Serum Biomarker Analysis in Pediatric ADHD: Implications of Homocysteine, Vitamin B12, Vitamin D, Ferritin, and Iron Levels
Abstract
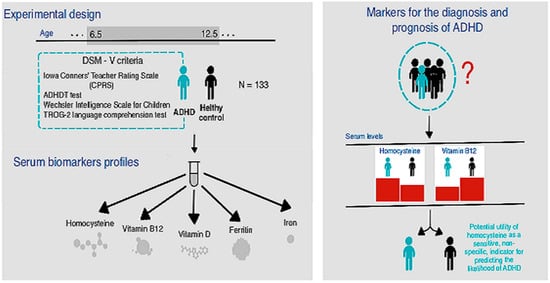
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Polanczyk, G.; Silva de Lima, M.; Horta, B.L.; Biederman, J.; Rohde, L.A. The worldwide prevalence of ADHD: A systematic review and metaregression analysis. Am. J. Psychiatry 2007, 164, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Couture, J. A review of the pathophysiology, etiology, and treatment of attention-deficit hyperactivity disorder (ADHD). Ann. Pharmacother. 2014, 48, 209–225. [Google Scholar] [CrossRef]
- Thapar, A.; Cooper, M. Attention deficit hyperactivity disorder. Lancet 2015, 387, 10024. [Google Scholar]
- Heijer, A.E.D.; Groen, Y.; Tucha, L.; Fuermaier, A.B.M.; Koerts, J.; Lange, K.W.; Thome, J.; Tucha, O. Sweat it out? The effects of physical exercise on cognition and behavior in children and adults with ADHD: A systematic literature review. J. Neural Transm. 2017, 124, 3–26. [Google Scholar] [CrossRef]
- Kooij, S.J.; Bejerot, S.; Blackwell, A.; Caci, H.; Casas-Brugué, M.; Carpentier, P.J.; Edvinsson, D.; Fayyad, J.; Foeken, K.; Fitzgerald, M.; et al. European consensus statement on diagnosis and treatment of adult ADHD: The European Network Adult ADHD. BMC Psychiatry 2010, 10, 67. [Google Scholar] [CrossRef]
- Lukovac, T.; Hil, O.A.; Popović, M.; Savić, T.; Pavlović, A.M.; Pavlović, D. Serum levels of glucose, thyroid stimulating hormone, and free thyroxine in boys diagnosed with attention deficit hyperactivity disorder: A cross-sectional pilot study. BMC Neurol. 2024, 24, 76. [Google Scholar] [CrossRef]
- Caylak, E. Biochemical and genetic analyses of childhood attention deficit/hyperactivity disorder. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2012, 159, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Ugarte, G.; Piña, R.; Contreras, D.; Godoy, F.; Rubio, D.; Rozas, C.; Zeise, M.; Vidal, R.; Escobar, J.; Morales, B. Attention Deficit-Hyperactivity Disorder (ADHD): From Abnormal Behavior to Impairment in Synaptic Plasticity. Biology 2023, 12, 1241. [Google Scholar] [CrossRef] [PubMed]
- Deb, S.; Dhaliwal, A.J.; Roy, M. The usefulness of Conners’ Rating Scales-Revised in screening for attention deficit hyperactivity disorder in children with intellectual disabilities and borderline intelligence. J. Intellect. Disabil. Res. 2008, 52, 950–965. [Google Scholar] [CrossRef]
- Cainelli, E.; Bisiacchi, P. Neurodevelopmental Disorders: Past, Present, and Future. Children 2022, 10, 31. [Google Scholar] [CrossRef]
- Alvarez-Arellano, L.; González-García, N.; Salazar-García, M.; Corona, J.C. Antioxidants as a potential target against inflammation and oxidative stress in attention-deficit/hyperactivity disorder. Antioxidants 2020, 9, 176. [Google Scholar] [CrossRef] [PubMed]
- Clemow, D.B. Misuse of methylphenidate. Non-Med. Illicit. Use Psychoact. Drugs 2017, 34, 99–124. [Google Scholar]
- Verlaet, A.A.J.; Maasakkers, C.M.; Hermans, N.; Savelkoul, H.F.J. Rationale for dietary antioxidant treatment of ADHD. Nutrients 2018, 10, 405. [Google Scholar] [CrossRef]
- Toomey, S.L.; Sox, C.M.; Rusinak, D.; Finkelstein, J.A. Why do children with ADHD discontinue their medication? Clin. Pediatr. 2012, 51, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.A.; Nigg, J.T.; Sullivan, E.L. Neuroinflammation as a risk factor for attention deficit hyperactivity disorder. Pharmacol. Biochem. Behav. 2019, 182, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Roehr, B. American Psychiatric Association explains DSM-5. BMJ Br. Med. J. 2013, 346, f3591. [Google Scholar] [CrossRef]
- Pinto, S.; Correia-De-Sá, T.; Sampaio-Maia, B.; Vasconcelos, C.; Moreira, P.; Ferreira-Gomes, J. Eating patterns and dietary interventions in ADHD: A narrative review. Nutrients 2022, 14, 4332. [Google Scholar] [CrossRef]
- Corona, J.C. Natural compounds for the management of Parkinson’s disease and attention-deficit/hyperactivity disorder. BioMed Res. Int. 2018, 2018, 4067597. [Google Scholar] [CrossRef]
- Miller, A.L. The methionine-homocysteine cycle and its effects on cognitive diseases. (Homocysteine & Cognitive). Altern. Med. Rev. 2003, 8, 7–20. [Google Scholar]
- Ioniță-Radu, F.; Nicolau, I.-N.; Petrache, O.-G.; Groșeanu, M.-L.; Bojincă, V.-C.; Negru, M.-M.; Bucurică, S.; Anghel, D. Correlation between Trabecular Bone Score and Homocysteine Level in Rheumatoid Arthritis Patients on Anti-TNF Inhibitors. Life 2024, 14, 463. [Google Scholar] [CrossRef]
- Kim, H.; Lee, K.J. Serum homocysteine levels are correlated with behavioral and psychological symptoms of Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2014, 10, 1887–1896. [Google Scholar] [PubMed]
- Kałużna-Czaplińska, J.; Michalska, M.; Rynkowski, J. Homocysteine level in urine of autistic and healthy children. Acta Biochim. Pol. 2011, 58, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Stanger, O.; Fowler, B.; Piertzik, K.; Huemer, M.; Haschke-Becher, E.; Semmler, A.; Lorenzl, S.; Linnebank, M. Homocysteine, folate and vitamin B12 in neuropsychiatric diseases: Review and treatment recommendations. Expert Rev. Neurother. 2009, 9, 1393–1412. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Shea, T.B. Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends Neurosci. 2003, 26, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Bottiglieri, T. Folate, vitamin B12, and neuropsychiatric disorders. Nutr. Rev. 1996, 54, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Luzzi, S.; Cherubini, V.; Falsetti, L.; Viticchi, G.; Silvestrini, M.; Toraldo, A. Homocysteine, cognitive functions, and degenerative dementias: State of the art. Biomedicines 2022, 10, 2741. [Google Scholar] [CrossRef] [PubMed]
- Cavalca, V.; Cighetti, G.; Bamonti, F.; Loaldi, A.; Bortone, L.; Novembrino, C.; De Franceschi, M.; Belardinelli, R.; Guazzi, M.D. Oxidative stress and homocysteine in coronary artery disease. Clin. Chem. 2001, 47, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R.; Herrmann, W. Mechanisms of homocysteine neurotoxicity in neurodegenerative diseases with special reference to dementia. FEBS Lett. 2006, 580, 2994–3005. [Google Scholar] [CrossRef] [PubMed]
- Kataria, N.; Yadav, P.; Kumar, R.; Kumar, N.; Singh, M.; Kant, R.; Kalyani, V. Effect of vitamin B6, B9, and B12 supplementation on homocysteine level and cardiovascular outcomes in stroke patients: A meta-analysis of randomized controlled trials. Cureus 2021, 13, e14958. [Google Scholar] [CrossRef]
- Gilfix, B.M. Vitamin B12 and homocysteine. CMAJ 2005, 173, 1360. [Google Scholar] [CrossRef]
- Yektaş, Ç.; Alpay, M.; Tufan, A.E. Comparison of serum B12, folate and homocysteine concentrations in children with autism spectrum disorder or attention deficit hyperactivity disorder and healthy controls. Neuropsychiatr. Dis. Treat. 2019, 15, 2213–2219. [Google Scholar] [CrossRef] [PubMed]
- Fuller, J.A.; Burrell, M.H.; Yee, A.G.; Liyanagama, K.; Lipski, J.; Wickens, J.R.; Hyland, B.I. Role of homeostatic feedback mechanisms in modulating methylphenidate actions on phasic dopamine signaling in the striatum of awake behaving rats. Prog. Neurobiol. 2019, 182, 101681. [Google Scholar] [CrossRef]
- Miranda, A.; Tárraga, R.; Fernández, M.I.; Colomer, C.; Pastor, G. Parenting stress in families of children with autism spectrum disorder and ADHD. Except. Child. 2015, 82, 81–95. [Google Scholar] [CrossRef]
- Bressenot, A.; Pooya, S.; Bossenmeyer-Pourie, C.; Gauchotte, G.; Germain, A.; Chevaux, J.-B.; Coste, F.; Vignaud, J.-M.; Guéant, J.-L.; Peyrin-Biroulet, L. Methyl donor deficiency affects small-intestinal differentiation and barrier function in rats. Br. J. Nutr. 2013, 109, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Poulose, S.M.; Miller, M.G.; Scott, T.; Shukitt-Hale, B. Nutritional factors affecting adult neurogenesis and cognitive function. Adv. Nutr. 2017, 8, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.-L.; Chen, Y.-J.; Shang, C.-Y.; Tseng, W.-Y.I.; Gau, S.S.-F. Different neural substrates for executive functions in youths with ADHD: A diffusion spectrum imaging tractography study. Psychol. Med. 2016, 46, 1225–1238. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.; Becker, A.; Sundermann, J.; Rothenberger, A.; Herrmann-Lingen, C. Attention deficit-hyperactivity disorder is associated with reduced blood pressure and serum vitamin D levels: Results from the nationwide German Health Interview and Examination Survey for Children and Adolescents (KiGGS). Eur. Child Adolesc. Psychiatry 2017, 26, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.; Bener, A.; Ehlayel, M.S. Is high prevalence of vitamin D deficiency a correlate for attention deficit hyperactivity disorder? ADHD Atten. Deficit Hyperact. Disord. 2014, 6, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Józefowicz, O.; Rabe-Jabłońska, J.; Woźniacka, A.; Strzelecki, D. Analysis of vitamin D status in major depression. J. Psychiatr. Pract. 2014, 20, 329–337. [Google Scholar] [CrossRef]
- Bradstreet, J.J.; Smith, S.; Baral, M.; Rossignol, D.A. Biomarker-guided interventions of clinically relevant conditions associated with autism spectrum disorders and attention deficit hyperactivity disorder. Altern. Med. Rev. 2010, 15, 15–32. [Google Scholar]
- Fabrazzo, M.; Agnese, S.; Cipolla, S.; Di Vincenzo, M.; Mancuso, E.; Volpicelli, A.; Perris, F.; Sampogna, G.; Catapano, F.; Fiorillo, A.; et al. Vitamin D Deficiency and Risk Factors Related to Acute Psychiatric Relapses in Patients with Severe Mental Disorders: A Preliminary Study. Brain Sci. 2022, 12, 973. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.W.; Smith, S.; Kinobe, R.; Hewison, M.; McGrath, J.J. Distribution of the vitamin D receptor and 1α-hydroxylase in human brain. J. Chem. Neuroanat. 2005, 29, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Neyestani, T.R.; Hajifaraji, M.; Omidvar, N.; Eshraghian, M.R.; Shariatzadeh, N.; Kalayi, A.; Gharavi, A.; Khalaji, N.; Haidari, H.; Zowghi, T.; et al. High prevalence of vitamin D deficiency in school-age children in Tehran, 2008: A red alert. Public Health Nutr. 2012, 15, 324–330. [Google Scholar] [CrossRef]
- Mohammadpour, N.; Jazayeri, S.; Tehrani-Doost, M.; Djalali, M.; Hosseini, M.; Effatpanah, M.; Davari-Ashtiani, R.; Karami, E. Effect of vitamin D supplementation as adjunctive therapy to methylphenidate on ADHD symptoms: A randomized, double blind, placebo-controlled trial. Nutr. Neurosci. 2018, 21, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Robberecht, H.; Verlaet, A.A.J.; Breynaert, A.; De Bruyne, T.; Hermans, N. Magnesium, iron, zinc, copper and selenium status in attention-deficit/hyperactivity disorder (ADHD). Molecules 2020, 25, 4440. [Google Scholar] [CrossRef] [PubMed]
- Lozoff, B.; Beard, J.; Connor, J.; Felt, B.; Georgieff, M.; Schallert, T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr. Rev. 2006, 64 (Suppl. S2), S34–S43. [Google Scholar] [CrossRef] [PubMed]
- Santa-Marina, L.; Lertxundi, N.; Andiarena, A.; Irizar, A.; Sunyer, J.; Molinuevo, A.; Llop, S.; Julvez, J.; Beneito, A.; Ibarluzea, J.; et al. Maternal ferritin levels during pregnancy and ADHD symptoms in 4-year-old children: Results from the INMA–infancia y medio ambiente (environment and childhood) prospective birth cohort study. Int. J. Environ. Res. Public Health 2020, 17, 7704. [Google Scholar] [CrossRef] [PubMed]
- McDowell, L. Reevaluation of the metabolic essentiality of the vitamins-review. Asian-Australas. J. Anim. Sci. 2000, 13, 115–125. [Google Scholar] [CrossRef]
- Dereboy, C.; Senol, S.; Sener, S.; Dereboy, F. Validation of the Turkish versions of the short-form Conners’ teacher and parent rating scales. Turk Psikiyatr. Derg. 2007, 18, 48. [Google Scholar]
- Franzen, M.D. The Wechsler Intelligence Scales for Children—The WISC-R, WISC-III, and WPPSI-R. In Reliability and Validity in Neuropsychological Assessment; Springer: Berlin/Heidelberg, Germany, 2000; pp. 71–89. [Google Scholar]
- Bishop, D.V. Test for Reception of Grammar: Test for Reception of Grammar Version TROG-2 Stimulus Book, 1st ed.; Harcourt Assessment: San Antonio, TX, USA, 2003. [Google Scholar]
- Mutlu, G.Y.; Hatun, Ş. Use of vitamin D in children and adults: Frequently asked questions. J. Clin. Res. Pediatr. Endocrinol. 2018, 10, 301. [Google Scholar]
- Moretti, R.; Caruso, P. The controversial role of homocysteine in neurology: From labs to clinical practice. Int. J. Mol. Sci. 2019, 20, 231. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Siracusa, R.; Fusco, R.; Cuzzocrea, S.; Di Paola, R.; Impellizzeri, D. Involvements of hyperhomocysteinemia in neurological disorders. Metabolites 2021, 11, 37. [Google Scholar] [CrossRef]
- Kruman, I.I.; Culmsee, C.; Chan, S.L.; Kruman, Y.; Guo, Z.; Penix, L.; Mattson, M.P. Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity. J. Neurosci. 2000, 20, 6920–6926. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.I.; Ortiz, D.; Rogers, E.; Shea, T.B. Multiple aspects of homocysteine neurotoxicity: Glutamate excitotoxicity, kinase hyperactivation and DNA damage. J. Neurosci. Res. 2002, 70, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, P.; Singh, N. Homocysteine excess: Delineating the possible mechanism of neurotoxicity and depression. Fundam. Clin. Pharmacol. 2015, 29, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.K.; Aboud, K.S.; Nguyen, T.Q.; Cutting, L.E. Applying a network framework to the neurobiology of reading and dyslexia. J. Neurodev. Disord. 2018, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.M.; Boy, E.; Miller, J.W.; Green, R.; Sabel, J.C.; Allen, L.H. High prevalence of cobalamin deficiency in Guatemalan schoolchildren: Associations with low plasma holotranscobalamin II and elevated serum methylmalonic acid and plasma homocysteine concentrations. Am. J. Clin. Nutr. 2003, 77, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Refsum, H.; Ueland, P.M.; Nygård, O.; Vollset, S.E. Homocysteine and cardiovascular disease. Annu. Rev. Med. 1998, 49, 31–62. [Google Scholar] [CrossRef] [PubMed]
- Altun, H.; Sahin, N.; Kurutas, E.B.; Gungor, O. Homocysteine, pyridoxine, folate and vitamin B12 levels in children with attention deficit hyperactivity disorder. Psychiatr. Danub. 2018, 30, 310–316. [Google Scholar] [CrossRef]
- Azzini, E.; Ruggeri, S.; Polito, A. Homocysteine: Its possible emerging role in at-risk population groups. Int. J. Mol. Sci. 2020, 21, 1421. [Google Scholar] [CrossRef]
- Saha, T.; Chatterjee, M.; Verma, D.; Ray, A.; Sinha, S.; Rajamma, U.; Mukhopadhyay, K. Genetic variants of the folate metabolic system and mild hyperhomocysteinemia may affect ADHD associated behavioral problems. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 84, 1–10. [Google Scholar] [CrossRef]
- De la Torre-Iturbe, S.; Vázquez-Roque, R.A.; De la Cruz-López, F.; Flores, G.; Garcés-Ramírez, L. Dendritic and behavioral changes in rats neonatally treated with homocysteine; A proposal as an animal model to study the attention deficit hyperactivity disorder. J. Chem. Neuroanat. 2022, 119, 102057. [Google Scholar] [CrossRef]
- Deth, R.; Muratore, C.; Benzecry, J.; Power-Charnitsky, V.-A.; Waly, M. How environmental and genetic factors combine to cause autism: A redox/methylation hypothesis. Neurotoxicology 2008, 29, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Pavlović, D. Vitamin B12, Vitamin D and Homocysteine–Trio of Health and Disease; OrionArt: Belgrade, Serbia, 2018; p. 152. [Google Scholar]
- Reynolds, E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol. 2006, 5, 949–960. [Google Scholar] [CrossRef]
- Lukovac, T.; Pavlović, D. Attention deficit hyperactivity disorder and micronutrities. Engrami 2019, 41, 46–59. [Google Scholar] [CrossRef]
- Oh, R.; Brown, D.L. Vitamin B12 Deficiency. Am. Fam. Physician. 2003, 67, 979–986. [Google Scholar] [PubMed]
- Aparicio-Ugarriza, R.; Palacios, G.; Alder, M.; González-Gross, M. A review of the cut-off points for the diagnosis of vitamin B12 deficiency in the general population. Clin. Chem. Lab. Med. 2015, 53, 1149–1159. [Google Scholar] [CrossRef]
- Bala, K.A.; Doğan, M.; Kaba, S.; Mutluer, T.; Aslan, O.; Doğan, S.Z. Hormone disorder and vitamin deficiency in attention deficit hyperactivity disorder (ADHD) and autism spectrum disorders (ASDs). J. Pediatr. Endocrinol. Metab. 2016, 29, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xie, Z.; Gu, P.; Li, X.; Zhang, Y.; Wang, X.; Chen, Z.; Deng, S.; Shu, Y.; Li, J.-D. Cry1Δ11 mutation induces ADHD-like symptoms through hyperactive dopamine D1 receptor signaling. JCI Insight 2023, 8, e170434. [Google Scholar] [CrossRef]
- Mathew, A.R.; Di Matteo, G.; La Rosa, P.; Barbati, S.A.; Mannina, L.; Moreno, S.; Tata, A.M.; Cavallucci, V.; Fidaleo, M. Vitamin B12 Deficiency and the Nervous System: Beyond Metabolic Decompensation—Comparing Biological Models and Gaining New Insights into Molecular and Cellular Mechanisms. Int. J. Mol. Sci. 2024, 25, 590. [Google Scholar] [CrossRef]
- Monasso, G.S.; Hoang, T.T.; Mancano, G.; Fernández-Barrés, S.; Dou, J.; Jaddoe, V.W.; Page, C.M.; Johnson, L.; Bustamante, M.; Bakulski, K.M.; et al. A meta-analysis of epigenome-wide association studies on pregnancy vitamin B12 concentrations and offspring DNA methylation. Epigenetics 2023, 18, 2202835. [Google Scholar] [CrossRef] [PubMed]
- Pino, J.M.V.; da Luz, M.H.M.; Antunes, H.K.M.; Giampá, S.Q.d.C.; Martins, V.R.; Lee, K.S. Iron-restricted diet affects brain ferritin levels, dopamine metabolism and cellular prion protein in a region-specific manner. Front. Mol. Neurosci. 2017, 10, 145. [Google Scholar] [CrossRef] [PubMed]
- Lange, K.W.; Lange, K.M.; Nakamura, Y.; Reissmann, A. Nutrition in the management of ADHD: A review of recent research. Curr. Nutr. Rep. 2023, 12, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, J.; Johansson, J.; Falahati, F.; Andersson, M.; Papenberg, G.; Avelar-Pereira, B.; Bäckman, L.; Kalpouzos, G.; Salami, A. The iron-dopamine D1 coupling modulates neural signatures of working memory across adult lifespan. NeuroImage 2023, 279, 120323. [Google Scholar] [CrossRef] [PubMed]
- Konofal, E.; Lecendreux, M.; Arnulf, I.; Mouren, M.-C. Iron deficiency in children with attention-deficit/hyperactivity disorder. Arch. Pediatr. Adolesc. Med. 2004, 158, 1113–1115. [Google Scholar] [CrossRef] [PubMed]
- Tseng, P.-T.; Cheng, Y.-S.; Yen, C.-F.; Chen, Y.-W.; Stubbs, B.; Whiteley, P.; Carvalho, A.F.; Li, D.-J.; Chen, T.-Y.; Tang, C.-H.; et al. Peripheral iron levels in children with attention-deficit hyperactivity disorder: A systematic review and meta-analysis. Sci. Rep. 2018, 8, 788. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Darakhshan, R.; Bagherian, A. Is there an association between early childhood caries and serum iron and serum ferritin levels? Dent. Res. J. 2012, 9, 294. [Google Scholar]
- Öztürk, Y.; Topal, Z.; Demir, N.; Tufan, A.E. Iron and Ferritin Levels of Children and Adolescents with Attention Deficit Hyperactivity Disorder and Attention Deficit Hyperactivity Disorder-Not Otherwise Specified. J. Pediatr. Res. 2020, 7, 216–222. [Google Scholar] [CrossRef]
- Donfrancesco, R.; Parisi, P.; Vanacore, N.; Martines, F.; Sargentini, V.; Cortese, S. Iron and ADHD: Time to move beyond serum ferritin levels. J. Atten. Disord. 2013, 17, 347–357. [Google Scholar] [CrossRef]
- Kwon, H.J.; Lim, M.H.; Ha, M.; Kim, E.J.; Yoo, S.J.; Kim, J.W.; Paik, K.C. Transferrin in Korean children with attention deficit hyperactivity disorder. Psychiatry Investig. 2011, 8, 366. [Google Scholar] [CrossRef]
- Menegassi, M.; de Mello, E.D.; Guimarães, L.R.; Matte, B.C.; Driemeier, F.; Pedroso, G.L.; Rohde, L.A.; Schmitz, M. Food intake and serum levels of iron in children and adolescents with attention-deficit/hyperactivity disorder. Braz. J. Psychiatry 2010, 32, 132–138. [Google Scholar] [CrossRef]
- Mahmoud, M.M.; El-Mazary, A.-A.M.; Maher, R.M.; Saber, M.M. Zinc, ferritin, magnesium and copper in a group of Egyptian children with attention deficit hyperactivity disorder. Ital. J. Pediatr. 2011, 37, 60. [Google Scholar] [CrossRef] [PubMed]
- Juneja, M.; Jain, R.; Singh, V.; Mallika, V. Iron deficiency in Indian children with attention deficit hyperactivity disorder. Indian Pediatr. 2010, 47, 955–958. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, L.; Zhang, L.; Qu, Y.; Mu, D. Iron status in attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0169145. [Google Scholar] [CrossRef] [PubMed]
- Degremont, A.; Jain, R.; Philippou, E.; Latunde-Dada, G.O. Brain iron concentrations in the pathophysiology of children with attention deficit/hyperactivity disorder: A systematic review. Nutr. Rev. 2021, 79, 615–626. [Google Scholar] [CrossRef]
- Szodoray, P.; Nakken, B.; Gaal, J.; Jonsson, R.; Szegedi, A.; Zold, E.; Szegedi, G.; Brun, J.G.; Gesztelyi, R.; Zeher, M.; et al. The complex role of vitamin D in autoimmune diseases. Scand. J. Immunol. 2008, 68, 261–269. [Google Scholar] [CrossRef]
- Saidi, L.; Hammou, H.; Sicard, F.; Landrier, J.-F.; Mounien, L. Maternal vitamin D deficiency and brain functions: A never-ending story. Food Funct. 2023, 14, 6290–6301. [Google Scholar] [CrossRef]
- Berridge, M.J. Vitamin D deficiency: Infertility and neurodevelopmental diseases (attention deficit hyperactivity disorder, autism, and schizophrenia). Am. J. Physiol.-Cell Physiol. 2018, 314, C135–C151. [Google Scholar] [CrossRef] [PubMed]
- Massoodi, A.; Koutanaei, S.J.; Faraz, Z.; Geraili, Z.; Zavarmousavi, S.M. Comparison of serum vitamin D levels between healthy and ADHD children. Casp. J. Intern. Med. 2023, 14, 681. [Google Scholar]
- Pavlović, D.M.; Pavlovic, A.M. Vitamin D across the life span. Spec. Edukac. I Rehabil. 2014, 13, 117–132. [Google Scholar]
- Gouveri, E.; Papanas, N.; Hatzitolios, A.; Maltezos, E. Hypovitaminosis D and peripheral arterial disease: Emerging link beyond cardiovascular risk factors. Eur. J. Intern. Med. 2012, 23, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Wang, Z.; Wang, J.; Xu, H.; Zhou, H. Serum vitamin D levels among children aged 0–12 years in the First Affiliated Hospital of Harbin Medical University, China. J. Public Health 2018, 40, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, P.; Rylander, L.; Lindh, C.H.; Jönsson, B.A.G.; Ode, A.; Olofsson, P.; Ivarsson, S.A.; Rignell-Hydbom, A.; Haglund, N.; Källén, K. Vitamin D status at birth and future risk of attention deficit/hyperactivity disorder (ADHD). PLoS ONE 2015, 10, e0140164. [Google Scholar] [CrossRef] [PubMed]
- Sharif, M.R.; Madani, M.; Tabatabaei, F.; Tabatabaee, Z. The relationship between serum vitamin D level and attention deficit hyperactivity disorder. Iran. J. Child Neurol. 2015, 9, 48. [Google Scholar] [PubMed]
- Goksugur, S.B.; Tufan, A.E.; Semiz, M.; Gunes, C.; Bekdas, M.; Tosun, M.; Demircioglu, F. Vitamin D status in children with attention-deficit–hyperactivity disorder. Pediatr. Int. 2014, 56, 515–519. [Google Scholar] [CrossRef]
- Sahin, N.; Altun, H.; Kurutas, E.B.; Balkan, D. Vitamin D and vitamin D receptor levels in children with attention-deficit/hyperactivity disorder. Neuropsychiatr. Dis. Treat. 2018, 14, 581–585. [Google Scholar] [CrossRef]
- Chaplin, G.; Jablonski, N.G. Vitamin D and the Evolution of Human Depigmentation; Wiley-Liss Div John Wiley & Sons Inc.: Hoboken, NJ, USA, 2009; pp. 451–461. [Google Scholar]
| Parameter | HC | ADHD |
|---|---|---|
| ADHDT | 63.15 ± 27.26 | 98.81 ± 16.05 |
| WISC-R scores | ||
| – Verbal IQ | 110.38 ± 12.84 | 102.79 ± 12.66 |
| – Non-verbal IQ | 112.65 ± 12.64 | 105.91 ± 12.90 |
| – Total IQ | 111.56 ± 12.05 | 104.04 ± 11.47 |
| Parameter | ADHDT | WISC-R Test | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Verbal IQ | Non-Verbal IQ | Total IQ | |||||||||||
| df | MS | F | p | MS | F | p | MS | F | p | MS | F | p | |
| Group | 1 | 33,835 | 66.491 | 3.153 × 10−13 *** | 1478 | 9.203 | 0.003 *** | 1861 | 12.103 | 0.001 *** | 1815 | 15.039 | 1.692 × 10−4 *** |
| Age | 3 | 337.3 | 0.663 | 0.576 | 296 | 1.845 | 0.142 | 222 | 1.443 | 0.233 | 279 | 2.312 | 0.079 |
| Group × age | 3 | 201 | 0.395 | 0.757 | 30 | 0.19 | 0.903 | 291 | 1.894 | 0.134 | 110 | 0.915 | 0.436 |
| Error | 125 | 508.9 | 161 | 154 | 121 | ||||||||
| Parameter | HC | ADHD |
|---|---|---|
| Iron (μmol/L) | 13.75 ± 3.78 | 16.99 ± 5.69 |
| Ferritin (ng/mL) | 32.72 ± 15.51 | 40.39 ± 18.67 |
| Hcy (μmol/L) | 6.58 ± 1.15 | 8.78 ± 1.75 |
| Vitamin B12 (pg/L) | 469.87 ± 174.37 | 359.66 ± 174.58 |
| Vitamin D (nmol/L) | 64.97 ± 15.52 | 64.16 ± 22.59 |
| Parameter | Age | ADHDT | WISC-R Test | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IQ Verbal | IQ Non-Verbal | IQ Total | ||||||||
| r | p | r | p | r | p | r | p | r | p | |
| Iron | 0.050 | 0.689 | −0.102 | 0.410 | 0.086 | 0.487 | 0.039 | 0.753 | 0.105 | 0.400 |
| Ferritin | −0.085 | 0.496 | −0.021 | 0.863 | 0.055 | 0.660 | −0.164 | 0.185 | −0.062 | 0.620 |
| Hcy | 0.211 | 0.086 | −0.233 | 0.058 | −0.048 | 0.703 | 0.061 | 0.626 | −0.006 | 0.960 |
| Vitamin B12 | −0.135 | 0.276 | 0.186 | 0.132 | −0.039 | 0.756 | −0.029 | 0.819 | −0.057 | 0.647 |
| Vitamin D | −0.093 | 0.456 | −0.267 | 0.029 * | −0.098 | 0.430 | −0.207 | 0.092 | −0.216 | 0.079 |
| Parameter | B | SE | Wald χ2 Test | df | p | Exp(B) |
|---|---|---|---|---|---|---|
| Iron | 0.145 | 0.056 | 6.792 | 1 | 0.009 ** | 1.156 |
| Ferritin | 0.028 | 0.015 | 3.393 | 1 | 0.065 | 1.029 |
| Hcy | 1.245 | 0.251 | 24.683 | 1 | 0.000 *** | 3.474 |
| Vitamin B12 | −0.003 | 0.002 | 2.879 | 1 | 0.090 | 0.997 |
| Vitamin D | −0.004 | 0.013 | 0.082 | 1 | 0.775 | 0.996 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukovac, T.; Hil, O.A.; Popović, M.; Jovanović, V.; Savić, T.; Pavlović, A.M.; Pavlović, D. Serum Biomarker Analysis in Pediatric ADHD: Implications of Homocysteine, Vitamin B12, Vitamin D, Ferritin, and Iron Levels. Children 2024, 11, 497. https://doi.org/10.3390/children11040497
Lukovac T, Hil OA, Popović M, Jovanović V, Savić T, Pavlović AM, Pavlović D. Serum Biomarker Analysis in Pediatric ADHD: Implications of Homocysteine, Vitamin B12, Vitamin D, Ferritin, and Iron Levels. Children. 2024; 11(4):497. https://doi.org/10.3390/children11040497
Chicago/Turabian StyleLukovac, Tanja, Olivera Aleksić Hil, Milka Popović, Vitomir Jovanović, Tatjana Savić, Aleksandra M. Pavlović, and Dragan Pavlović. 2024. "Serum Biomarker Analysis in Pediatric ADHD: Implications of Homocysteine, Vitamin B12, Vitamin D, Ferritin, and Iron Levels" Children 11, no. 4: 497. https://doi.org/10.3390/children11040497
APA StyleLukovac, T., Hil, O. A., Popović, M., Jovanović, V., Savić, T., Pavlović, A. M., & Pavlović, D. (2024). Serum Biomarker Analysis in Pediatric ADHD: Implications of Homocysteine, Vitamin B12, Vitamin D, Ferritin, and Iron Levels. Children, 11(4), 497. https://doi.org/10.3390/children11040497