Update on Fever of Unknown Origin in Children: Focus on Etiologies and Clinical Approach
Abstract
:1. Introduction
2. Etiology
2.1. Infections
2.2. Autoimmune Inflammatory Diseases
2.3. Neoplastic Diseases
2.4. Miscellaneous
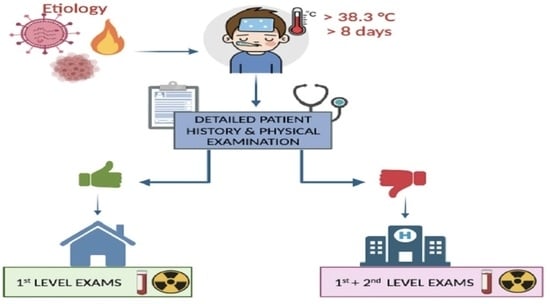
3. Clinical Approach to a Child with FUO
3.1. Focused History
3.2. Physical Examination
4. First-Level Investigations
4.1. Hematochemical Tests
4.1.1. Complete Blood Cell Count (CBC)
4.1.2. Inflammatory Biomarkers
4.1.3. Urine Examination
4.1.4. Culture Tests
4.2. Imaging
5. Second-Level Investigations
5.1. Second-Line Laboratory Tests
5.2. Imaging
6. Management and Prognosis
7. Discussion
8. Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diaz Kane, M.M. “The Fever Is Still There!”: Workup and Management of Prolonged Fever. Pediatr. Ann. 2023, 52, e124–e126. [Google Scholar] [CrossRef] [PubMed]
- Statler, V.A.; Marshall, G.S. Evaluation of Prolonged and Recurrent Unexplained Fevers. Pediatr. Ann. 2018, 47, e347–e353. [Google Scholar] [CrossRef] [PubMed]
- Antoon, J.W.; Potisek, N.M.; Lohr, J.A. Pediatric Fever of Unknown Origin. Pediatr. Rev. 2015, 36, 380–391. [Google Scholar] [CrossRef]
- Chusid, M.J. Fever of Unknown Origin in Childhood. Pediatr. Clin. N. Am. 2017, 64, 205–230. [Google Scholar] [CrossRef] [PubMed]
- Petersdorf, R.G.; Beeson, P.B. Fever of unexplained origin: Report on 100 cases. Medicine 1961, 40, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Marshall, G.S. Prolonged and Recurrent Fevers in Children. J. Infect. 2014, 68, S83–S93. [Google Scholar] [CrossRef]
- Graaf, S.; Keuning, M.W.; Pajkrt, D.; Plötz, F.B. Fever without a Source in Children: International Comparison of Guidelines. World J. Pediatr. 2023, 19, 120–128. [Google Scholar] [CrossRef]
- Lohr, J.A.; Hendley, J.O. Diagnostic Review: Prolonged Fever of Unknown Origin. Clin. Pediatr. 1977, 16, 768–773. [Google Scholar] [CrossRef]
- Pizzo, P.A.; Lovejoy, F.H.; Smith, D.H. Prolonged Fever in Children: Review of 100 Cases. Pediatrics 1975, 55, 468–473. [Google Scholar] [CrossRef]
- McClung, H.J. Prolonged Fever of Unknown Origin in Children. Arch. Pediatr. Adolesc. Med. 1972, 124, 544. [Google Scholar] [CrossRef]
- Steele, R.W.; Jones, S.M.; Lowe, B.A.; Glasier, C.M. Usefulness of Scanning Procedures for Diagnosis of Fever of Unknown Origin in Children. J. Pediatr. 1991, 119, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, R.F.; Schutze, G.E. Bartonella Henselae as a Cause of Prolonged Fever and Fever of Unknown Origin in Children. Clin. Infect. Dis. 1998, 26, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Chantada, G.; Casak, S.; Plata, J.D.; Pociecha, J.; Bologna, R. Children with Fever of Unknown Origin in Argentina. Pediatr. Infect. Dis. J. 1994, 13, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Cogulu, O.; Koturoglu, G.; Kurugol, Z.; Ozkinay, F.; Vardar, F.; Ozkinay, C. Evaluation of 80 Children with Prolonged Fever. Pediatr. Int. 2003, 45, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Çiftçi, E.; İnce, E.; Doğru, Ü. Pyrexia of Unknown Origin in Children: A Review of 102 Patients from Turkey. Ann. Trop. Paediatr. 2003, 23, 259–263. [Google Scholar] [CrossRef]
- Pasic, S.; Minic, A.; Djuric, P.; Micic, D.; Kuzmanovic, M.; Sarjanovic, L.; Markovic, M. Fever of Unknown Origin in 185 Paediatric Patients: A Single-Centre Experience. Acta Paediatr. 2006, 95, 463–466. [Google Scholar] [CrossRef]
- Joshi, N.; Rajeshwari, K.; Dubey, A.P.; Singh, T.; Kaur, R. Clinical Spectrum of Fever of Unknown Origin among Indian Children. Ann. Trop. Paediatr. 2008, 28, 261–266. [Google Scholar] [CrossRef]
- Tezer, H.; Ceyhan, M.; Kara, A.; Cengiz, A.B.; Devrim, İ.; Seçmeer, G. Fever of Unknown Origin in Children: The Experience of One Center in Turkey. Turk. J. Pediatr. 2012, 54, 583–589. [Google Scholar]
- Mahmoudi, S.; Mehrazmay, A.; Sales, M.; Mamishi, S. Fever of Unknown Origin: A Retrospective Study of 95 Children in an Iranian Referral Hospital. Br. J. Biomed. Sci. 2014, 71, 40–42. [Google Scholar] [CrossRef]
- Hassan, R.H.; Fouda, A.E.; Kandil, S.M. Fever of Unknown Origin in Children: A 6 Year-Experience in a Tertiary Pediatric Egyptian Hospital. Int. J. Health Sci. 2014, 8, 13–19. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Kim, K.-R.; Kang, J.-M.; Kim, J.-M.; Kim, Y.-J. Etiology and Clinical Characteristics of Fever of Unknown Origin in Children: A 15-Year Experience in a Single Center. Korean J. Pediatr. 2017, 60, 77. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.-L.; Huang, F.-L.; Huang, C.-M.; Chen, P.-Y. Clinical Approach to Fever of Unknown Origin in Children. J. Microbiol. Immunol. Infect. 2017, 50, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.-Y.; Lai, C.-C.; Lee, M.-L.; Hsu, C.-L.; Chen, C.-J.; Chang, L.-Y.; Lo, C.-W.; Chiang, S.-F.; Wu, K.-G. Clinical Analysis of Fever of Unknown Origin in Children: A 10-Year Experience in a Northern Taiwan Medical Center. J. Microbiol. Immunol. Infect. 2017, 50, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Antoon, J.W.; Peritz, D.C.; Parsons, M.R.; Skinner, A.C.; Lohr, J.A. Etiology and Resource Use of Fever of Unknown Origin in Hospitalized Children. Hosp. Pediatr. 2018, 8, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Szymanski, A.M.; Clifford, H.; Ronis, T. Fever of Unknown Origin: A Retrospective Review of Pediatric Patients from an Urban, Tertiary Care Center in Washington, DC. World J. Pediatr. 2020, 16, 177–184. [Google Scholar] [CrossRef]
- Xu, J.; Ye, Y.Z.; Ye, L.J.; Han, S.Z.; Wu, X.; Wang, C.; Yu, H. Etiology and Clinical Characteristics of Fever of Unknown Origin in 357 Pediatric Patients. Chin. J. Pediatr. 2022, 60, 41–45. [Google Scholar] [CrossRef]
- Hu, B.; Chen, T.-M.; Liu, S.-P.; Hu, H.-L.; Guo, L.-Y.; Chen, H.-Y.; Li, S.-Y.; Liu, G. Fever of Unknown Origin (FUO) in Children: A Single-Centre Experience from Beijing, China. BMJ Open 2022, 12, e049840. [Google Scholar] [CrossRef]
- Attard, L.; Tadolini, M.; De Rose, D.U.; Cattalini, M. Overview of Fever of Unknown Origin in Adult and Paediatric Patients. Clin. Exp. Rheumatol. 2018, 36 (Suppl. 110), 10–24. [Google Scholar]
- Akpede, G.O.; Akenzua, G.I. Management of Children with Prolonged Fever of Unknown Origin and Difficulties in the Management of Fever of Unknown Origin in Children in Developing Countries. Paediatr. Drugs 2001, 3, 247–262. [Google Scholar] [CrossRef]
- Chow, A.; Robinson, J.L. Fever of Unknown Origin in Children: A Systematic Review. World J. Pediatr. 2011, 7, 5–10. [Google Scholar] [CrossRef]
- Yachie, A. Clinical Perspectives and Therapeutic Strategies: Pediatric Autoinflammatory Disease—A Multi-Faceted Approach to Fever of Unknown Origin of Childhood. Inflamm. Regen. 2022, 42, 21. [Google Scholar] [CrossRef] [PubMed]
- Scolfaro, C.; Leunga, G.G.K.; Bezzio, S.; Chiapello, N.; Riva, C.; Balbo, L.; Bertaina, C.; Tovo, P.-A. Prolonged Follow up of Seven Patients Affected by Hepatosplenic Granulomata Due to Cat-Scratch Disease. Eur. J. Pediatr. 2008, 167, 471–473. [Google Scholar] [CrossRef]
- Amarnani, S.; Ranjan, A. Lemierre’s Syndrome: A Lethal Complication of Acute Tonsillitis. Cureus 2022, 14, e30072. [Google Scholar] [CrossRef] [PubMed]
- Hicks, E.D.; Agada, N.O.; Yates, T.R.; Kelly, M.S.; Tam, J.S.; Ferdman, R.M.; Dibernardo, L.R.; Madden, J.F.; Moody, M.A.; Markert, M.L. Case Report: Nontuberculous Mycobacterial Infections in Children with Complete DiGeorge Anomaly. Front. Immunol. 2023, 14, 1078976. [Google Scholar] [CrossRef] [PubMed]
- Kalata, K.E.; Osborne, C.; Willis, A.; Navarro, K.; Fenton, L.Z.; Smith, C. Disseminated Histoplasmosis as an AIDS-Defining Illness Presenting as Fever of Unknown Origin in an 11-Year-Old Female. Case Rep. Pediatr. 2019, 2019, 9417102. [Google Scholar] [CrossRef] [PubMed]
- Dayal, R.; Agarwal, D. Fever in Children and Fever of Unknown Origin. Indian. J. Pediatr. 2016, 83, 38–43. [Google Scholar] [CrossRef] [PubMed]
- De Jong, E.P.; van den Beuken, M.G.A.; van Elzakker, E.P.M.; Wolthers, K.C.; Sprij, A.J.; Lopriore, E.; Walther, F.J.; Brus, F. Epidemiology of Sepsis-like Illness in Young Infants. Pediatr. Infect. Dis. J. 2018, 37, 113–118. [Google Scholar] [CrossRef]
- Bhattacharya, S.K.; Sur, D.; Karbwang, J. Childhood Visceral Leishmaniasis. Indian. J. Med. Res. 2006, 123, 353–356. [Google Scholar]
- Scaggs Huang, F.A.; Schlaudecker, E. Fever in the Returning Traveler. Infect. Dis. Clin. N. Am. 2018, 32, 163–188. [Google Scholar] [CrossRef]
- Chen, K.; Zeng, H.; Togizbayev, G.; Martini, A.; Zeng, H. New Classification Criteria for Juvenile Idiopathic Arthritis. Int. J. Rheum. Dis. 2023, 26, 1889–1892. [Google Scholar] [CrossRef]
- McCrindle, B.W.; Rowley, A.H.; Newburger, J.W.; Burns, J.C.; Bolger, A.F.; Gewitz, M.; Baker, A.L.; Jackson, M.A.; Takahashi, M.; Shah, P.B.; et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association. Circulation 2017, 135, e927–e999. [Google Scholar] [CrossRef] [PubMed]
- Freed, G.L. Wegener’s Granulomatosis Presenting as Fever of Unknown Origin. Clin. Pediatr. 1994, 33, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.C.; Sisson, B.A.; Tucker, L.B.; Schaller, J.G. Prolonged Fevers of Unknown Origin in Children: Patterns of Presentation and Outcome. J. Pediatr. 1996, 129, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Watts, S.; Diaz, M.; Teller, C.; Hamby, T.; Guirola, R.; Perez, M.; Eames, G.; Howrey, R.; Rios, A.; Trinkman, H.; et al. Pediatric Hemophagocytic Lymphohistiocytosis: Formation of an Interdisciplinary HLH Working Group at a Single Institution. J. Pediatr. Hematol. Oncol. 2023, 45, e328–e333. [Google Scholar] [CrossRef]
- Henter, J.-I.; Horne, A.; Aricó, M.; Egeler, R.M.; Filipovich, A.H.; Imashuku, S.; Ladisch, S.; McClain, K.; Webb, D.; Winiarski, J.; et al. HLH-2004: Diagnostic and Therapeutic Guidelines for Hemophagocytic Lymphohistiocytosis. Pediatr. Blood Cancer 2007, 48, 124–131. [Google Scholar] [CrossRef]
- Ravelli, A.; Davì, S.; Minoia, F.; Martini, A.; Cron, R.Q. Macrophage Activation Syndrome. Hematol. Oncol. Clin. N. Am. 2015, 29, 927–941. [Google Scholar] [CrossRef]
- Smith, C.; Lee-Miller, C.; Dishop, M.K.; Cost, C.; Wang, M.; Asturias, E.J. Multicentric Castleman Disease Presenting with Fever. J. Pediatr. 2014, 165, 1261–1265. [Google Scholar] [CrossRef]
- Patel, R.A.; Gallagher, J.C. Drug Fever. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2010, 30, 57–69. [Google Scholar] [CrossRef]
- McDermott, M.F.; Aksentijevich, I.; Galon, J.; McDermott, E.M.; Ogunkolade, B.W.; Centola, M.; Mansfield, E.; Gadina, M.; Karenko, L.; Pettersson, T.; et al. Germline Mutations in the Extracellular Domains of the 55 KDa TNF Receptor, TNFR1, Define a Family of Dominantly Inherited Autoinflammatory Syndromes. Cell 1999, 97, 133–144. [Google Scholar] [CrossRef]
- Kastner, D.L.; Aksentijevich, I.; Goldbach-Mansky, R. Autoinflammatory Disease Reloaded: A Clinical Perspective. Cell 2010, 140, 784–790. [Google Scholar] [CrossRef]
- Kim, L.; Tatarina-Numlan, O.; Yin, Y.D.; John, M.; Sundaram, R. A Case of Kikuchi-Fujimoto Disease in a 7-Year-Old African American Patient: A Case Report and Review of Literature. Am. J. Case Rep. 2020, 21, e922784-1. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, J. North American Zoonoses. Pediatr. Ann. 2009, 38, 193. [Google Scholar] [CrossRef] [PubMed]
- Mathews, D.; McMahon, P.; Bolton, M. Unusual Cause of Prolonged Fever of Unknown Origin. Clin. Pediatr. 2018, 57, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Seashore, C.J.; Lohr, J.A. Fever of Unknown Origin in Children. Pediatr. Ann. 2011, 40, 26–30. [Google Scholar] [CrossRef]
- Stein, R.C. The White Blood Cell Count in Fevers of Unknown Origin. Arch. Pediatr. Adolesc. Med. 1972, 124, 60. [Google Scholar] [CrossRef]
- Lelii, M.; Senatore, L.; Amodeo, I.; Pinzani, R.; Torretta, S.; Fiori, S.; Marchisio, P.; Bosis, S. Kikuchi-Fujimoto Disease in Children: Two Case Reports and a Review of the Literature. Ital. J. Pediatr. 2018, 44, 83. [Google Scholar] [CrossRef]
- Olaciregui, I.; Hernandez, U.; Munoz, J.A.; Emparanza, J.I.; Landa, J.J. Markers That Predict Serious Bacterial Infection in Infants under 3 Months of Age Presenting with Fever of Unknown Origin. Arch. Dis. Child. 2009, 94, 501–505. [Google Scholar] [CrossRef]
- Otrock, Z.K.; Hock, K.G.; Riley, S.B.; de Witte, T.; Eby, C.S.; Scott, M.G. Elevated Serum Ferritin Is Not Specific for Hemophagocytic Lymphohistiocytosis. Ann. Hematol. 2017, 96, 1667–1672. [Google Scholar] [CrossRef]
- Neuman, M.I.; Hall, M.; Lipsett, S.C.; Hersh, A.L.; Williams, D.J.; Gerber, J.S.; Brogan, T.V.; Blaschke, A.J.; Grijalva, C.G.; Parikh, K.; et al. Utility of Blood Culture Among Children Hospitalized with Community-Acquired Pneumonia. Pediatrics 2017, 140, e20171013. [Google Scholar] [CrossRef]
- Cameron, L.H.; Cruz, A.T. Childhood Tuberculosis. Curr. Opin. Infect. Dis. 2022, 35, 477–483. [Google Scholar] [CrossRef]
- Sun, B.; Yang, M.; Hou, J.; Wang, W.; Ying, W.; Hui, X.; Zhou, Q.; Yao, H.; Sun, J.; Wang, X. Chromosomal Abnormalities Related to Fever of Unknown Origin in a Chinese Pediatric Cohort and Literature Review. Orphanet J. Rare Dis. 2022, 17, 292. [Google Scholar] [CrossRef] [PubMed]
- Hayani, A.; Mahoney, D.H.; Fernbach, D.J. Role of Bone Marrow Examination in the Child with Prolonged Fever. J. Pediatr. 1990, 116, 919–920. [Google Scholar] [CrossRef] [PubMed]
- Chamroonrat, W. PET/Computed Tomography in the Evaluation of Fever of Unknown Origin and Infectious/Inflammatory Disease in Pediatric Patients. PET Clin. 2020, 15, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Delgado, J.; Chauvin, N.A.; Bedoya, M.A.; Patel, S.J.; Anupindi, S.A. Whole-Body Magnetic Resonance Imaging in the Evaluation of Children with Fever without a Focus. Pediatr. Radiol. 2021, 51, 605–613. [Google Scholar] [CrossRef]
- Damasio, M.B.; Magnaguagno, F.; Stagnaro, G. Whole-Body MRI: Non-Oncological Applications in Paediatrics. Radiol. Med. 2016, 121, 454–461. [Google Scholar] [CrossRef]
- Palestro, C.J.; Brandon, D.C.; Dibble, E.H.; Keidar, Z.; Kwak, J.J. FDG PET in Evaluation of Patients with Fever of Unknown Origin: AJR Expert Panel Narrative Review. Am. J. Roentgenol. 2023, 221, 151–162. [Google Scholar] [CrossRef]
- Pijl, J.P.; Kwee, T.C.; Legger, G.E.; Peters, H.J.H.; Armbrust, W.; Schölvinck, E.H.; Glaudemans, A.W.J.M. Role of FDG-PET/CT in Children with Fever of Unknown Origin. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1596–1604. [Google Scholar] [CrossRef]
- Li, Q.; Tian, R.; Wang, H.; Li, L.; Wu, T.; Ren, Y.; Su, M.; Zou, K.; Sun, X. Quantifying the Contribution of 18F-FDG PET to the Diagnostic Assessment of Pediatric Patients with Fever of Unknown Origin: A Systematic Review and Meta-Analysis. Pediatr. Radiol. 2022, 52, 1500–1511. [Google Scholar] [CrossRef]
- Khalatbari, H.; Shulkin, B.L.; Parisi, M.T. Emerging Trends in Radionuclide Imaging of Infection and Inflammation in Pediatrics: Focus on FDG PET/CT and Immune Reactivity. Semin. Nucl. Med. 2023, 53, 18–36. [Google Scholar] [CrossRef]
- Sherman, J.M.; Sood, S.K. Current Challenges in the Diagnosis and Management of Fever. Curr. Opin. Pediatr. 2012, 24, 400–406. [Google Scholar] [CrossRef]
- Talano, J.-A.M.; Katz, B.Z. Long-Term Follow-up of Children with Fever of Unknown Origin. Clin. Pediatr. 2000, 39, 715–717. [Google Scholar] [CrossRef] [PubMed]
- Tolan, R.W. Fever of Unknown Origin: A Diagnostic Approach to This Vexing Problem. Clin. Pediatr. 2010, 49, 207–213. [Google Scholar] [CrossRef]
- Park, C.; Miranda-Garcia, M.; Berendes, R.; Horneff, G.; Kuemmerle-Deschner, J.; Ganser, G.; Huppertz, H.-I.; Minden, K.; Haas, J.-P.; Jansson, A.F.; et al. MRP8/14 Serum Levels as Diagnostic Markers for Systemic Juvenile Idiopathic Arthritis in Children with Prolonged Fever. Rheumatology 2022, 61, 3082–3092. [Google Scholar] [CrossRef] [PubMed]
- Foell, D.; Saers, M.; Park, C.; Brix, N.; Glerup, M.; Kessel, C.; Wittkowski, H.; Hinze, C.; Berntson, L.; Fasth, A.; et al. A Novel Serum Calprotectin (MRP8/14) Particle-Enhanced Immuno-Turbidimetric Assay (SCAL Turbo) Helps to Differentiate Systemic Juvenile Idiopathic Arthritis from Other Diseases in Routine Clinical Laboratory Settings. Mol. Cell Pediatr. 2023, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhu, K.; Wang, X.; Yang, Q.; Yu, S.; Zhang, Y.; Fu, Z.; Wang, H.; Zhao, Y.; Lin, K.; et al. Utility of Clinical Metagenomics in Diagnosing Malignancies in a Cohort of Patients with Epstein-Barr Virus Positivity. Front. Cell Infect. Microbiol. 2023, 13, 1211732. [Google Scholar] [CrossRef]
- Chen, H.; Tang, M.; Yao, L.; Zhang, D.; Zhang, Y.; Zhao, Y.; Xia, H.; Chen, T.; Zheng, J. Early Application of Metagenomics Next-Generation Sequencing May Significantly Reduce Unnecessary Consumption of Antibiotics in Patients with Fever of Unknown Origin. BMC Infect. Dis. 2023, 23, 478. [Google Scholar] [CrossRef]


| Infectious Causes | Noninfectious Causes | |
|---|---|---|
| Bacterial | Neoplastic Causes | |
| Localized | Abscesses | Leukemia |
| Mastoiditis | Lymphoma | |
| Sinusitis | Wilms tumor | |
| Osteomyelitis | Neuroblastoma | |
| Chronic meningitis/encephalitis | Pheochromocytoma | |
| Pyelonephritis/urinary infection | Soft-tissue sarcoma | |
| Pneumonia/empyema | Hepatocarcinoma | |
| Pericarditis | Langerhans cell histiocytosis | |
| Endocarditis | Hemophagocytic lymphohistiocytosis | |
| Mediastinitis | Macrophage activation syndrome | |
| Systemic | Bartonella henselae | Autoimmune/Inflammatory |
| Staphylococcus spp. | Juvenile idiopathic arthritis | |
| Streptococcus A and B | Systemic lupus erythematosus | |
| Pseudomonas | Kawasaki disease | |
| Streptococcus pneumoniae | Polyarteritis nodosa | |
| Hemophilus influenzae | Behçet disease | |
| Brucella | Rheumatic fever | |
| Fusobacterium | Wegener granulomatosis | |
| Kingella kingae | Sarcoidosis | |
| Mycobacteria | Antiphospholipid syndrome | |
| Mycoplasma pneumoniae | Takayasu arteritis | |
| Francisella tularensis | Ankylosing spondylitis | |
| Coxiella burneti | Chronic noninfectious osteomyelitis | |
| Salmonella sp. | Inflammatory bowel disease | |
| Enterococcus spp. | Thyroiditis | |
| Borrelia burgdoferi | ||
| Viral | Miscellaneous | |
| Cytomegalovirus (CMV) | Drug fever | |
| Epstein Barr Virus (EBV) | Diabetes insipidus | |
| Human immunodeficiency virus (HIV) | Familial dysautonomia | |
| Parvovirus B-19 (HPV-B19) | Autoinflammatory diseases | |
| Adenovirus | Kikuchi–Fujimoto disease | |
| Herpes virus (HSV) | Castleman disease | |
| Enterovirus | Sick-serum disease | |
| Hepatitis virus A, B, C, E | Sweet syndrome | |
| Influenza virus A and B | Ectodermal dysplasia | |
| SARS-CoV-2 | Pancreatitis | |
| Arbovirus | Pediatric multisystem inflammatory syndrome | |
| Fungal | Central nervous system dysfunction | |
| Blastomycosis | Immunodeficiencies | |
| Cryptococcosis | Fictitious fever | |
| Histoplasmosis | ||
| Candidiasis | ||
| Aspergillosis | ||
| Coccidioidomycosis | ||
| Parasitic | ||
| Leishmania | ||
| Toxoplasma | ||
| Malaria | ||
| Amoeba | ||
| Giardia | ||
| Family history | Familial disease | Familial dysautonomia |
| Brothers/sisters (age) | Viral infections | |
| Physiological history | Ethnicity | Familial Mediterranean Fever |
| Adoption | TB, HIV, hepatitis B and/or C, malaria, typhoid fever | |
| Kindergarten attendance | Viral infections, bacterial infections, Kingella kingae | |
| Food habits | Unpasteurized milk: TB, Brucella, Listeria, E. coli, Campylobacter Goat’s milk: Brucella, Salmonella, Listeriawater (well): Salmonella, Giardia, Campylobacter Undercooked meat: toxoplasma Picacism: Toxocara | |
| Vaccinations | Absence: invasive infections | |
| Sexual activity | Sexually transmitted infections, pelvic disease | |
| Piercings and tattoos | HIV, hepatitis B and/or C, infectious endocarditis | |
| Past medical history | Comorbidities | Diabetes, autoimmunity, nephrotic syndrome, etc. |
| Supports/devices | Bacterial infections | |
| Drugs in chronic | Drug-induced fever | |
| Travel/bathing (rivers and lakes) | Malaria, hepatitis A and/or E, Dengue, Leptospira, TB, visceral | |
| Recent history | leishmania, schistosomiasis, Lyme Disease, rickettsia, tularemia, Chikungunya, Vibrionaceae | |
| Animals/insect bites/ticks | Zoonoses | |
| At risk contacts | TB | |
| Recurrent infections | UTIs, pharyngotonsillitis, skin infections | |
| Surgical interventions | Abdominal or pelvic abscesses | |
| Drugs | Drug-induced fever | |
| Anorexia | Leukemia, lymphoma | |
| Weight loss | Leukemia, lymphoma, IBD, TB, HIV | |
| Itching, night sweats | Lymphoma | |
| Severe asthenia | EBV, leukemia, lymphoma, systemic JIA, infective endocarditis |
| Suspected Infectious Disease | Specific Test |
|---|---|
| TB exposure, travel, immigration | Mantoux test and IGRA |
| Travel to countries with malaria endemics | Thick and thin drop smearPCR plasmodium, Malaric antigen detection |
| Asthenia, lymphopenia, high AST and ALT | EBV serology |
| Contact with cats, lymphadenopathy | Bartonella serology |
| Consumption of unpasteurized dairy products | Brucella, listeria, E. coli serology |
| Picacism, ingestion of contaminated food | Toxocara, toxoplasma serology |
| Raw/undercooked meat, animal contact | Toxoplasma, tularemia serology |
| Asthenia, sexual activity, cytopenia | HIV test |
| Travel, hepatosplenomegaly | Mantoux test, stool culture, malaria research |
| Osteoarticular swelling/pain | Synovial fluid cultures, X-ray, MRI |
| Abdominal pain (abscess or UTI) | Ultrasound, CT scan |
| Suspected inflammatory disease | |
| JIA | ANA, slit lamp, ultrasound abdomen |
| SLE | ANA, C3 and C4, anti-dsDNA |
| Kawasaki disease | EKG and Echocardiogram |
| IBD | ANCA, ASCA, calprotectin, fecal occult blood test, bowel US, GI endoscopy |
| Suspected neoplasm | |
| Leukemia, HLH | LDH, uricemia, peripheral smear, myeloaspirate |
| Lymphoma | Lymph node biopsy |
| Neuroblastoma | Urinary VMA |
| Nonlocalized malignancy | Scintigraphy, WB-MRI |
| Wilms tumor | Abdomen ultrasound, CT scan of the abdomen |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trapani, S.; Fiordelisi, A.; Stinco, M.; Resti, M. Update on Fever of Unknown Origin in Children: Focus on Etiologies and Clinical Approach. Children 2024, 11, 20. https://doi.org/10.3390/children11010020
Trapani S, Fiordelisi A, Stinco M, Resti M. Update on Fever of Unknown Origin in Children: Focus on Etiologies and Clinical Approach. Children. 2024; 11(1):20. https://doi.org/10.3390/children11010020
Chicago/Turabian StyleTrapani, Sandra, Adele Fiordelisi, Mariangela Stinco, and Massimo Resti. 2024. "Update on Fever of Unknown Origin in Children: Focus on Etiologies and Clinical Approach" Children 11, no. 1: 20. https://doi.org/10.3390/children11010020
APA StyleTrapani, S., Fiordelisi, A., Stinco, M., & Resti, M. (2024). Update on Fever of Unknown Origin in Children: Focus on Etiologies and Clinical Approach. Children, 11(1), 20. https://doi.org/10.3390/children11010020