Emerging Links between Microbiome Composition and Skin Immunology in Diaper Dermatitis: A Narrative Review
Abstract
:1. Introduction
2. Methods and Materials
3. Results
3.1. Early Fetal and Postnatal Microbiome
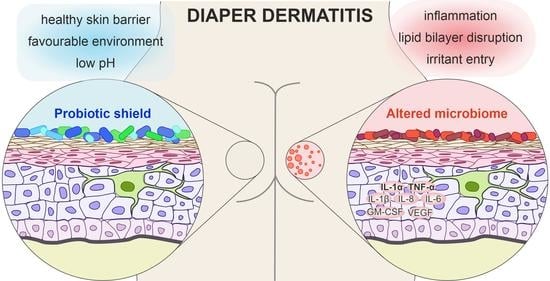
3.2. The Microbiome in Diaper Dermatitis
3.3. Inflammation in Diaper Dermatitis
3.4. Role of Skin pH
3.5. Inflammatory Signaling
3.6. Probiotics as a “Protective Shield” against Skin Inflammation
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benitez Ojeda, A.B.; Mendez, M.D. Diaper Dermatitis. In StatPearls Publishing. Updated 21 July 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK559067/ (accessed on 13 January 2021).
- Klunk, C.; Domingues, E.; Wiss, K. An update on diaper dermatitis. Clin. Dermatol. 2014, 32, 477–487. [Google Scholar] [CrossRef]
- Tüzün, Y.; Wolf, R.; Bağlam, S.; Engin, B. Diaper (napkin) dermatitis: A fold (intertriginous) dermatosis. Clin. Dermatol. 2015, 33, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Blume-Peytavi, U.; Kanti, V. Prevention and treatment of diaper dermatitis. Pediatr. Dermatol. 2018, 35, s19–s23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, B. Differential diagnosis of diaper dermatitis. Clin. Pediatr. 2017, 56 (Suppl. 5), 16S–22S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atherton, D.J. Understanding irritant napkin dermatitis. Int. J. Dermatol. 2016, 55 (Suppl. 1), 7–9. [Google Scholar] [CrossRef] [Green Version]
- Sukhneewat, C.; Chaiyarit, J.; Techasatian, L. Diaper dermatitis: A survey of risk factors in Thai children aged under 24 months. BMC Dermatol. 2019, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Garcia Bartels, N.; Massoudy, L.; Scheufele, R.; Dietz, E.; Proquitté, H.; Wauer, R.; Bertin, C.; Serrano, J.; Blume-Peytavi, U. Standardized diaper care regimen: A prospective, randomized pilot study on skin barrier function and epidermal IL-1α in newborns. Pediatr. Dermatol. 2012, 29, 270–276. [Google Scholar] [CrossRef]
- Garcia Bartels, N.; Lünnemann, L.; Stroux, A.; Kottner, J.; Serrano, J.; Blume-Peytavi, U. Effect of diaper cream and wet wipes on skin barrier properties in infants: A prospective randomized controlled trial. Pediatr. Dermatol. 2014, 31, 683–691. [Google Scholar] [CrossRef]
- Zhuang, L.; Gu, H.; Huang, Y.; Li, X.; Lu, Y.; Kaku, K. Development of a new diaper dermatitis-like reconstructed skin equivalent for testing children atopic dermatitis relieving cosmetics. Skin Res. Technol. 2019, 25, 839–845. [Google Scholar] [CrossRef]
- Perkins, M.A.; Osterhues, M.A.; Farage, M.A.; Robinson, M.K. A noninvasive method to assess skin irritation and compromised skin conditions using simple tape adsorption of molecular markers of inflammation. Skin Res. Technol. 2001, 7, 227–237. [Google Scholar] [CrossRef]
- Šikić Pogačar, M.; Maver, U.; Marčun Varda, N.; Mičetić-Turk, D. Diagnosis and management of diaper dermatitis in infants with emphasis on skin microbiota in the diaper area. Int. J. Dermatol. 2018, 57, 265–275. [Google Scholar] [CrossRef]
- Schommer, N.N.; Gallo, R.L. Structure and function of the human skin microbiome. Trends Microbiol. 2013, 21, 660–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, A.M.; Nelson, A.M. Skin microbiota: Friend or foe in pediatric skin health and skin disease. Pediatr. Dermatol. 2019, 36, 815–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoch, J.J.; Monir, R.L.; Satcher, K.G.; Harris, J.; Triplett, E.; Neu, J. The infantile cutaneous microbiome: A review. Pediatr. Dermatol. 2019, 36, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Casterline, B.W.; Paller, A.S. Early development of the skin microbiome: Therapeutic opportunities. Pediatr. Res. 2021, 90, 731–737. [Google Scholar] [CrossRef]
- Younge, N.; McCann, J.R.; Ballard, J.; Plunkett, C.; Akhtar, S.; Araújo-Pérez, F.; Murtha, A.; Brandon, D.; Seed, P.C. Fetal exposure to the maternal microbiota in humans and mice. JCI Insight 2019, 4, e127806. [Google Scholar] [CrossRef] [Green Version]
- Krieger, Y.; Horev, A.; Wainstock, T.; Sheiner, E.; Walfisch, A. Meconium-stained amniotic fluid as a protective factor against childhood dermatitis and skin rash-related hospitalization in the offspring—A population-based cohort analysis. J. Eur. Acad Dermatol. Venereol. 2020, 34, 319–324. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, E.A.; Connolly, J.; Hourihane, J.O.; Fallon, P.; McLean, W.I.; Murray, D.; Jo, J.-H.; Segre, J.A.; Kong, H.H.; Irvine, A.D. Skin microbiome before development of atopic dermatitis: Early colonization with commensal staphylococci at 2 months is associated with a lower risk of atopic dermatitis at 1 year. J. Allergy Clin. Immunol. 2017, 139, 166–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capone, K.A.; Dowd, S.E.; Stamatas, G.N.; Nikolovski, J. Diversity of the human skin microbiome early in life. J. Investig. Dermatol. 2011, 131, 2026–2032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef]
- Chu, D.M.; Ma, J.; Prince, A.L.; Antony, K.M.; Seferovic, M.D.; Aagaard, K.M. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat. Med. 2017, 23, 314–326. [Google Scholar] [CrossRef] [Green Version]
- Gaitanis, G.; Tsiouri, G.; Spyridonos, P.; Stefos, T.; Stamatas, G.N.; Velegraki, A.; Bassukas, I.D. Variation of cultured skin microbiota in mothers and their infants during the first year postpartum. Pediatr. Dermatol. 2019, 36, 460–465. [Google Scholar] [CrossRef]
- Mattila-Sandholm, T.; Blum, S. Probiotics: Towards demonstrating efficacy. Trends Food Sci. Technol. 1999, 10, 393–399. [Google Scholar] [CrossRef]
- Harmsen, H.J.M.; Wildeboer–Veloo, A.C.M.; Raangs, G.C.; Wagendorp, A.A.; Klijn, N.; Bindels, J.G.; Welling, G.W. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J. Pediatr. Gastroenterol. Nutr. 2000, 30, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Caramia, G.; Atzei, A.; Fanos, V. Probiotics and the skin. Clin. Dermatol. 2008, 26, 4–11. [Google Scholar] [CrossRef]
- Mičetić Turk, D.; Turk, E.; Šikić Pogačar, M. Historical overview of breastfeeding in Slovenia. Acta Med.-Biotech. 2017, 10, 18–24. [Google Scholar]
- Kumbhare, S.V.; Patangia, D.V.; Patil, R.H.; Shouche, Y.S.; Patil, N.P. Factors influencing the gut microbiome in children: From infancy to childhood. J. Biosci. 2019, 44, 49. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, Q.; Ma, L.; Chen, Y.; Gao, Y.; Zhang, G.; Cui, S.; Liang, H.; Song, L.; He, C. Shifts in the skin microbiome associated with diaper dermatitis and emollient treatment amongst infants and toddlers in China. Exp. Dermatol. 2019, 28, 1289–1297. [Google Scholar] [CrossRef]
- Goto, T.; Yamashita, A.; Hirakawa, H.; Matsutani, M.; Todo, K.; Ohshima, K.; Toh, H.; Miyamoto, K.; Kuhara, S.; Hattori, M.; et al. Complete genome sequence of Finegoldia magna, an anaerobic opportunistic pathogen. DNA Res. 2008, 15, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Ferrazzini, G.; Kaiser, R.R.; Hirsig Cheng, S.-K.; Wehrli, M.; Della Casa, V.; Pohlig, G.; Gonser, S.; Graf, F.; Jörg, W. Microbiological aspects of diaper dermatitis. Dermatology 2003, 206, 136–141. [Google Scholar] [CrossRef]
- Teufel, A.; Howard, B.; Hu, P.; Carr, A.N. Characterization of the microbiome in the infant diapered area: Insights from healthy and damaged skin. Exp. Dermatol. 2021, 30, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Bäsler, K.; Brandner, J.M. Tight junctions in skin inflammation. Pflugers Arch. Eur. J. Physiol. 2017, 469, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.K.; Campbell, K.L.; Lavergne, S.N. Contact dermatitis: A comparative and translational review of the literature. Vet. Dermatol. 2015, 26, 314-e67. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Piessens, S.; Bloem, A.; Pronk, H.; Finkel, P. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int. J. Cosmet. Sci. 2006, 28, 359–370. [Google Scholar] [CrossRef]
- Rippke, F.; Berardesca, E.; Weber, T.M. pH and microbial infections. Curr. Probl. Dermatol. 2018, 54, 87–94. [Google Scholar] [PubMed]
- Schmid-Wendtner, M.-H.; Korting, H.C. The pH of the skin surface and its impact on the barrier function. Skin Pharmacol. Physiol. 2006, 19, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Hoeger, P.H.; Enzmann, C.C. Skin physiology of the neonate and young infant: A prospective study of functional skin parameters during early infancy. Pediatr. Dermatol. 2002, 19, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.S.; Cho, J.S. Immunity against Staphylococcus aureus cutaneous infections. Nat. Rev. Immunol. 2011, 11, 505–518. [Google Scholar] [CrossRef] [Green Version]
- Campois, T.; Zucoloto, A.Z.; Araujo, E.J.D.A.; Svidizinski, T.I.E.; Almeida, R.S.; Quirino, G.F.D.S.; Harano, R.M.; Conchon-Costa, I.; Felipe, I. Immunological and histopathological characterization of cutaneous candidiasis. J. Med. Microbiol. 2015, 64, 810–817. [Google Scholar] [CrossRef] [Green Version]
- Visscher, M.O. Recent advances in diaper dermatitis: Etiology and treatment. Pediatr. Health 2009, 3, 81–98. [Google Scholar] [CrossRef]
- Lee, H.Y.; Stieger, M.; Yawalkar, N.; Kakeda, M. Cytokines and chemokines in irritant contact dermatitis. Mediat. Inflamm. 2013, 2013, 916497. [Google Scholar] [CrossRef]
- Nosbaum, A.; Vocanson, M.; Rozieres, A.; Hennino, A.; Nicolas, J.F. Allergic and irritant contact dermatitis. Eur. J. Dermatol. 2009, 19, 325–332. [Google Scholar] [CrossRef]
- Koudounas, S. Investigation of the Underlying Mechanisms Leading to the Development of Incontinence-Associated Dermatitis. Ph.D. Thesis, University of Southampton, Southampton, UK, 2019. Available online: http://eprints.soton.ac.uk/id/eprint/433262 (accessed on 10 January 2022).
- Gosenca, M.; Gašperlin, M.; Kristl, J. Irritative contact dermatitis: From mechanism of irritation to irritants’ assessment. Farm. Vestn. 2012, 63, 145–152. [Google Scholar]
- Landeck, L.; Visser, M.; Kezic, S.; John, S.M. Impact of tumour necrosis factor-α polymorphisms on irritant contact dermatitis. Contact Derm. 2012, 66, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Landeck, L.; Visser, M.; Kezic, S.; John, S.M. IL1A-889 C/T gene polymorphism in irritant contact dermatitis. J. Eur. Acad Dermatol. Venereol. 2013, 27, 1040–1043. [Google Scholar] [CrossRef] [PubMed]
- Yucesoy, B.; Talzhanov, Y.; Barmada, M.M.; Johnson, V.J.; Kashon, M.L.; Baron, E.; Wilson, N.W.; Frye, B.; Wang, W.; Fluharty, K.; et al. Genetic basis of irritant susceptibility in health care workers. J. Occup. Environ. Med. 2016, 58, 753–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joint FAO/WHO Working Group. Guidelines for the Evaluation of Probiotics in Food; Food and Agriculture Organization of the United Nations, WHO: London, ON, Canada, 2002. [Google Scholar]
- Yu, Y.; Dunaway, S.; Champer, J.; Kim, J.; Alikhan, A. Changing our microbiome: Probiotics in dermatology. Br. J. Dermatol. 2020, 182, 39–46. [Google Scholar] [CrossRef]
- Roudsari, M.R.; Karimi, R.; Sohrabvandi, S.; Mortazavian, A.M. Health effects of probiotics on the skin. Crit. Rev. Food Sci. Nutr. 2015, 55, 1219–1240. [Google Scholar] [CrossRef]
- Cinque, B.; La Torre, C.; Melchiorre, E.; Marchesani, G.; Zoccali, G.; Palumbo, P.; Di Marzio, L.; Masci, A.; Mosca, L.; Mastromarino, P.; et al. Use of probiotics for dermal applications. Probiotics 2011, 21, 221–241. [Google Scholar]
- Saavedra, J.; Abi-Hanna, A.; Moore, N.; Yolken, R. Effect of long term consumption of infant formulas with Bifidobacteria (B) and S. thermophilus (ST) on stool patterns and diaper rash in infants. J. Pediatr. Gastroenterol. Nutr. 1998, 27, 483. [Google Scholar] [CrossRef]
- Dimitratos, S.M.; Brown, H.; Shafizadeh, T.; Kazi, S.; Altmann, T.; Ostrer, B. Symptomatic relief from at-home use of activated Bifidobacterium infantis EVC001 probiotic in infants: Results from a consumer survey on the effects on diaper rash, colic symptoms, and sleep. Benef. Microbes 2021, 12, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Tan-Lim, C.S.C.; Esteban-Ipac, N.A.R.; Recto, M.S.T.; Castor, M.A.R.; Casis-Hao, R.J.; Nano, A.L.M. Comparative effectiveness of probiotic strains on the prevention of pediatric atopic dermatitis: A systematic review and network meta-analysis. Pediatr. Allergy Immunol. 2021, 32, 1255–1270. [Google Scholar] [CrossRef]
- Mansfield, J.A.; Bergin, S.W.; Cooper, J.R.; Olsen, C.H. Comparative probiotic strain efficacy in the prevention of eczema in infants and children: A systematic review and meta-analysis. Mil. Med. 2014, 179, 580–592. [Google Scholar] [CrossRef] [Green Version]
- Boyle, R.J.; Bath-Hextall, F.J.; Leonardi-Bee, J.; Murrell, D.F.; Tang, M.L. Probiotics for treating eczema. Cochrane Database Syst. Rev. 2018, 11. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Thyssen, J.P.; Paller, A.S.; Drucker, A.M.; Wollenberg, A.; Lee, K.H.; Kabashima, K.; Todd, G.; Schmid-Grendelmeier, P.; Bieber, T. What’s in a name? Atopic dermatitis or atopic eczema, but not eczema alone. Allergy 2017, 72, 2026–2030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lolou, V.; Panayiotidis, M.I. Functional role of probiotics and prebiotics on skin health and disease. Fermentation 2019, 5, 41. [Google Scholar] [CrossRef] [Green Version]
- Salem, I.; Ramser, A.; Isham, N.; Ghannoum, M.A. The gut microbiome as a major regulator of the gut-skin axis. Front. Microbiol. 2018, 9, 1459. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, C.A.; Monteleone, G.; McLaughlin, J.T.; Paus, R. The gut-skin axis in health and disease: A paradigm with therapeutic implications. Bioessays 2016, 38, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Saiki, A.; Matsui, Y.; Tsuchimoto, N.; Nakakita, Y.; Takata, Y.; Nakamura, T. Effects of oral intake of heat-killed Lactobacillus brevis SBC8803 (SBL88TM) on dry skin conditions: A randomized, double-blind, placebo-controlled study. Exp. Ther. Med. 2016, 12, 3863. [Google Scholar] [CrossRef] [PubMed]
- Gueniche, A.; Philippe, D.; Bastien, P.; Reuteler, G.; Blum, S.; Castiel-Higounenc, I.; Breton, L.; Benyacoub, J. Randomised double-blind placebo-controlled study of the effect of Lactobacillus paracasei NCC 2461 on skin reactivity. Benef. Microbes 2014, 5, 137–145. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hertiš Petek, T.; Petek, M.; Petek, T.; Marčun Varda, N. Emerging Links between Microbiome Composition and Skin Immunology in Diaper Dermatitis: A Narrative Review. Children 2022, 9, 112. https://doi.org/10.3390/children9010112
Hertiš Petek T, Petek M, Petek T, Marčun Varda N. Emerging Links between Microbiome Composition and Skin Immunology in Diaper Dermatitis: A Narrative Review. Children. 2022; 9(1):112. https://doi.org/10.3390/children9010112
Chicago/Turabian StyleHertiš Petek, Tjaša, Maya Petek, Tadej Petek, and Nataša Marčun Varda. 2022. "Emerging Links between Microbiome Composition and Skin Immunology in Diaper Dermatitis: A Narrative Review" Children 9, no. 1: 112. https://doi.org/10.3390/children9010112
APA StyleHertiš Petek, T., Petek, M., Petek, T., & Marčun Varda, N. (2022). Emerging Links between Microbiome Composition and Skin Immunology in Diaper Dermatitis: A Narrative Review. Children, 9(1), 112. https://doi.org/10.3390/children9010112