García, and et al. 2023. "Analysis of Adverse Drug Reactions in Pediatric Patients with Epilepsy: An Intensive Pharmacovigilance Study" Children 10, no. 11: 1775. https://doi.org/10.3390/children10111775
APA StyleHernández García, E., Naranjo, L., Pichardo-Macías, L. A., Bernad Bernad, M. J., Castro-Pastrana, L. I., Ruíz García, M., García Bernal, T. A., Mendoza Solís, J. L., Calderón Guzmán, D., Díaz-García, L., Mendoza-Torreblanca, J. G., & Chávez Pacheco, J. L. (2023). Analysis of Adverse Drug Reactions in Pediatric Patients with Epilepsy: An Intensive Pharmacovigilance Study. Children, 10(11), 1775. https://doi.org/10.3390/children10111775