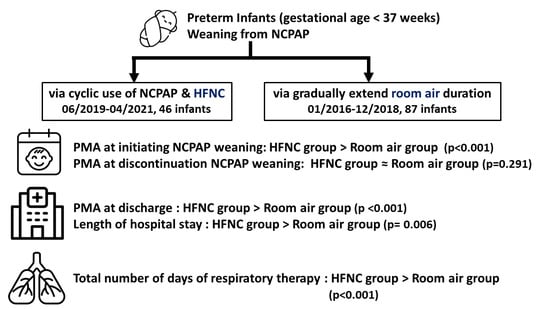
Comparison of Two Methods for Weaning from Nasal Continuous Positive Airway Pressure via the Cyclic Use of High-Flow Nasal Cannula or Room Air in Preterm Infants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Methods of NCPAP Weaning
2.2.1. HFNC Group
2.2.2. Room Air Group
2.3. Study Design
2.4. Statistics Analyses
2.5. Ethics Approval
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amatya, S.; Rastogi, D.; Bhutada, A.; Rastogi, S. Weaning of nasal CPAP in preterm infants: Who, when and how? A systematic review of the literature. World J. Pediatr. 2015, 11, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Manley, B.J.; Owen, L.S. High-Flow Nasal Cannula: Mechanisms, Evidence and Recommendations. Semin. Fetal Neonatal Med. 2016, 21, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Al-Alaiyan, S.; Dawoud, M.; Al-Hazzani, F. Positive Distending Pressure Produced by Heated, Humidified High Flow Nasal Cannula as Compared to Nasal Continuous Positive Airway Pressure in Premature Infants. J. Neonatal Perinatal. Med. 2014, 7, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.; Andersen, C.; O’Donnell, C.P.; De Paoli, A.G.; Manley, B.J. High Flow Nasal Cannula for Respiratory Support in Preterm Infants. Cochrane Database Syst. Rev. 2016, 2, CD006405. [Google Scholar] [CrossRef] [PubMed]
- Kayton, A.; Timoney, P.; Vargo, L.; Perez, J.A. A Review of Oxygen Physiology and Appropriate Management of Oxygen Levels in Premature Neonates. Adv. Neonatal Care 2018, 18, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.E.; Richardson, D.K.; McCormick, M.C.; Workman-Daniels, K.; Goldmann, D.A. Neonatal therapeutic intervention scoring system: A therapy-based severity-of-illness index. Pediatrics 1992, 90, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Weiner, G.M. Textbook of Neonatal Resuscitation®, 8th ed.; American Academy of Pediatrics: Itasca, IL, USA, 2021. [Google Scholar]
- Chen, I.L.; Chen, H.L. Impact of Illness Severity and Interventions on Successful Weaning from Nasal CPAP in Very Preterm Neonates: An Observational Study. Children 2022, 9, 673. [Google Scholar] [CrossRef] [PubMed]
- Badiee, Z.; Eshghi, A.; Mohammadizadeh, M. High flow nasal cannula as a method for rapid weaning from nasal continuous positive airway pressure. Int. J. Prev. Med. 2015, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Amatya, S.; Macomber, M.; Bhutada, A.; Rastogi, D.; Rastogi, S. Sudden versus gradual pressure wean from Nasal CPAP in preterm infants: A randomized controlled trial. J. Perinatol. 2017, 37, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Reid, S.; Lutz, T.; Malcolm, G.; Oliver, S.; Osborn, D.A. Randomised controlled trial of weaning strategies for preterm infants on nasal continuous positive airway pressure. BMC Pediatr. 2015, 15, 147. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hady, H.; Shouman, B.; Aly, H. Early weaning from CPAP to high flow nasal cannula in preterm infants is associated with prolonged oxygen requirement: A randomized controlled trial. Early Hum. Dev. 2011, 87, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Habas, F.; Durand, S.; Milési, C.; Mesnage, R.; Combes, C.; Gavotto, A.; Picaud, J.C.; Cambonie, G. 15-Year trends in respiratory care of extremely preterm infants: Contributing factors and consequences on health and growth during hospitalization. Pediatr. Pulmonol. 2020, 55, 1946–1954. [Google Scholar] [CrossRef] [PubMed]
- Su, B.H.; Lin, H.Y.; Chiu, H.Y.; Tsai, M.L.; Chen, Y.T.; Lu, I.C. Therapeutic strategy of patent ductus arteriosus in extremely preterm infants. Pediatr. Neonatol. 2020, 61, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.; Rajasekhar, H.; Gupta, A.; Bhutada, A.; Rastogi, D.; Wung, J.T. Factors Affecting the Weaning from Nasal CPAP in Preterm Neonates. Int. J. Pediatr. 2012, 2012, 416073. [Google Scholar] [CrossRef] [PubMed]
- Meister, A.L.; Doheny, K.K.; Travagli, R.A. Necrotizing enterocolitis: It’s not all in the gut. Exp. Biol. Med. 2020, 245, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Taiwan Society of Neonatology. Taiwan Neonatal Network Data 2017–2019; Taiwan Society of Neonatology: Taichung City, Taiwan, 2020. [Google Scholar]
- Wilson, A.; Gardner, M.N.; Armstrong, M.A.; Folck, B.F.; Escobar, G.J. Neonatal assisted ventilation: Predictors, frequency, and duration in a mature managed care organization. Pediatrics 2000, 105, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Murki, S.; Vardhelli, V.; Deshabhotla, S.; Sharma, D.; Pawale, D.; Kulkarni, D.; Kumar, P.; Kabra, N.S.; Sundaram, M.; Plakkal, N.; et al. Predictors of length of hospital stay among preterm infants admitted to neonatal intensive care unit: Data from a multicentre collaborative network from India (INNC: Indian National Neonatal Collaborative). J. Paediatr. Child Health 2020, 56, 1584–1589. [Google Scholar] [CrossRef] [PubMed]
- Promelle, V.; Milazzo, S. Retinopathy of Prematurity. J. Fr. Ophtalmol. 2017, 40, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Fortes Filho, J.B.; Eckert, G.U.; Valiatti, F.B.; Dos Santos, P.G.; da Costa, M.C.; Procianoy, R.S. The influence of gestational age on the dynamic behavior of other risk factors associated with retinopathy of prematurity. Graefes Arch. Clin. Exp. Ophthalmol. 2010, 248, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Behera, J.R.; Behera, G.; Sahu, S.K. Factors Influencing the Age at Discharge of Very Low Birth Weight Preterm Neonates from a Neonatal Intensive Care Unit in Eastern India: A Cohort Study. Cureus 2020, 12, e11889. [Google Scholar] [CrossRef] [PubMed]
| Item | HFNC Group (n = 46) | Room Air Group (n = 87) | p Value |
|---|---|---|---|
| GA (weeks) (mean ± SD) | 28.7 ± 2.6 | 28.4 ± 1.9 | 0.433 |
| BBW (g) (mean ± SD) | 1181 ± 354 | 1099 ± 240 | 0.165 |
| Male, n (%) | 27 (59) | 42 (48) | 0.253 |
| Vaginal delivery, n (%) | 29 (63) | 49 (56) | 0.454 |
| NTISS (mean ± SD) | 19 ± 4 | 20 ± 8 | 0.737 |
| 1′ Apgar score (mean ± SD) | 4 ± 2 | 5 ± 2 | 0.093 |
| 5′ Apgar score (mean ± SD) | 6 ± 2 | 7 ± 2 | 0.272 |
| RDS grade (mean ± SD) | 3 ± 1 | 3 ± 1 | 0.064 |
| Surfactant usage, n (%) | 23 (50) | 33 (38) | 0.180 |
| Intubation after birth, n (%) | 20 (43) | 24 (28) | 0.064 |
| Medicine for apnea, n (%) | 36 (78) | 80 (92) | 0.025 * |
| Theophylline, n (%) | 20 (43) | 67 (77) | 0.001 * |
| Caffeine, n (%) | 16 (35) | 13 (15) | 0.001 * |
| Packed RBC transfusion, n (%) | 36 (38) | 59 (62) | 0.205 |
| Moderate and severe BPD, n (%) | 20 (43) | 31 (36) | 0.376 |
| PDA, n (%) | 30 (65) | 52 (60) | 0.539 |
| Without Tx, n (%) | 6 (11) | 21 (24) | 0.130 |
| With conservative Tx, n (%) | 24 (52) | 31 (36) | 0.065 |
| Late-onset sepsis, n (%) | 16 (35) | 25 (29) | 0.473 |
| Blood culture proved, n (%) | 9 (20) | 9 (10) | 0.139 |
| Clinical diagnosis, n (%) | 7 (15) | 16 (18) | 0.645 |
| Hypotension, n (%) | 12 (26) | 8 (10) | 0.018 * |
| IVH ≥ grade III, n (%) | 3 (7) | 3 (3) | 0.417 |
| ROP ≥ stage III, n (%) | 7 (15) | 19 (22) | 0.359 |
| NEC ≥ stage II, n (%) | 1 (2) | 1 (1) | 0.644 |
| Item | Mean ± SD | p Value | |
|---|---|---|---|
| Weaned by HFNC (n = 46) | Weaned by Room Air (n = 87) | ||
| Length of Hospital Stay at Initiating NCPAP Weaning (days) | 48 ± 28 | 34 ± 26 | 0.004 * |
| PMA at Initiating NCPAP Weaning (weeks) | 35.6 ± 2.3 | 33.2 ± 2.5 | <0.001 * |
| Duration of Weaning from NCPAP to HFNC in HFNC group or to Room Air in Room Air Group (days) # | 7 ± 10 | 20 ± 11 | <0.001 * |
| PMA at Discontinuation of NCPAP (weeks) | 36.6 ± 3.0 | 36.1 ± 2.4 | 0.291 |
| Duration from Initiating NCPAP Weaning to Achieving Complete Room Air (days) | 30 ± 21 | 20 ± 11 | 0.003 * |
| Length of Hospital Stay (days) | 96 ± 38 | 78 ± 28 | 0.006 * |
| PMA at Discharge (weeks) | 42.4 ± 4.1 | 39.4 ± 2.6 | <0.001 * |
| Total Number of Days of Respiratory Therapy ^ | 80 ± 37 | 56 ± 25 | <0.001 * |
| Item | PMA at Initiating NCPAP Weaning (Weeks) | The Duration of Weaning from NCPAP to HFNC in HFNC Group or to Room Air in Room Air Group (Days) | ||||||
|---|---|---|---|---|---|---|---|---|
| Regression Coefficient | Lower 95% CI | Upper 95% CI | p Value | Regression Coefficient | Lower 95% CI | Upper 95% CI | p Value | |
| Room air–HFNC @ | −2.324 | −3.195 | −1.453 | <0.001 * | 12.796 | 8.888 | 16.706 | <0.001 * |
| GA | −0.129 | −0.342 | 0.082 | 0.229 | −0.211 | −1.210 | 0.788 | 0.676 |
| BBW | −0.001 | −0.003 | 9.948 | 0.066 | −0.001 | −0.009 | 0.006 | 0.787 |
| Female–Male ! | −0.063 | −0.976 | 0.849 | 0.891 | 1.509 | −2.759 | 5.777 | 0.486 |
| C/S-VD # | −0.348 | −1.273 | 0.576 | 0.458 | 0.899 | −3.436 | 5.235 | 0.682 |
| NTISS | 0.006 | −0.056 | 0.068 | 0.856 | 0.183 | −0.105 | 0.473 | 0.210 |
| 1′ Apgar score | −0.103 | −0.349 | 0.144 | 0.412 | 0.550 | −0.602 | 1.702 | 0.347 |
| 5′ Apgar score | −0.078 | −0.307 | 0.151 | 0.503 | 0.210 | −0.864 | 1.285 | 0.699 |
| RDS grade | 0.572 | −0.073 | 1.218 | 0.082 | −0.596 | −3.652 | 2.461 | 0.701 |
| Surfactant usage | 1.367 | 0.474 | 2.261 | 0.003 * | −0.136 | −4.463 | 4.191 | 0.950 |
| Intubation after birth | 0.666 | −0.297 | 1.629 | 0.174 | −1.365 | −5.899 | 3.169 | 0.553 |
| Apnea medicine | ||||||||
| Used–not used $ | −0.181 | −1.584 | 1.221 | 0.799 | 5.611 | −0.884 | 12.11 | 0.089 |
| Caffeine- Theophylline ^ | 1.582 | 0.494 | 2.669 | 0.005 * | −6.759 | −12.08 | −1.442 | 0.013 * |
| pRBC transfusion | 1.247 | 0.260 | 2.234 | 0.014 * | −1.858 | −6.576 | 2.860 | 0.437 |
| BPD ≥ moderate | −0.119 | −1.058 | 0.818 | 0.801 | −1.916 | −6.297 | 2.466 | 0.389 |
| PDA | ||||||||
| Without Tx | 0.009 | −1.125 | 1.144 | 0.987 | −2.871 | −8.159 | 2.417 | 0.285 |
| Conservative Tx | 1.543 | 0.656 | 2.430 | <0.001 * | −4.588 | −8.853 | −0.323 | 0.035 * |
| Late-onset sepsis | ||||||||
| B/C proved | 0.069 | −1.265 | 1.403 | 0.919 | 2.644 | −3.585 | 8.873 | 0.403 |
| Clinical diagnosis | 0.749 | −0.449 | 1.949 | 0.218 | −2.104 | −7.742 | 3.533 | 0.462 |
| Hypotension | −0.314 | −1.564 | 0.936 | 0.620 | −2.158 | −8.005 | 3.689 | 0.467 |
| IVH ≥ grade III | 0.865 | −1.328 | 3.059 | 0.436 | −4.995 | −15.25 | 5.262 | 0.337 |
| ROP ≥ stage III | 1.957 | −0.858 | 3.057 | 0.001 * | 0.032 | −5.356 | 5.419 | 0.991 |
| NEC ≥ stage II | 0.314 | −3.435 | 4.063 | 0.869 | 19.86 | 2.647 | 37.08 | 0.024 * |
| Item | Regression Coefficient | Lower 95% CI | Upper 95% CI | p Value | VIF | |
|---|---|---|---|---|---|---|
| PMA at Initiating NCPAP Weaning (weeks) | Room air-HFNC @ | −1.592 | −2.599 | −0.586 | 0.002 * | 1.158 |
| Surfactant usage | 0.717 | −0.227 | 1.661 | 0.135 | 1.177 | |
| Caffeine-Theophylline ^ | 0.918 | −0.216 | 2.053 | 0.112 | 1.289 | |
| pRBC transfusion | 0.636 | −0.649 | 1.922 | 0.329 | 1.729 | |
| PDA with Tx $ | 0.961 | 0.003 | 1.919 | 0.049 * | 1.202 | |
| ROP ≥ stage III | 0.119 | −1.178 | 1.416 | 0.856 | 1.428 | |
| Duration of Weaning from NCPAP to HFNC in HFNC Group or to Room Air in Room Air Group (days) | Room air-HFNC @ | 12.215 | 8.156 | 16.274 | <0.001 * | 1.134 |
| Caffeine-Theophylline ^ | −0.974 | −6.213 | 4.265 | 0.713 | 1.424 | |
| PDA with Tx $ | −2.409 | −6.577 | 1.758 | 0.255 | 1.282 | |
| NEC ≥ stage II | 21.569 | 6.542 | 36.598 | 0.005 * | 1.018 |
| Item | Duration from Initiating NCPAP Weaning to Achieving Complete Room Air (Days) | Total Number of Days of Respiratory Therapy | ||||||
|---|---|---|---|---|---|---|---|---|
| Regression Coefficient | Lower 95% CI | Upper 95% CI | p Value | Regression Coefficient | Lower 95% CI | Upper 95% CI | p Value | |
| Room air-HFNC @ | −10.468 | −16.077 | −4.858 | <0.001 * | −24.194 | −34.948 | −13.440 | <0.001 * |
| GA | 0.003 | −1.309 | 1.315 | 0.996 | −9.884 | −11.801 | −7.966 | <0.001 * |
| BBW | 0.005 | −0.005 | 0.014 | 0.356 | −0.067 | −0.082 | −0.052 | <0.001 * |
| Female-Male ! | −2.273 | −7.869 | 3.324 | 0.423 | −1.205 | −12.187 | 9.777 | 0.829 |
| C/S-VD # | −4.952 | −10.580 | 0.675 | 0.084 | 1.023 | −10.119 | 12.166 | 0.856 |
| NTISS | 0.048 | −0.333 | 0.429 | 0.805 | 0.712 | −0.025 | 1.448 | 0.058 |
| 1′ Apgar score | −1.410 | −2.907 | 0.087 | 0.065 | −5.738 | −8.537 | −2.939 | <0.001 * |
| 5′ Apgar score | −0.806 | −2.210 | 0.598 | 0.258 | −4.594 | −7.239 | −1.948 | <0.001 * |
| RDS grade | 3.677 | −0.284 | 7.639 | 0.069 | 9.489 | 1.807 | 17.171 | 0.016 * |
| Surfactant usage | 1.259 | −4.414 | 6.933 | 0.661 | 12.461 | 1.556 | 23.366 | 0.025 * |
| Intubation after birth | 7.329 | 1.507 | 13.151 | 0.014 * | 11.921 | 0.440 | 23.402 | 0.042 * |
| Apnea medicine | ||||||||
| Used–not used $ | −3.793 | −12.385 | 4.799 | 0.384 | 1.492 | −15.377 | 18.360 | 0.861 |
| Caffeine- Theophylline ^ | −2.264 | −8.559 | 4.029 | 0.478 | 14.977 | 1.774 | 28.180 | 0.027 * |
| pRBC transfusion | 1.758 | −4.440 | 7.956 | 0.576 | 6.226 | −5.875 | 18.327 | 0.311 |
| PDA | ||||||||
| Without Tx | −4.144 | −11.076 | 2.789 | 0.239 | −8.179 | −21.749 | 5.392 | 0.235 |
| Conservative Tx | −3.557 | −9.217 | 2.102 | 0.216 | 3.968 | −7.155 | 15.091 | 0.482 |
| BPD ≥ moderate | 0.188 | −5.578 | 5.954 | 0.949 | −0.604 | −11.891 | 10.683 | 0.916 |
| IVH ≥ grade III | −6.193 | −19.658 | 7.272 | 0.365 | 3.316 | −23.120 | 29.753 | 0.804 |
| Late-onset sepsis | ||||||||
| B/C proved | 4.019 | −4.146 | 12.185 | 0.332 | 2.634 | −13.403 | 18.671 | 0.746 |
| Clinical diagnosis | −2.392 | −9.794 | 5.009 | 0.524 | 3.259 | −11.242 | 17.760 | 0.657 |
| Hypotension | 3.863 | −3.796 | 11.522 | 0.320 | 3.449 | −11.589 | 18.488 | 0.651 |
| ROP ≥ stage III | −3.335 | −10.380 | 3.711 | 0.351 | 26.772 | 13.729 | 39.814 | <0.001 * |
| NEC ≥ stage II | 18.363 | −4.453 | 41.178 | 0.114 | −28.935 | −73.752 | 15.882 | 0.204 |
| Item | Regression Coefficient | Lower 95% CI | Upper 95% CI | p Value | VIF | |
|---|---|---|---|---|---|---|
| Duration from Initiating NCPAP Weaning to Achieving Complete Room Air (days) | Room air–HFNC @ | −9.549 | −15.168 | −3.931 | 0.001 * | 1.026 |
| Intubation after birth | 5.778 | 0.099 | 11.458 | 0.046 * | 1.026 | |
| Total Number of Days of Respiratory Therapy | Room air-HFNC @ | −28.667 | −37.396 | −19.938 | <0.001 * | 1.261 |
| GA | −7.241 | −10.129 | −4.352 | <0.001 * | 2.827 | |
| BBW | −0.031 | −0.053 | −0.009 | 0.004 * | 2.874 | |
| 1′ Apgar score | −2.176 | −6.357 | 2.005 | 0.305 | 4.567 | |
| 5′ Apgar score | 2.055 | −1.599 | 5.709 | 0.268 | 4.293 | |
| RDS grade | 3.522 | −3.211 | 10.254 | 0.302 | 1.789 | |
| Surfactant usage | 0.906 | −9.598 | 11.409 | 0.865 | 2.110 | |
| Intubation after birth | −4.601 | −13.973 | 4.771 | 0.333 | 1.475 | |
| Caffeine-Theophylline ^ | 3.053 | −6.319 | 12.426 | 0.519 | 1.274 | |
| ROP ≥ stage III | 0.830 | −8.570 | 10.231 | 0.861 | 1.086 |
| Item | Length of Hospital Stay (Days) | PMA at Discharge (Weeks) | ||||||
|---|---|---|---|---|---|---|---|---|
| Regression Coefficient | Lower 95% CI | Upper 95% CI | p Value | Regression Coefficient | Lower 95% CI | Upper 95% CI | p Value | |
| Room air-HFNC @ | −18.033 | −29.616 | −6.451 | 0.003 * | −3.044 | −4.234 | −1.853 | <0.001 * |
| GA | −6.911 | −9.298 | −4.524 | <0.001 * | −0.595 | −0.866 | −0.324 | <0.001 * |
| BBW | −0.039 | −0.058 | −0.021 | <0.001 * | −0.005 | −0.007 | −0.003 | <0.001 * |
| Female-Male ! | 1.929 | −9.484 | 13.343 | 0.739 | −0.216 | −1.454 | 1.022 | 0.731 |
| C/S-VD # | −6.329 | −17.823 | 5.204 | 0.279 | −0.006 | −1.263 | 1.251 | 0.992 |
| NTISS | 1.409 | 0.672 | 2.146 | <0.001 * | 0.035 | −0.049 | 0.119 | 0.418 |
| 1′ Apgar score | −4.976 | −7.940 | −2.011 | 0.001 * | −0.603 | −0.921 | −0.284 | <0.001 * |
| 5′ Apgar score | −3.811 | −6.606 | −1.016 | 0.008 * | −0.479 | −0.779 | −0.179 | 0.002 * |
| RDS grade | 10.305 | 2.335 | 18.274 | 0.012 * | 1.135 | 0.271 | 1.999 | 0.010 * |
| Surfactant usage | 11.148 | −0.246 | 22.541 | 0.055 | 1.097 | −0.142 | 2.337 | 0.082 |
| Intubation after birth | 32.621 | 21.886 | 43.356 | <0.001 * | 1.251 | −0.046 | 2.549 | 0.059 |
| Apnea medicine | ||||||||
| Used-not used $ | −7.408 | −24.898 | 10.083 | 0.404 | −0.639 | −2.539 | 1.259 | 0.506 |
| Caffeine- Theophylline ^ | −0.805 | −14.741 | 13.132 | 0.909 | 1.625 | 0.128 | 3.123 | 0.034 * |
| pRBC transfusion | 1.179 | −11.448 | 13.806 | 0.854 | 0.809 | −0.554 | 2.173 | 0.242 |
| PDA | 0.554 | −0.715 | 1.824 | 0.389 | ||||
| Without Tx | −5.501 | −19.652 | 8.651 | 0.443 | ||||
| Conservative Tx | −6.174 | −17.710 | 5.361 | 0.292 | −0.759 | −2.292 | 0.774 | 0.329 |
| BPD ≥ moderate | 6.599 | −5.078 | 18.278 | 0.266 | 0.710 | −0.541 | 1.961 | 0.264 |
| IVH ≥ grade III | −12.484 | −39.887 | 14.919 | 0.369 | ||||
| Late-onset sepsis | 0.967 | −0.835 | 2.769 | 0.291 | ||||
| B/C proved | −8.454 | −25.067 | 8.159 | 0.316 | −0.337 | −1.973 | 1.299 | 0.684 |
| Clinical diagnosis | 1.909 | −13.173 | 16.991 | 0.803 | 0.229 | −1.468 | 1.926 | 0.789 |
| Hypotension | −14.411 | −29.857 | 1.036 | 0.067 | −0.154 | −3.137 | 2.827 | 0.919 |
| ROP ≥ stage III | 19.173 | 5.174 | 33.172 | 0.008 * | 2.706 | 1.217 | 4.195 | <0.001 * |
| NEC ≥ stage II | −22.763 | −69.476 | 23.949 | 0.337 | −2.163 | −7.236 | 2.909 | 0.400 |
| Item | Regression Coefficient | Lower 95% CI | Upper 95% CI | p Value | VIF | |
|---|---|---|---|---|---|---|
| Length of Hospital Stay (days) | Room air-HFNC @ | −19.761 | −29.393 | −10.129 | <0.001 * | 1.141 |
| GA | −4.424 | −7.916 | −0.932 | 0.013 * | 3.027 | |
| BBW | −0.013 | −0.038 | 0.012 | 0.301 | 2.770 | |
| NTISS | 0.759 | 0.105 | 1.412 | 0.023 * | 1.254 | |
| 1′ Apgar score | −0.137 | −4.789 | 4.516 | 0.954 | 4.019 | |
| 5′ Apgar score | 3.069 | −1.154 | 7.294 | 0.153 | 3.828 | |
| RDS grade | −1.139 | −7.893 | 5.616 | 0.739 | 1.211 | |
| Intubation after birth | 28.435 | 17.476 | 39.394 | <0.001 * | 1.445 | |
| ROP ≥ stage III | 12.059 | 0.153 | 23.966 | 0.047 * | 1.212 | |
| PMA at Discharge (weeks) | Room air-HFNC @ | −3.284 | −4.567 | −2.001 | <0.001 * | 1.249 |
| GA | 0.005 | −0.421 | 0.431 | 0.979 | 2.816 | |
| BBW | −0.006 | −0.009 | −0.003 | <0.001 * | 2.773 | |
| 1′ Apgar score | −0.210 | −0.800 | 0.379 | 0.481 | 4.166 | |
| 5′ Apgar score | 0.018 | −0.506 | 0.542 | 0.945 | 4.042 | |
| RDS grade | 0.575 | −0.215 | 1.365 | 0.152 | 1.129 | |
| Caffeine-Theophylline ^ | −0.072 | −1.451 | 1.306 | 0.917 | 1.262 | |
| ROP ≥ stage III | −0.448 | −1.826 | 0.929 | 0.520 | 1.068 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.-T.; Chung, H.-W.; Chen, H.-L. Comparison of Two Methods for Weaning from Nasal Continuous Positive Airway Pressure via the Cyclic Use of High-Flow Nasal Cannula or Room Air in Preterm Infants. Children 2024, 11, 351. https://doi.org/10.3390/children11030351
Yang S-T, Chung H-W, Chen H-L. Comparison of Two Methods for Weaning from Nasal Continuous Positive Airway Pressure via the Cyclic Use of High-Flow Nasal Cannula or Room Air in Preterm Infants. Children. 2024; 11(3):351. https://doi.org/10.3390/children11030351
Chicago/Turabian StyleYang, Shu-Ting, Hao-Wei Chung, and Hsiu-Lin Chen. 2024. "Comparison of Two Methods for Weaning from Nasal Continuous Positive Airway Pressure via the Cyclic Use of High-Flow Nasal Cannula or Room Air in Preterm Infants" Children 11, no. 3: 351. https://doi.org/10.3390/children11030351
APA StyleYang, S. -T., Chung, H. -W., & Chen, H. -L. (2024). Comparison of Two Methods for Weaning from Nasal Continuous Positive Airway Pressure via the Cyclic Use of High-Flow Nasal Cannula or Room Air in Preterm Infants. Children, 11(3), 351. https://doi.org/10.3390/children11030351