Isolation and In Vitro Stability Studies of Edible Plant-Seed Derived (Raphani Semen) Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
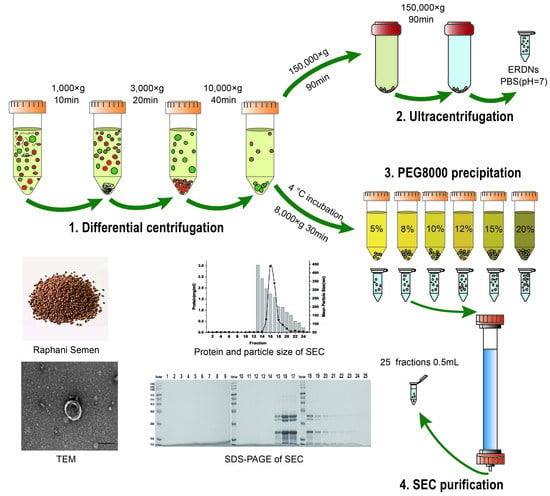
2.1. Differential Ultracentrifugation for the Isolation of ERDNs
2.2. Polyethylene Glycol Solution for Precipitation ERDNs
2.3. Physical and Chemical Characterization of ERDNs
2.4. Stability of ERDNs In Vitro Simulated Digestion
2.5. Purification of ERDNs by Size Exclusion Chromatography
2.6. SDS-PAGE Analysis of ERDNs
2.7. Statistical Analysis
3. Results and Discussion
3.1. ERDNs Isolated by Ultracentrifugation
3.2. PEG8000 Can Be Used for the Enrichment of ERDNs
3.3. Sephacryl S-500 Column for Purification of ERDNs
3.4. Stability of ERDNs in Simulated Gastrointestinal Digestion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Shehbaz, I.A.; Beilstein, M.; Kellogg, E. Systematics and phylogeny of the Brassicaceae (Cruciferae): An overview. Plant Syst. Evol. 2006, 259, 89–120. [Google Scholar] [CrossRef]
- Ağagündüz, D.; Şahin, T.; Yılmaz, B.; Ekenci, K.D.; Özer, Ş.D.; Capasso, R. Cruciferous Vegetables and Their Bioactive Metabolites: From Prevention to Novel Therapies of Colorectal Cancer. Evid. Based Complement. Altern. Med 2022, 2022, 1534083. [Google Scholar] [CrossRef] [PubMed]
- Melim, C.; Lauro, M.R.; Pires, I.M.; Oliveira, P.J.; Cabral, C. The Role of Glucosinolates from Cruciferous Vegetables (Brassicaceae) in Gastrointestinal Cancers: From Prevention to Therapeutics. Pharmaceutics 2022, 14, 190. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wu, X.; Zhuang, W.; Wu, C.; Rao, Z.; Du, L.; Zhou, Y. Cruciferous vegetable and isothiocyanate intake and multiple health outcomes. Food Chem. 2022, 375, 131816. [Google Scholar] [CrossRef]
- Björkman, M.; Klingen, I.; Birch, A.N.; Bones, A.M.; Bruce, T.J.; Johansen, T.J.; Meadow, R.; Mølmann, J.; Seljåsen, R.; Smart, L.E.; et al. Phytochemicals of Brassicaceae in plant protection and human health—Influences of climate, environment and agronomic practice. Phytochemistry 2011, 72, 538–556. [Google Scholar] [CrossRef]
- Poddar, K.H.; Sikand, G.; Kalra, D.; Wong, N.; Duell, P.B. Mustard oil and cardiovascular health: Why the controversy? J. Clin. Lipidol. 2022, 16, 13–22. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science Publisher: Beijing, China, 2020.
- Gao, L.; Li, H.; Li, B.; Shao, H.; Yu, X.; Miao, Z.; Zhang, L.; Zhu, L.; Sheng, H. Traditional uses, phytochemistry, transformation of ingredients and pharmacology of the dried seeds of Raphanus sativus L. (Raphani Semen): A comprehensive review. J. Ethnopharmacol. 2022, 294, 115387. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, C.S.; Park, Y.J.; Moon, E.; Choi, S.U.; Lee, J.H.; Kim, S.Y.; Lee, K.R. Anti-inflammatory and antitumor phenylpropanoid sucrosides from the seeds of Raphanus sativus. Bioorg. Med. Chem. Lett. 2015, 25, 96–99. [Google Scholar] [CrossRef]
- Kim, K.H.; Moon, E.; Lee, S.R.; Park, K.J.; Kim, S.Y.; Choi, S.U.; Lee, K.R. Chemical Constituents of the Seeds of Raphanus sativus and their Biological Activity. J. Braz. Chem. Soc. 2015, 26, 2307–2312. [Google Scholar]
- Choi, K.-C.; Cho, S.-W.; Kook, S.-H.; Chun, S.-R.; Bhattarai, G.; Poudel, S.B.; Kim, M.-K.; Lee, K.-Y.; Lee, J.-C. Intestinal anti-inflammatory activity of the seeds of Raphanus sativus L. in experimental ulcerative colitis models. J. Ethnopharmacol. 2016, 179, 55–65. [Google Scholar] [CrossRef]
- Park, W.Y.; Song, G.; Noh, J.H.; Kim, T.; Kim, J.J.; Hong, S.; Park, J.; Um, J.-Y. Raphani Semen (Raphanus sativus L.) Ameliorates Alcoholic Fatty Liver Disease by Regulating De Novo Lipogenesis. Nutrients 2021, 13, 448. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.; Pan, Y.; Cai, Z.; Jing, P. Phytochemical composition, antioxidant capacity and ACE-inhibitory activity of China-grown radish seeds. J. Appl. Bot. Food Qual. 2017, 90, 315–322. [Google Scholar]
- Gao, S.; Wang, J.; Cheng, L.; Fan, Y.; Qin, W.; Wang, Y.; Guo, Y.; Zhang, X.; Yang, Y. Evaluation of the Effects of Processing Technique on Chemical Components in Raphani Semen by HPLC and UPLC-Q-TOF-MS. Int. J. Anal. Chem. 2022, 2022, 8279839. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.T.; Johnstone, R.M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell 1983, 33, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Halperin, W.; Jensen, W.A. Ultrastructural changes during growth and embryogenesis in carrot cell cultures. J. Ultrastruct. Res. 1967, 18, 428–443. [Google Scholar] [CrossRef]
- Man, F.; Wang, J.; Lu, R. Techniques and Applications of Animal- and Plant-Derived Exosome-Based Drug Delivery System. J. Biomed. Nanotechnol. 2020, 16, 1543–1569. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Sfragano, P.S.; Pillozzi, S.; Condorelli, G.; Palchetti, I. Practical tips and new trends in electrochemical biosensing of cancer-related extracellular vesicles. Anal. Bioanal. Chem. 2023, 415, 1087–1106. [Google Scholar] [CrossRef]
- Sadeghi, S.; Tehrani, F.R.; Tahmasebi, S.; Shafiee, A.; Hashemi, S.M. Exosome engineering in cell therapy and drug delivery. Inflammopharmacology 2023, 31, 145–169. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, M.; Merlin, D. Advances in Plant-derived Edible Nanoparticle-based lipid Nano-drug Delivery Systems as Therapeutic Nanomedicines. J. Mater. Chem. B 2018, 6, 1312–1321. [Google Scholar] [CrossRef]
- Mu, J.; Zhuang, X.; Wang, Q.; Jiang, H.; Deng, Z.B.; Wang, B.; Zhang, L.; Kakar, S.; Jun, Y.; Miller, D.; et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol. Nutr. Food Res. 2014, 58, 1561–1573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldini, N.; Torreggiani, E.; Roncuzzi, L.; Perut, F.; Zini, N.; Avnet, S. Exosome-like Nanovesicles Isolated from Citrus limon L. Exert Antioxidative Effect. Curr. Pharm. Biotechnol. 2018, 19, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Rong, Y.; Teng, Y.; Mu, J.; Zhuang, X.; Tseng, M.; Samykutty, A.; Zhang, L.; Yan, J.; Miller, D.; et al. Broccoli-Derived Nanoparticle Inhibits Mouse Colitis by Activating Dendritic Cell AMP-Activated Protein Kinase. Mol. Ther. 2017, 25, 1641–1654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Lu, Y.; Chen, X.; Muthuraj, P.G.; Li, X.; Pattabiraman, M.; Zempleni, J.; Kachman, S.D.; Natarajan, S.K.; Yu, J. Protective Role of Shiitake Mushroom-Derived Exosome-Like Nanoparticles in D-Galactosamine and Lipopolysaccharide-Induced Acute Liver Injury in Mice. Nutrients 2020, 12, 477. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Hou, D.; Chen, X.; Li, D.; Zhu, L.; Zhang, Y.; Li, J.; Bian, Z.; Liang, X.; Cai, X.; et al. Exogenous plant MIR168a specifically targets mammalian LDLRAP1: Evidence of cross-kingdom regulation by microRNA. Cell Res. 2012, 22, 107–126. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef] [Green Version]
- Teng, Y.; Ren, Y.; Sayed, M.; Hu, X.; Lei, C.; Kumar, A.; Hutchins, E.; Mu, J.; Deng, Z.; Luo, C.; et al. Plant-Derived Exosomal MicroRNAs Shape the Gut Microbiota. Cell Host Microbe 2018, 24, 637–652.e8. [Google Scholar] [CrossRef] [Green Version]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Wong, D.K.; Hong, K.Y.; Raffai, R.L. Cushioned-Density Gradient Ultracentrifugation (C-DGUC): A Refined and High Performance Method for the Isolation, Characterization, and Use of Exosomes. Methods Mol. Biol. 2018, 1740, 69–83. [Google Scholar]
- Zeringer, E.; Barta, T.; Li, M.; Vlassov, A.V. Strategies for isolation of exosomes. Cold Spring Harb. Protoc. 2015, 2015, 319–323. [Google Scholar] [CrossRef] [Green Version]
- Linares, R.; Tan, S.; Gounou, C.; Arraud, N.; Brisson, A.R. High-speed centrifugation induces aggregation of extracellular vesicles. J. Extracell. Vesicles 2015, 4, 29509. [Google Scholar] [CrossRef] [PubMed]
- Kalarikkal, S.P.; Prasad, D.; Kasiappan, R.; Chaudhari, S.R.; Sundaram, G.M. A cost-effective polyethylene glycol-based method for the isolation of functional edible nanoparticles from ginger rhizomes. Sci. Rep. 2020, 10, 4456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; McNamara, R.; Dittmer, D. Purification Methods and the Presence of RNA in Virus Particles and Extracellular Vesicles. Viruses 2020, 12, 917. [Google Scholar] [CrossRef] [PubMed]
- Deregibus, M.C.; Figliolini, F.; D′Antico, S.; Manzini, P.M.; Pasquino, C.; De Lena, M.; Tetta, C.; Brizzi, M.F.; Camussi, G. Charge-based precipitation of extracellular vesicles. Int. J. Mol. Med. 2016, 38, 1359–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, J.Y.; Kang, S.J.; Rhee, W.J. Isolation of cabbage exosome-like nanovesicles and investigation of their biological activities in human cells. Bioact. Mater. 2021, 6, 4321–4332. [Google Scholar] [CrossRef] [PubMed]
- Regente, M.; Corti-Monzón, G.; Maldonado, A.M.; Pinedo, M.; Jorrín, J.; de la Canal, L. Vesicular fractions of sunflower apoplastic fluids are associated with potential exosome marker proteins. FEBS Lett. 2009, 583, 3363–3366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, F.; Mu, J.; Teng, Y.; Zhang, X.; Sundaram, K.; Sriwastva, M.K.; Kumar, A.; Lei, C.; Zhang, L.; Liu, Q.M.; et al. Restoring Oat Nanoparticles Mediated Brain Memory Function of Mice Fed Alcohol by Sorting Inflammatory Dectin-1 Complex Into Microglial Exosomes. Small 2022, 18, e2105385. [Google Scholar] [CrossRef]
- Peters, D.L.; Harris, G.; Davis, C.M.; Dennis, J.J.; Chen, W. Bacteriophage Isolation, Purification, and Characterization Techniques Against Ubiquitous Opportunistic Pathogens. Curr. Protoc. 2022, 2, e594. [Google Scholar] [CrossRef]
- Dumke, R.; Geissler, M.; Skupin, A.; Helm, B.; Mayer, R.; Schubert, S.; Oertel, R.; Renner, B.; Dalpke, A. Simultaneous Detection of SARS-CoV-2 and Influenza Virus in Wastewater of Two Cities in Southeastern Germany, January to May 2022. Int. J. Environ. Res. Public Health 2022, 19, 13374. [Google Scholar] [CrossRef]
- Pandey, M.; Chawla, G. Purification of exosome-enriched proteins produced in a Drosophila cell line by size exclusion chromatography. STAR Protoc. 2022, 3, 101834. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alić, V.K.; Malenica, M.; Biberić, M.; Zrna, S.; Valenčić, L.; Šuput, A.; Fabris, L.K.; Wechtersbach, K.; Kojc, N.; Kurtjak, M.; et al. Extracellular Vesicles from Human Cerebrospinal Fluid Are Effectively Separated by Sepharose CL-6B-Comparison of Four Gravity-Flow Size Exclusion Chromatography Methods. Biomedicines 2022, 10, 785. [Google Scholar] [CrossRef] [PubMed]
- An, M.; Wu, J.; Zhu, J.; Lubman, D.M. Comparison of an Optimized Ultracentrifugation Method versus Size-Exclusion Chromatography for Isolation of Exosomes from Human Serum. J. Proteome Res. 2018, 17, 3599–3605. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Ma, J.; Zhou, Y.; Lu, R. Focusing on Future Applications and Current Challenges of Plant Derived Extracellular Vesicles. Pharmaceuticals 2022, 15, 708. [Google Scholar] [CrossRef]
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.-B.; Wang, B.; Zhang, L.; et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol. Ther. 2013, 21, 1345–1357. [Google Scholar] [CrossRef] [Green Version]
- Rome, S. Biological properties of plant-derived extracellular vesicles. Food Funct. 2019, 10, 529–538. [Google Scholar] [CrossRef]
- Yang, M.; Luo, Q.; Chen, X.; Chen, F. Bitter melon derived extracellular vesicles enhance the therapeutic effects and reduce the drug resistance of 5-fluorouracil on oral squamous cell carcinoma. J. Nanobiotechnol. 2021, 19, 259. [Google Scholar] [CrossRef]
- Wang, B.; Zhuang, X.; Deng, Z.-B.; Jiang, H.; Mu, J.; Wang, Q.; Xiang, X.; Guo, H.; Zhang, L.; Dryden, G.; et al. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol. Ther. 2014, 22, 522–534. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Li, S.; Zhang, S.; Wang, J. Plant-derived exosome-like nanoparticles and their therapeutic activities. Asian J. Pharm. Sci. 2022, 17, 53–69. [Google Scholar] [CrossRef]
- Zu, M.; Xie, D.; Canup, B.S.; Chen, N.; Wang, Y.; Sun, R.; Zhang, Z.; Fu, Y.; Dai, F.; Xiao, B. ‘Green’ nanotherapeutics from tea leaves for orally targeted prevention and alleviation of colon diseases. Biomaterials 2021, 279, 121178. [Google Scholar] [CrossRef]
- Ludwig, A.-K.; De Miroschedji, K.; Doeppner, T.R.; Börger, V.; Ruesing, J.; Rebmann, V.; Durst, S.; Jansen, S.; Bremer, M.; Behrmann, E.; et al. Precipitation with polyethylene glycol followed by washing and pelleting by ultracentrifugation enriches extracellular vesicles from tissue culture supernatants in small and large scales. J. Extracell. Vesicles 2018, 7, 1528109. [Google Scholar] [CrossRef] [Green Version]
- Weng, Y.; Sui, Z.; Shan, Y.; Hu, Y.; Chen, Y.; Zhang, L.; Zhang, Y. Effective isolation of exosomes with polyethylene glycol from cell culture supernatant for in-depth proteome profiling. Analyst 2016, 141, 4640–4646. [Google Scholar] [CrossRef] [PubMed]
- Matrajt, G.; Naughton, B.; Bandyopadhyay, A.S.; Meschke, J.S. A Review of the Most Commonly Used Methods for Sample Collection in Environmental Surveillance of Poliovirus. Clin. Infect. Dis. 2018, 67 (Suppl. S1), S90–S97. [Google Scholar] [CrossRef] [Green Version]
- Cong, M.; Tan, S.; Li, S.; Gao, L.; Huang, L.; Zhang, H.G.; Qiao, H. Technology insight: Plant-derived vesicles-How far from the clinical biotherapeutics and therapeutic drug carriers? Adv. Drug Deliv. Rev. 2022, 182, 114108. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, A.A.; Shegokar, R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.R.; Alberts, B.M.; Benzinger, R.; Lawhorne, L.; Treiber, G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology 1970, 40, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.J.; Booth, A.M.; Hildreth, J.E.K. The Trojan exosome hypothesis. Proc. Natl. Acad. Sci. USA 2003, 100, 10592–10597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rider, M.A.; Hurwitz, S.N.; Meckes, D.G., Jr. ExtraPEG: A Polyethylene Glycol-Based Method for Enrichment of Extracellular Vesicles. Sci. Rep. 2016, 6, 23978. [Google Scholar] [CrossRef] [PubMed]
- Madera-Contreras, A.M.; Solano-Texta, R.; Cisneros-Sarabia, A.; Bautista-Santos, I.; Vences-Velázquez, G.; Vences-Velázquez, A.; Cortés-Sarabia, K. Optimized method for the extraction of contaminant-free IgY antibodies from egg yolk using PEG 6000. MethodsX 2022, 9, 101874. [Google Scholar] [CrossRef]
- Sunoqrot, S.; Al-Bakri, A.G.; Ibrahim, L.H.; Aldaken, N. Amphotericin B-Loaded Plant-Inspired Polyphenol Nanoparticles Enhance Its Antifungal Activity and Biocompatibility. ACS Appl. Bio. Mater. 2022, 5, 5156–5164. [Google Scholar] [CrossRef]
- Carroll-Portillo, A.; Coffman, C.; Varga, M.; Alcock, J.; Singh, S.; Lin, H. Standard Bacteriophage Purification Procedures Cause Loss in Numbers and Activity. Viruses 2021, 13, 328. [Google Scholar] [CrossRef] [PubMed]
- Yun, L.; Wu, T.; Mao, Z.; Li, W.; Zhang, M.; Sun, X. A novel wheat germ polysaccharide: Structural characterization, potential antioxidant activities and mechanism. Int. J. Biol. Macromol. 2020, 165 Pt B, 1978–1987. [Google Scholar] [CrossRef]
- Shao, K.; Sun, J.; Lin, Y.; Zhi, H.; Wang, X.; Fu, Y.; Xu, J.; Liu, Z. Green Synthesis and Antimicrobial Study on Functionalized Chestnut-Shell-Extract Ag Nanoparticles. Antibiotics 2023, 12, 201. [Google Scholar] [CrossRef] [PubMed]
- Cassari, L.; Zamuner, A.; Messina, G.M.L.; Marsotto, M.; Chen, H.; Gonnella, G.; Coward, T.; Battocchio, C.; Huang, J.; Iucci, G.; et al. Bioactive PEEK: Surface Enrichment of Vitronectin-Derived Adhesive Peptides. Biomolecules 2023, 13, 246. [Google Scholar] [CrossRef] [PubMed]
- Suresh, A.P.; Kalarikkal, S.P.; Pullareddy, B.; Sundaram, G.M. Low pH-Based Method to Increase the Yield of Plant-Derived Nanoparticles from Fresh Ginger Rhizomes. ACS Omega 2021, 6, 17635–17641. [Google Scholar] [CrossRef]
- Samak, Y.O.; Santhanes, D.; El-Massik, M.A.; Coombes, A.G.A. Formulation strategies for achieving high delivery efficiency of thymoquinone-containing Nigella sativa extract to the colon based on oral alginate microcapsules for treatment of inflammatory bowel disease. J. Microencapsul. 2019, 36, 204–214. [Google Scholar] [CrossRef]
- Qin, X.; Wang, X.; Xu, K.; Zhang, Y.; Ren, X.; Qi, B.; Liang, Q.; Yang, X.; Li, L.; Li, S. Digestion of Plant Dietary miRNAs Starts in the Mouth under the Protection of Coingested Food Components and Plant-Derived Exosome-like Nanoparticles. J. Agric. Food Chem. 2022, 70, 4316–4327. [Google Scholar] [CrossRef]
- del Pozo-Acebo, L.; de Las Hazas, M.C.; Tomé-Carneiro, J.; del Saz-Lara, A.; Gil-Zamorano, J.; Balaguer, L.; Chapado, L.A.; Busto, R.; Visioli, F.; Dávalos, A. Therapeutic potential of broccoli-derived extracellular vesicles as nanocarriers of exogenous miRNAs. Pharmacol. Res. 2022, 185, 106472. [Google Scholar] [CrossRef]
- Li, Z.; Wang, H.; Yin, H.; Bennett, C.; Zhang, H.; Guo, P. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J. Extracell. Vesicles 2015, 4, 28713. [Google Scholar]








| SSF(pH = 7) | SGF(pH = 3) | SIF(pH = 7) | ||||||
|---|---|---|---|---|---|---|---|---|
| Constituent | Stock Solution | Volume | Concentration | Volume | Concentration | Volume | Concentration | |
| g/500 mL | mol/L | mL | mmol/L | mL | mmol/L | mL | mmol/L | |
| KCl | 18.5 | 0.5 | 3.02 | 15.1 | 1.38 | 6.9 | 1.36 | 6.8 |
| KH2PO4 | 34 | 0.5 | 0.74 | 3.7 | 0.18 | 0.9 | 0.16 | 0.8 |
| NaHCO3 | 42 | 1 | 1.37 | 13.7 | 2.55 | 25.5 | 8.5 | 85 |
| NaCl | 58.5 | 2 | 2.36 | 47.2 | 1.92 | 38.4 | ||
| MgCl2(H2O)6 | 15.25 | 0.15 | 0.1 | 0.15 | 0.08 | 0.1 | 0.22 | 0.33 |
| NH4Cl | 26.75 | 1 | 0.012 | 0.12 | 0.1 | 1 | ||
| Add H2O2 to | 80 | 80 | 80 | |||||
| Composition of the final solution | ||||||||
| Electrolyte stock solution | 40 | 40 | 40 | |||||
| CaCl2(H2O2)2 | 44.1 | 0.3 | 0.25 | 1.5 | 0.025 | 0.15 | 0.2 | 0.6 |
| α-amylase | 0.0025 g | 150 U/mL | ||||||
| Pepsin | 0.8 g | 4000 U/mL | ||||||
| Pancreatin | 0.05 g | 200 U/mL | ||||||
| Bile | 0.629 g | 20 mM | ||||||
| Add H2O2 to | 50 | 50 | 50 | |||||
| UC | Binding Energy(eV) | Atomic% |
| C1s | 285.27 | 64.67 |
| N1s | 399.72 | 13.84 |
| O1s | 531.53 | 21.49 |
| PEG | Binding Energy (eV) | Atomic% |
| C 1s | 284.85 | 64.79 |
| N 1s | 399.56 | 15.4 |
| O 1s | 531.3 | 19.81 |
| PEG+SEC | Binding Energy (eV) | Atomic% |
| C 1s | 284.8 | 67.26 |
| N 1s | 399.63 | 12.42 |
| O 1s | 531.29 | 20.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, J.; Zhu, Y. Isolation and In Vitro Stability Studies of Edible Plant-Seed Derived (Raphani Semen) Nanoparticles. Separations 2023, 10, 218. https://doi.org/10.3390/separations10030218
An J, Zhu Y. Isolation and In Vitro Stability Studies of Edible Plant-Seed Derived (Raphani Semen) Nanoparticles. Separations. 2023; 10(3):218. https://doi.org/10.3390/separations10030218
Chicago/Turabian StyleAn, Jiahui, and Yi Zhu. 2023. "Isolation and In Vitro Stability Studies of Edible Plant-Seed Derived (Raphani Semen) Nanoparticles" Separations 10, no. 3: 218. https://doi.org/10.3390/separations10030218
APA StyleAn, J., & Zhu, Y. (2023). Isolation and In Vitro Stability Studies of Edible Plant-Seed Derived (Raphani Semen) Nanoparticles. Separations, 10(3), 218. https://doi.org/10.3390/separations10030218