Study of Orofacial Function in Preschool Children Born Prematurely
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Menon, R. Preterm birth: A global burden on maternal and child health. Pathog. Glob. Health 2012, 106, 139–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breslau, N.; Chilcoat, H.D.; Johnson, E.O.; Andreski, P.; Lucia, V.C. Neurologic soft signs and low birthweight: Their association and neuropsychiatric implications. Biol. Psychiatry 2000, 47, 71–79. [Google Scholar] [CrossRef]
- Anderson, P.; Doyle, L.W.; Victorian Infant Collaborative Study Group. Neurobehavioral outcomes of school-age children born extremely low birth weight or very preterm in the 1990s. JAMA 2003, 289, 3264–3272. [Google Scholar] [CrossRef] [Green Version]
- Kunnari, S.; Yliherva, A.; Paavola, L.; Peltoniemi, O.M. Expressive language skills in Finnish two-year-old extremely-and very-low-birth-weight preterm children. Folia Phoniatr. Logop. 2012, 64, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Gayraud, F.; Kern, S. Influence of preterm birth on early lexical and grammatical acquisition. First Lang. 2007, 27, 159–173. [Google Scholar] [CrossRef]
- Leme, M.S.; Barbosa, T.D.S.; Gavião, M.B.D. Assessment of orofacial functions in Brazilian children using the Nordic Orofacial Test-Screening (NOT-S). Rev. Odonto Ciência 2012, 27, 108–114. [Google Scholar] [CrossRef] [Green Version]
- Bakke, M.; Bergendal, B.; McAllister, A.; Sjögreen, L.; Asten, P. Development and evaluation of a comprehensive screening for orofacial dysfunction. Swed. Dent. J. 2007, 31, 75–84. [Google Scholar]
- Gustavsson, C.; Skoglund, C.; Thelin, H. Normering av Nordiskt Orofacialt Test-Screening (NOT-S) för Barn i Åldrarna 3 Till 6 år; Institutionen för Nervsystem och Rörelseorgan: Linköping, Sweden, 2007. [Google Scholar]
- Trulsson, U.; Klingberg, G. Living with a child with a severe orofacial handicap: Experiences from the perspectives of parents. Eur. J. Oral Sci. 2003, 111, 19–25. [Google Scholar] [CrossRef]
- McAllister, A.; Lundeborg, I. Oral sensorimotor functions in typically developing children 3 to 8 years old; assessed by the Nordic orofacial test, NOT-S. J. Med. Speech Lang. Pathol. 2013, 21, 51–59. [Google Scholar]
- Buswell, C.A.; Leslie, P.; Embleton, N.D.; Drinnan, M.J. Oral-motor dysfunction at 10 months corrected gestational age in infants born less than 37 weeks preterm. Dysphagia 2009, 24, 20–25. [Google Scholar] [CrossRef]
- Pridham, K.; Steward, D.; Thoyre, S.; Brown, R.; Brown, L. Feeding skill performance in premature infants during the first year. Early Hum. Dev. 2007, 83, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Bergendal, B.; Bakke, M.; McAllister, A.; Sjögreen, L.; Åsten, P. Profiles of orofacial dysfunction in different diagnostic groups using the Nordic Orofacial Test (NOT-S)—A review. Acta Odontol. Scand. 2014, 72, 578–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coulthard, H.; Harris, G.; Emmett, P. Delayed introduction of lumpy foods to children during the complementary feeding period affects child’s food acceptance and feeding at 7 years of age. Matern. Child Nutr. 2009, 5, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, M.; van Doorn, J.; van den Berg, J. Parents’ perceptions of eating skills of pre-term vs full-term infants from birth to 3 years. Int. J. Speech Lang. Pathol. 2013, 15, 604–612. [Google Scholar] [CrossRef]
- Bertoncelli, N.; Cuomo, G.; Cattani, S.; Mazzi, C.; Pugliese, M.; Coccolini, E.; Zagni, P.; Mordini, B.; Ferrari, F. Oral feeding competences of healthy preterm infants: A review. Int. J. Pediatr. 2012, 2012, 896257. [Google Scholar] [CrossRef] [Green Version]
- Braid, S.; Harvey, E.M.; Bernstein, J.; Matoba, N. Early introduction of complementary foods in preterm infants. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 811–818. [Google Scholar] [CrossRef]
- Migraine, A.; Nicklaus, S.; Parnet, P.; Lange, C.; Monnery-Patris, S.; Robert, C.D.; Darmaun, D.; Flamant, C.; Amarger, V.; Rozé, J. Effect of preterm birth and birth weight on eating behavior at 2 y of age. Am. J. Clin. Nutr. 2013, 97, 1270–1277. [Google Scholar] [CrossRef] [Green Version]
- Shahraki, N.; Yassaei, S.; Moghadam, M.G. Abnormal oral habits: A review. J. Dent. Oral Hyg. 2012, 4, 12–15. [Google Scholar] [CrossRef]
- Farsi, N.M.; Salama, F.; Pedo, C. Sucking habits in Saudi children: Prevalence, contributing factors and effects on the primary dentition. Pediatr. Dent. 1997, 19, 28–33. [Google Scholar]
- Ferrini, F.R.D.O.; Marba, S.T.M.; Gavião, M.B.D. Oral conditions in very low and extremely low birth weight children. J. Dent. Child. 2008, 75, 235–242. [Google Scholar]
- Fernández Gallardo, M.A.; Rojas Contreras, D.P.; Vargas Keith, J.F. Development of feeding skills in preterm infants: A critical literature review. Rev. CEFAC 2017, 19, 539–550. [Google Scholar] [CrossRef] [Green Version]
- Törölä, H.; Lehtihalmes, M.; Yliherva, A.; Olsén, P. Feeding skill milestones of preterm infants born with extremely low birth weight (ELBW). Inf. Behav. Dev. 2012, 35, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.; Sheena, H.R.; Shulman, R.J.; Schanler, R.J. Oral feeding in low birth weight infants. J. Pediatr. 1997, 130, 561–569. [Google Scholar] [CrossRef]
- Lau, C.; Smith, E.; Schanler, R. Coordination of suck-swallow and swallow respiration in preterm infants. Acta Paediatr. 2003, 92, 721–727. [Google Scholar] [CrossRef]
- Tsai, H.H.; Tan, C.T. Morphology of the palatal vault of primary dentition in transverse view. Angle Orthodont. 2004, 74, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Le Révérend, B.J.; Edelson, L.R.; Loret, C. Anatomical, functional, physiological and behavioural aspects of the development of mastication in early childhood. Br. J. Nutr. 2014, 111, 403–414. [Google Scholar] [CrossRef] [Green Version]
- Stevenson, P.; Bax, M.; Stevenson, J. The evaluation of home based speech therapy for language delayed pre-school children in an inner city area. Int. J. Lang. Commun. Disord. 1982, 17, 141–148. [Google Scholar] [CrossRef]
- Brown, B.B.; Bendersky, M.; Chapman, T. The early utterances of preterm infants. Br. J. Disord. Commun. 1986, 21, 307–319. [Google Scholar] [CrossRef]
- Arvedson, J.; Clark, H.; Lazarus, C.; Schooling, T.; Frymark, T. Evidence-based systematic review: Effects of oral motor interventions on feeding and swallowing in preterm infants. Am. J. Speech Lang. Pathol. 2010, 19, 321–340. [Google Scholar] [CrossRef] [Green Version]
- Dodrill, P.; McMahon, S.; Ward, E.; Weir, K.; Donovan, T.; Riddle, B. Long-term oral sensitivity and feeding skills of low-risk pre-term infants. Early Hum. Dev. 2004, 76, 23–37. [Google Scholar] [CrossRef]
- Bier, J.A.B.; Ferguson, A.; Cho, C.; Oh, W.; Vohr, B.R. The oral motor development of low-birth-weight infants who underwent orotracheal intubation during the neonatal period. Am. J. Dis. Child. 1993, 147, 858–862. [Google Scholar] [CrossRef] [PubMed]
- LaTuga, M.S.; Mittelstaedt, G.; Moon, J.Y.; Kim, M.; Murray-Keane, L.; Si, W.; Havranek, T. Clinical characteristics of premature infants who orally feed on continuous positive airway pressure. Early Hum. Dev. 2019, 139, 104833. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, L.; Bidiwala, A.; Sher, I.; Pirzada, M.; Barlev, D.; Islam, S.; Rosenfeld, W.; Crowley, C.C.; Hanna, N. Effect of nasal continuous positive airway pressure on the pharyngeal swallow in neonates. J. Perinatol. 2017, 37, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Montgomery-Downs, H.E.; Young, M.E.; Ross, M.A.; Polak, M.J.; Ritchie, S.K.; Lynch, S.K. Sleep-disordered breathing symptoms frequency and growth among prematurely born infants. Sleep Med. 2010, 11, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Samson, N.; Michaud, A.; Othman, R.; Nadeau, C.; Nault, S.; Cantin, D.; Sage, M.; Catelin, C.; Praud, J.P. Nasal continuous positive airway pressure influences bottle-feeding in preterm lambs. Pediatr. Res. 2017, 82, 926–933. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Reid, S.; Lutz, T.; Malcolm, G.; Oliver, S.; Osborn, D.A. Randomised controlled trial of weaning strategies for preterm infants on nasal continuous positive airway pressure. BMC Pediatr. 2015, 15, 147. [Google Scholar] [CrossRef] [Green Version]
- Brogårdh-Roth, S.; Paulsson, L.; Larsson, P.; Ekberg, E. Do preterm-born adolescents have a poorer oral health-related quality of life? BMC Oral Health 2021, 21, 440. [Google Scholar] [CrossRef]
- Tighe, D.; Tighe, R.; Petrick, L.; Cobourne, M.T.; Rabe, H. Palatal Development and Orofacial Function: Possible Effects of Preterm Care. Neoreviews 2011, 12, e308–e314. [Google Scholar] [CrossRef]
| Variable | Characteristics | Categories |
|---|---|---|
| Gulping | Swallowing food after chewing | |
| Swallowing food without chewing | ||
| Choke (water and liquid classes) | Non-choke: No coughing during eating | |
| Choke: Coughing more than 3 times during eating | ||
| The amount of open mouth | 21–30 mm | |
| 31 mm or more | ||
| Occlusal force | Normal: Can chew ordinary diet | |
| Abnormal: Can only chew soft food | ||
| Open bite | ≦2 mm | |
| >4 mm | ||
| Dental arch form | Circular shape | |
| Non-Circular shape | ||
| Palate vault | Normal Palate | |
| Abnormal Palate | ||
| Dysarthria | Normal: clear articulation of phonemes | |
| Abnormal: poor articulation of phonemes | ||
| Conscious cough | Good: Strong contraction of abdominal muscles, exercise accompanied by cough sounds | |
| Poor: Abdominal muscle contraction, coughing sound without accompanying movement | ||
| Modified Water Swallowing Test (MWST) | Normal: 3 mL water, swallow more than 3 times within 30 s | |
| Abnormal: 3 mL of water, swallowed less than 2 times within 30 s |
| Variable | GA < 37 Weeks | GA ≥ 37 Weeks | p-Value | BBW < 1501 g | BBW > 1500 g | p-Value |
|---|---|---|---|---|---|---|
| (N = 101) | (N = 142) | (N = 65) | (N = 178) | |||
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||
| Average age of the first time eating pureed food (Month) | ||||||
| Fruits | 4.0 ± 1.3 | 5.1 ± 0.9 | <0.001 | 4.1 ± 1.3 | 4.8 ± 1.1 | <0.001 |
| Minced-Toast | 6.5 ± 2.5 | 7.5 ± 2.1 | <0.001 | 6.7 ± 2.9 | 7.2 ± 2.1 | 0.025 |
| Vegetable | 8.0 ± 2.8 | 9.4 ± 2.2 | <0.001 | 8.1 ± 3.1 | 9.1 ± 2.3 | 0.006 |
| Meat | 10.2 ± 3.6 | 11.0 ± 2.0 | <0.001 | 10.3 ± 4.2 | 10.8 ± 2.1 | 0.006 |
| Current status of development | ||||||
| Weight growth curve (%) | 33.4 ± 30.2 | 46.0 ± 31.9 | 0.002 | 33.0 ± 30.4 | 45.0 ± 31.3 | 0.003 |
| Weight growth curve < 3% 1 | 14 (13.9) | 10 (7.0) | 0.079 | 12 (18.5) | 12 (6.7) | 0.007 |
| Height growth curve (%) | 33.3 ± 28.7 | 47.8 ± 31.5 | <0.001 | 36.8 ± 32.8 | 42.2 ± 31.5 | 0.161 |
| Height growth curve < 3% 1 | 23 (22.8) | 15 (10.6) | 0.010 | 14 (21.5) | 24 (13.5) | 0.126 |
| BMI growth curve (%) | 42.7 ± 32.0 | 52.4 ± 33.5 | 0.033 | 40.0 ± 31.8 | 51.4 ± 33.6 | 0.018 |
| BMI growth curve < 3% 1 | 12 (11.9) | 14 (9.9) | 0.615 | 11 (16.9) | 15 (8.4) | 0.058 |
| Variable | Total | BBW 1001–1500 g | BBW < 1001 g | p-Value 1 |
|---|---|---|---|---|
| (N = 65) | (N = 50) | (N = 15) | ||
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| Apgar 1 min | 5.9 ± 1.6 | 6.2 ± 1.3 | 5.1 ± 1.9 | 0.043 |
| Apgar 5 min | 7.8 ± 1.2 | 7.9 ± 1.3 | 7.2 ± 1.0 | 0.011 |
| Day of intubation | 12.7 ± 19.7 | 6.5 ± 15.0 | 26.7 ± 22.7 | <0.001 |
| Day of NCPAP | 26.7 ± 19.7 | 22.9 ± 17.9 | 39.5 ± 20.4 | 0.005 |
| Day of NG tube placement | 51.9 ± 31.0 | 43.6 ± 24.1 | 79.6 ± 35.8 | <0.001 |
| Day of hospitalization | 71.5 ± 333.8 | 60.5 ± 26.7 | 108.2 ± 29.3 | <0.001 |
| Variable | GA < 37 Weeks | GA ≥ 37 Weeks | p-Value | BBW < 1501 g | BBW > 1500 g | p-Value |
|---|---|---|---|---|---|---|
| (N = 101) | (N = 142) | (N = 65) | (N = 178) | |||
| N (%) | N (%) | N (%) | N (%) | |||
| NOT-S | ||||||
| Breathing-snoring while sleeping | 12 (11.9) | 20 (14.1) | 0.617 | 9 (13.8) | 23 (12.9) | 0.850 |
| Habits (oral habits) | 36 (35.6) | 76 (53.5) | 0.006 | 21 (32.3) | 91 (51.1) | 0.009 |
| Grinding teeth while sleeping | 15 (14.9) | 38 (26.8) | 0.027 | 7 (10.8) | 46 (25.8) | 0.012 |
| Abnormal chewing and swallowing | 61 (60.4) | 97 (68.3) | 0.202 | 39 (60.0) | 119 (66.9) | 0.321 |
| Speech- Pronunciation is not standard | 22 (21.8) | 19 (13.4) | 0.085 | 16 (24.6) | 25 (14.0) | 0.051 |
| Assessment of chewing and swallowing in children | ||||||
| Abnormal gulping | 19 (18.8) | 46 (32.4) | 0.018 | 10 (15.4) | 55 (30.9) | 0.016 |
| Choke | 12 (11.9) | 5 (3.5) | 0.012 | 11 (16.9) | 6 (3.4) | <0.001 |
| The abnormal amount of open mouth | 6 (5.9) | 0 (0.0) | 0.003 | 6 (9.2) | 0 (0.0) | <0.001 |
| Abnormal occlusal force | 43 (42.6) | 51 (35.9) | 0.294 | 34 (52.3) | 60 (33.7) | 0.008 |
| Abnormal open bite | 6 (5.9) | 5 (3.5) | 0.371 | 4 (6.2) | 7 (3.9) | 0.461 |
| Abnormal dental arch form | 29 (28.7) | 21 (14.8) | 0.008 | 24 (36.9) | 26 (14.6) | <0.001 |
| Abnormal palate vault | 25 (24.8) | 14 (9.9) | 0.002 | 21 (32.3) | 18 (10.1) | <0.001 |
| Dysarthria | 21 (20.8) | 15 (10.6) | 0.027 | 16 (24.6) | 20 (11.2) | 0.009 |
| Abnormal conscious cough | 26 (25.7) | 49 (34.5) | 0.145 | 13 (20.0) | 62 (34.8) | 0.270 |
| Abnormal MWST | 34 (33.7) | 49 (34.5) | 0.891 | 19 (29.2) | 64 (36.6) | 0.328 |
| Variable | Gestational Age (Weeks) | p -Value | Birth Weight (g) | p -Value | ||
|---|---|---|---|---|---|---|
| Negative | Positive | Negative | Positive | |||
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||
| NOT-S | ||||||
| Breathing-snoring while sleeping | 35.9 ± 4.1 (211) | 35.6 ± 4.9 (32) | 0.742 | 2505.2 ± 890.2 (211) | 2530.3 ± 957.0 (32) | 0.883 |
| Bad oral habits | 35.3 ± 4.3 (131) | 36.5 ± 4.0 (112) | 0.018 | 2364.2 ± 899.1 (131) | 2677.4 ± 869.0 (112) | 0.006 |
| Grinding teeth during sleep | 35.7 ± 4.3 (190) | 36.7 ± 3.5 (53) | 0.044 | 2437.0 ± 922.5 (190) | 2765.2 ± 53.6 (53) | 0.018 |
| Chewing and swallowing | 35.5 ± 4.2 (85) | 36.1 ± 4.2 (158) | 0.311 | 2473.8 ± 912.2 (85) | 2527.2 ± 891.5 (158) | 0.659 |
| Speech—Pronunciation is not standard | 36.1 ± 4.0 (202) | 34.8 ± 4.9 (41) | 0.065 | 2558.8 ± 856.5 (202) | 2260.7 ± 1053.2 (41) | 0.052 |
| The assessment chewing and swallowing in children | ||||||
| Gulping | 35.5 ± 4.3 (178) | 36.9 ± 3.7 (65) | <0.017 | 2407.0 ± 909.8 (178) | 2786.7 ± 804.8 (65) | 0.003 |
| Choke | 36.0 ± 4.1 (226) | 32.8 ± 4.5 (17) | <0.001 | 2556.9 ± 875.8 (226) | 1865.6 ± 959.1 (17) | 0.002 |
| The abnormal amount of open mouth | 36.0 ± 4.0 (237) | 28.2 ± 2.3 (6) | <0.001 | 2544.8 ± 877.5 (237) | 1077.5 ± 318.2 (6) | <0.001 |
| Occlusal force | 36.3 ± 3.8 (149) | 35.2 ± 4.7 (94) | 0.057 | 2602.4 ± 818.1 (149) | 2359 ± 996.7 (94) | 0.040 |
| Open bite | 35.9 ± 4.1 (232) | 34.7 ± 4.8 (11) | 0.361 | 2522.6 ± 895.8 (232) | 2211.7 ± 919.0 (11) | 0.262 |
| Dental arch form | 36.3 ± 3.9 (193) | 34.1 ± 4.8 (50) | 0.001 | 2610.6 ± 847.4 (193) | 2114.4 ± 981.3 (50) | <0.001 |
| Palate vault | 36.3 ± 4.0 (204) | 33.6 ± 4.5 (39) | <0.001 | 2603.9 ± 840.7 (204) | 2009.7 ± 1023.7 (39) | <0.001 |
| Dysarthria | 36.1 ± 4.0 (207) | 34.3 ± 5.1 (36) | 0.014 | 2572.5 ± 860.4 (207) | 2140.5 ± 1022.9 (36) | 0.007 |
| Conscious cough | 35.5 ± 4.3 (168) | 36.7 ± 3.9 (75) | 0.041 | 2442.8 ± 937.2 (168) | 2140.5 ± 1022.9 (75) | 0.092 |
| MWST | 35.7 ± 4.2 (160) | 36.1 ± 4.0 (83) | 0.511 | 2466.3 ± 931.3 (160) | 2655.5 ± 787.3 (83) | 0.593 |
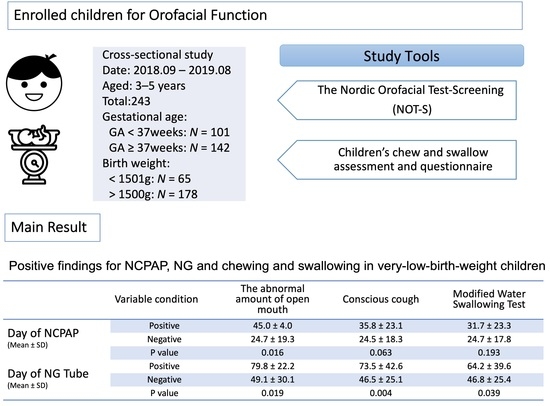
| Variable | Day of Intubation (N = 65) | p-Value | Day of NCPAP (N = 65) | p-Value | Day of NG Tube Placement (N = 65) | p-Value | |||
|---|---|---|---|---|---|---|---|---|---|
| Negative | Positive | Negative | Positive | Negative | Positive | ||||
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||||
| NOT-S | |||||||||
| Breathing-snoring while sleeping | 11.4 ± 17.4 | 21.3 ± 29.6 | 0.158 | 24.7 ± 18.1 | 39.6 ± 25.0 | 0.034 | 51.1 ± 32.7 | 53.7 ± 27.7 | 0.755 |
| Bad oral habits | 10.2 ± 17.4 | 13.1 ± 22.1 | 0.556 | 25.3 ± 20.3 | 29.7 ± 18.4 | 0.401 | 25.3 ± 20.3 | 29.7 ± 18.4 | 0.401 |
| Grinding teeth during sleep | 11.4 ± 19.9 | 9.0 ± 9.1 | 0.756 | 26.6 ± 20.2 | 28.0 ± 15.6 | 0.857 | 51.4 ± 32.2 | 56.4 ± 19.7 | 0.686 |
| Chewing and swallowing | 9.8 ± 17.3 | 12.0 ± 20.1 | 0.66 | 26.4 ± 20.3 | 26.9 ± 19.5 | 0.911 | 47.3 ± 23.7 | 55.0 ± 35.0 | 0.332 |
| Speech—Pronunciation is not standard | 9.4 ± 16.0 | 16.4 ± 25.8 | 0.203 | 26.6 ± 20.2 | 27.1 ± 18.5 | 0.937 | 53.0 ± 30.9 | 48.7 ± 32.2 | 0.636 |
| The assessment chewing and swallowing in children | |||||||||
| Gulping | 10.4 ± 16.3 | 14.9 ± 30.5 | 0.497 | 25.5 ± 18.1 | 33.4 ± 27.0 | 0.246 | 52.0 ± 31.1 | 51.5 ± 32.1 | 0.964 |
| Choke | 10.8 ± 18.8 | 15.6 ± 22.5 | 0.586 | 26.1 ± 19.5 | 34.8 ± 22.4 | 0.343 | 51.5 ± 31.1 | 57.4 ± 32.9 | 0.683 |
| The abnormal amount of open mouth | 10.6 ± 18.8 | 16.3 ± 22.5 | 0.483 | 24.7 ± 19.3 | 45.0 ± 4.0 | 0.016 | 49.1 ± 30.1 | 79.8 ± 22.2 | 0.019 |
| Occlusal force | 7.0 ± 14.4 | 14.9 ± 21.8 | 0.091 | 23.7 ± 18.5 | 29.5 ± 20.5 | 0.241 | 45.5 ± 21.7 | 57.836.9 | 0.109 |
| Open bite | 11.3 ± 19.2 | 8.3 ± 15.2 | 0.757 | 27.2 ± 20.0 | 19.3 ± 11.6 | 0.437 | 52.9 ± 31.4 | 37.3 ± 21.0 | 0.333 |
| Dental arch form | 12.5 ± 21.4 | 8.8 ± 13.8 | 0.451 | 26.7 ± 21.8 | 26.8 ± 15.7 | 0.983 | 50.7 ± 34.4 | 54.0 ± 24.6 | 0.666 |
| Palate vault | 10.9 ± 19.8 | 11.7 ± 17.4 | 0.874 | 28.0 ± 20.1 | 24.0 ± 16.9 | 0.437 | 49.5 ± 27.6 | 56.9 ± 37.4 | 0.378 |
| Dysarthria | 9.4 ± 16.0 | 16.4 ± 25.8 | 0.203 | 26.6 ± 20.2 | 27.1 ± 18.5 | 0.937 | 53.0 ± 30.8 | 48.7 ± 32.2 | 0.636 |
| Conscious cough | 11.1 ± 19.5 | 11.2 ± 17.1 | 0.955 | 24.5 ± 18.3 | 35.8 ± 23.1 | 0.063 | 46.5 ± 25.1 | 73.5 ± 42.6 | 0.004 |
| MWST | 11.6 ± 20.3 | 9.8 ± 15.2 | 0.718 | 24.7 ± 17.8 | 31.7 ± 23.3 | 0.193 | 46.8 ± 25.4 | 64.2 ± 39.6 | 0.039 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, M.-C.; Liu, H.-Y.; Huang, S.-T.; Chen, H.-L. Study of Orofacial Function in Preschool Children Born Prematurely. Children 2022, 9, 360. https://doi.org/10.3390/children9030360
Chang M-C, Liu H-Y, Huang S-T, Chen H-L. Study of Orofacial Function in Preschool Children Born Prematurely. Children. 2022; 9(3):360. https://doi.org/10.3390/children9030360
Chicago/Turabian StyleChang, Mei-Chen, Hsiu-Yueh Liu, Shun-Te Huang, and Hsiu-Lin Chen. 2022. "Study of Orofacial Function in Preschool Children Born Prematurely" Children 9, no. 3: 360. https://doi.org/10.3390/children9030360
APA StyleChang, M. -C., Liu, H. -Y., Huang, S. -T., & Chen, H. -L. (2022). Study of Orofacial Function in Preschool Children Born Prematurely. Children, 9(3), 360. https://doi.org/10.3390/children9030360