A Fixed-Bed Column with an Agro-Waste Biomass Composite for Controlled Separation of Sulfate from Aqueous Media
Abstract
:1. Introduction
2. Materials and Methods
2.1. Adsorbent Preparation
2.2. Sulfate Concentration Determination and Adsorption Experiments
2.3. Materials Characterization
2.3.1. Thermogravimetric Analysis (TGA)
2.3.2. FT-IR Spectroscopy
2.3.3. 13C Solids NMR Spectroscopy
2.3.4. Acid Stability
2.3.5. Mechanical Characteristics
2.3.6. SEM Imaging
2.3.7. Moisture Uptake and Density
3. Results and Discussion
3.1. FT-IR Spectral Characterization
3.2. 13C Solid State NMR Spectroscopy
3.3. Thermogravimetric Analysis
3.4. Mechanical Characteristics and Acid Stability
3.5. SEM Imaging
3.6. Equilibrium Adsorption and Sorbent Selection
3.6.1. Selection and Equilibrium Adsorption Experiment
3.6.2. Regeneration
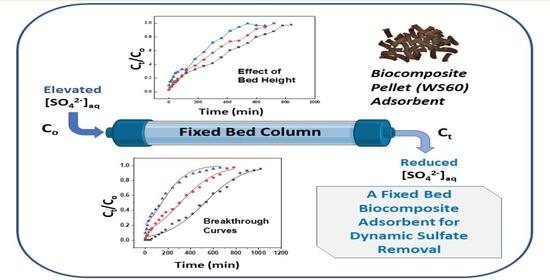
3.7. Fixed-Bed Column Adsorption Experiments
3.7.1. Effect of Initial Sulfate Concentration
3.7.2. Effect of Flow Rate
3.7.3. Effect of Bed Height
Thomas Model
3.7.4. Modeling of the Fixed-Bed Adsorption Results for Sulfate by the WS60 Pellet System
Yoon–Nelson Model
3.7.5. Error Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Environmental Protection Agency. Drinking Water Advisory: Consumer Acceptability Advice and Health Effects Analysis on Sulfate. Available online: https://www.epa.gov/sites/production/files/2014-09/documents/support_cc1_sulfate_healtheffects.pdf (accessed on 11 April 2023).
- Mossop, G.D.; Shetsen, I. Introduction to the Geological Atlas of the Western Canada Sedimentary Basin. Available online: https://ags.aer.ca/reports/atlas-of-the-western-canada-sedimentary-basin (accessed on 11 April 2023).
- Banks, P.J.; Banks, J.C. Relationship between Soil and Groundwater Salinity in the Western Canada Sedimentary Basin. Environ. Monit. Assess. 2019, 191, 761. [Google Scholar] [CrossRef]
- Feist, M.; Elford, C.; Bailey, P.; Campbell, J. A Review of Dugout and Well Water Tested for Livestock Quality in Southern Saskatchewan. Available online: https://pubsaskdev.blob.core.windows.net/pubsask-prod/106044/106044-Technical_Report_on_South_Saskatchewan_Water_Quality_-_March_2018.pdf (accessed on 11 April 2023).
- Bates, A.L.; Orem, W.H.; Harvey, J.W.; Spiker, E.C. Tracing Sources of Sulfur in the Florida Everglades. J. Environ. Qual. 2002, 31, 287–299. [Google Scholar] [CrossRef]
- Darbi, A.; Viraraghavan, T.; Jin, Y.-C.; Braul, L.; Corkal, D. Sulfate Removal from Water. Water Qual. Res. J. 2003, 38, 169–182. [Google Scholar] [CrossRef]
- Lee, D.-J.; Liu, X.; Weng, H.-L. Sulfate and Organic Carbon Removal by Microbial Fuel Cell with Sulfate-Reducing Bacteria and Sulfide-Oxidising Bacteria Anodic Biofilm. Bioresour. Technol. 2014, 156, 14–19. [Google Scholar] [CrossRef]
- Singh, N.B.; Nagpal, G.; Agrawal, S.; Rachna. Water Purification by Using Adsorbents: A Review. Environ. Technol. Innov. 2018, 11, 187–240. [Google Scholar] [CrossRef]
- Pincus, L.N.; Rudel, H.E.; Petrović, P.V.; Gupta, S.; Westerhoff, P.; Muhich, C.L.; Zimmerman, J.B. Exploring the Mechanisms of Selectivity for Environmentally Significant Oxo-Anion Removal during Water Treatment: A Review of Common Competing Oxo-Anions and Tools for Quantifying Selective Adsorption. Environ. Sci. Technol. 2020, 54, 9769–9790. [Google Scholar] [CrossRef]
- Hamad, H.N.; Idrus, S. Recent Developments in the Application of Bio-Waste-Derived Adsorbents for the Removal of Methylene Blue from Wastewater: A Review. Polymers 2022, 14, 783. [Google Scholar] [CrossRef]
- Desbrières, J.; Guibal, E. Chitosan for Wastewater Treatment. Polym. Int. 2018, 67, 7–14. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Conventional and Non-Conventional Adsorbents for Wastewater Treatment. Environ. Chem. Lett. 2019, 17, 195–213. [Google Scholar] [CrossRef]
- Solgi, M.; Tabil, L.G.; Wilson, L.D. Modified Biopolymer Adsorbents for Column Treatment of Sulfate Species in Saline Aquifers. Materials 2020, 13, 2408. [Google Scholar] [CrossRef]
- Zhou, H.; Margenot, A.J.; Li, Y.; Si, B.; Wang, T.; Zhang, Y.; Li, S.; Bhattarai, R. Phosphorus Pollution Control Using Waste-Based Adsorbents: Material Synthesis, Modification, and Sustainability. Crit. Rev. Environ. Sci. Technol. 2022, 52, 2023–2059. [Google Scholar] [CrossRef]
- UNESCO. Water Security and the Sustainable Development Goals, 1st ed.; Lim, K., Makarigakis, A.K., Sohn, O., Lee, B., Eds.; UNESCO: Paris, France, 2019. [Google Scholar]
- Elhafez, S.E.A.; Hamad, H.A.; Zaatout, A.A.; Malash, G.F. Management of Agricultural Waste for Removal of Heavy Metals from Aqueous Solution: Adsorption Behaviors, Adsorption Mechanisms, Environmental Protection, and Techno-Economic Analysis. Environ. Sci. Pollut. Res. 2017, 24, 1397–1415. [Google Scholar] [CrossRef]
- Ali, R.; Elsagan, Z.; AbdElhafez, S. Lignin from Agro-Industrial Waste to an Efficient Magnetic Adsorbent for Hazardous Crystal Violet Removal. Molecules 2022, 27, 1831. [Google Scholar] [CrossRef]
- Hamad, H.A.; AbdElhafez, S.E.; Elsenety, M.M.; Sorour, M.K.; Amin, N.K.; Abdelwahab, O.; El-Ashtoukhy, E.-S.Z. Fabrication and Characterization of Functionalized Lignin-Based Adsorbent Prepared from Black Liquor in the Paper Industry for Superior Removal of Toxic Dye. Fuel 2022, 323, 124288. [Google Scholar] [CrossRef]
- Kheilkordi, Z.; Mohammadi Ziarani, G.; Mohajer, F.; Badiei, A.; Varma, R.S. Waste-to-Wealth Transition: Application of Natural Waste Materials as Sustainable Catalysts in Multicomponent Reactions. Green Chem. 2022, 24, 4304–4327. [Google Scholar] [CrossRef]
- Jung, K.-W.; Jeong, T.-U.; Kang, H.-J.; Ahn, K.-H. Characteristics of Biochar Derived from Marine Macroalgae and Fabrication of Granular Biochar by Entrapment in Calcium-Alginate Beads for Phosphate Removal from Aqueous Solution. Bioresour. Technol. 2016, 211, 108–116. [Google Scholar] [CrossRef]
- Wang, Y.; Su, J.; Ali, A.; Chang, Q.; Bai, Y.; Gao, Z. Enhanced Nitrate, Manganese, and Phenol Removal by Polyvinyl Alcohol/Sodium Alginate with Biochar Gel Beads Immobilized Bioreactor: Performance, Mechanism, and Bacterial Diversity. Bioresour. Technol. 2022, 348, 126818. [Google Scholar] [CrossRef]
- Gubitosa, J.; Rizzi, V.; Cignolo, D.; Fini, P.; Fanelli, F.; Cosma, P. From Agricultural Wastes to a Resource: Kiwi Peels, as Long-Lasting, Recyclable Adsorbent, to Remove Emerging Pollutants from Water. The Case of Ciprofloxacin Removal. Sustain. Chem. Pharm. 2022, 29, 100749. [Google Scholar] [CrossRef]
- Hamidon, T.S.; Adnan, R.; Haafiz, M.K.M.; Hussin, M.H. Cationic Surfactant-Modified Cellulose Nanocrystal/Alginate Hydrogel Beads for Enhanced Adsorptive Removal of 4-Chlorophenol from Wastewater. J. Polym. Environ. 2022, 30, 5024–5048. [Google Scholar] [CrossRef]
- Mohamed, M.H.; Udoetok, I.A.; Solgi, M.; Steiger, B.G.K.; Zhou, Z.; Wilson, L.D. Design of Sustainable Biomaterial Composite Adsorbents for Point-of-Use Removal of Lead Ions From Water. Front. Water 2022, 4, 739492. [Google Scholar] [CrossRef]
- Steiger, B.G.K.; Zhou, Z.; Anisimov, Y.A.; Evitts, R.W.; Wilson, L.D. Valorization of Agro-Waste Biomass as Composite Adsorbents for Sustainable Wastewater Treatment. Ind. Crops Prod. 2023, 191, 115913. [Google Scholar] [CrossRef]
- Iwuozor, K.O.; Emenike, E.C.; Aniagor, C.O.; Iwuchukwu, F.U.; Ibitogbe, E.M.; Okikiola, T.B.; Omuku, P.E.; Adeniyi, A.G. Removal of Pollutants from Aqueous Media Using Cow Dung-Based Adsorbents. Curr. Res. Green Sustain. Chem. 2022, 5, 100300. [Google Scholar] [CrossRef]
- Jo, J.-Y.; Choi, J.-H.; Tsang, Y.F.; Baek, K. Pelletized Adsorbent of Alum Sludge and Bentonite for Removal of Arsenic. Environ. Pollut. 2021, 277, 116747. [Google Scholar] [CrossRef] [PubMed]
- Campbell, W.A. Adaptive Torrefaction of Stem Biomass in a Horizontal Moving Bed with Normalized Direct Measurement of Quality Characteristics. Ph.D. Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2018. Available online: http://hdl.handle.net/10388/11637 (accessed on 11 April 2023).
- IS 3025; (Part 24): Method of Sampling and Test (Physical and Chemical) for Water and Wastewater, Part 24: Sulphates (First Revision). Bureau of Indian Standards (BIS): Delhi, India, 2003.
- Morais, I.P.A.; Tóth, I.V.; Rangel, A.O.S.S. Turbidimetric and Nephelometric Flow Analysis: Concepts and Applications. Spectrosc. Lett. 2006, 39, 547–579. [Google Scholar] [CrossRef]
- Croker, D.M.; Kelly, D.M.; Horgan, D.E.; Hodnett, B.K.; Lawrence, S.E.; Moynihan, H.A.; Rasmuson, Å.C. Demonstrating the Influence of Solvent Choice and Crystallization Conditions on Phenacetin Crystal Habit and Particle Size Distribution. Org. Process Res. Dev. 2015, 19, 1826–1836. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Über Die Adsorption in Lösungen. Z. Phys. Chem. 1906, 57, 385–470. [Google Scholar] [CrossRef]
- Runtti, H.; Tolonen, E.-T.; Tuomikoski, S.; Luukkonen, T.; Lassi, U. How to Tackle the Stringent Sulfate Removal Requirements in Mine Water Treatment—A Review of Potential Methods. Environ. Res. 2018, 167, 207–222. [Google Scholar] [CrossRef]
- Fernando, W.A.M.; Ilankoon, I.M.S.K.; Syed, T.H.; Yellishetty, M. Challenges and Opportunities in the Removal of Sulphate Ions in Contaminated Mine Water: A Review. Miner. Eng. 2018, 117, 74–90. [Google Scholar] [CrossRef]
- Hong, S.; Cannon, F.S.; Hou, P.; Byrne, T.; Nieto-Delgado, C. Adsorptive Removal of Sulfate from Acid Mine Drainage by Polypyrrole Modified Activated Carbons: Effects of Polypyrrole Deposition Protocols and Activated Carbon Source. Chemosphere 2017, 184, 429–437. [Google Scholar] [CrossRef]
- Jozanikohan, G.; Abarghooei, M.N. The Fourier Transform Infrared Spectroscopy (FTIR) Analysis for the Clay Mineralogy Studies in a Clastic Reservoir. J. Pet. Explor. Prod. Technol. 2022, 12, 2093–2106. [Google Scholar] [CrossRef]
- Lim, S.-H.; Hudson, S.M. Synthesis and Antimicrobial Activity of a Water-Soluble Chitosan Derivative with a Fiber-Reactive Group. Carbohydr. Res. 2004, 339, 313–319. [Google Scholar] [CrossRef]
- Eddya, M.; Tbib, B.; EL-Hami, K. A Comparison of Chitosan Properties after Extraction from Shrimp Shells by Diluted and Concentrated Acids. Heliyon 2020, 6, e03486. [Google Scholar] [CrossRef]
- Paluszkiewicz, C.; Stodolak, E.; Hasik, M.; Blazewicz, M. FT-IR Study of Montmorillonite–Chitosan Nanocomposite Materials. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 784–788. [Google Scholar] [CrossRef]
- Santoni, I.; Callone, E.; Sandak, A.; Sandak, J.; Dirè, S. Solid State NMR and IR Characterization of Wood Polymer Structure in Relation to Tree Provenance. Carbohydr. Polym. 2015, 117, 710–721. [Google Scholar] [CrossRef]
- Hospodarova, V.; Singovszka, E.; Stevulova, N. Characterization of Cellulosic Fibers by FTIR Spectroscopy for Their Further Implementation to Building Materials. Am. J. Anal. Chem. 2018, 9, 303–310. [Google Scholar] [CrossRef]
- Karunarathna, M.S.; Smith, R.C. Valorization of Lignin as a Sustainable Component of Structural Materials and Composites: Advances from 2011 to 2019. Sustainability 2020, 12, 734. [Google Scholar] [CrossRef]
- Emiola-Sadiq, T.; Zhang, L.; Dalai, A.K. Thermal and Kinetic Studies on Biomass Degradation via Thermogravimetric Analysis: A Combination of Model-Fitting and Model-Free Approach. ACS Omega 2021, 6, 22233–22247. [Google Scholar] [CrossRef]
- Anisimov, Y.A.; Steiger, B.G.K.; Cree, D.E.; Wilson, L.D. Moisture Content and Mechanical Properties of Bio-Waste Pellets for Fuel and/or Water Remediation Applications. J. Compos. Sci. 2023, 7, 100. [Google Scholar] [CrossRef]
- Mohamed, M.H.; Udoetok, I.A.; Wilson, L.D. Animal Biopolymer-Plant Biomass Composites: Synergism and Improved Sorption Efficiency. J. Compos. Sci. 2020, 4, 15. [Google Scholar] [CrossRef]
- Esmaeili, H.; Foroutan, R.; Jafari, D.; Aghil Rezaei, M. Effect of Interfering Ions on Phosphate Removal from Aqueous Media Using Magnesium Oxide@ferric Molybdate Nanocomposite. Korean J. Chem. Eng. 2020, 37, 804–814. [Google Scholar] [CrossRef]
- Kalam, S.; Abu-Khamsin, S.A.; Kamal, M.S.; Patil, S. Surfactant Adsorption Isotherms: A Review. ACS Omega 2021, 6, 32342–32348. [Google Scholar] [CrossRef] [PubMed]
- Sumesh, K.; Saikrishnan, G.; Pandiyan, P.; Prabhu, L.; Gokulkumar, S.; Priya, A.; Spatenka, P.; Krishna, S. The Influence of Different Parameters in Tribological Characteristics of Pineapple/Sisal/TiO2 Filler Incorporation. J. Ind. Text. 2022, 51, 8626S–8644S. [Google Scholar] [CrossRef]
- Steiger, B.G.K.; Wilson, L.D. Modular Chitosan-Based Adsorbents for Tunable Uptake of Sulfate from Water. Int. J. Mol. Sci. 2020, 21, 7130. [Google Scholar] [CrossRef]
- Chen, C.; Chen, Z.; Shen, J.; Kang, J.; Zhao, S.; Wang, B.; Chen, Q.; Li, X. Dynamic Adsorption Models and Artificial Neural Network Prediction of Mercury Adsorption by a Dendrimer-Grafted Polyacrylonitrile Fiber in Fixed-Bed Column. J. Clean. Prod. 2021, 310, 127511. [Google Scholar] [CrossRef]
- Bacelo, H.; Santos, S.C.R.; Ribeiro, A.; Boaventura, R.A.R.; Botelho, C.M.S. Antimony Removal from Water by Pine Bark Tannin Resin: Batch and Fixed-Bed Adsorption. J. Environ. Manag. 2022, 302 Pt B, 114100. [Google Scholar] [CrossRef]
- Egbosiuba, T.C.; Abdulkareem, A.S. Highly Efficient As-Synthesized and Oxidized Multi-Walled Carbon Nanotubes for Copper(II) and Zinc(II) Ion Adsorption in a Batch and Fixed-Bed Process. J. Mater. Res. Technol. 2021, 15, 2848–2872. [Google Scholar] [CrossRef]
- Du, L.; Yang, J.; Xu, X. Highly Enhanced Adsorption of Dimethyl Disulfide from Model Oil on MOF-199/Attapulgite Composites. Ind. Eng. Chem. Res. 2019, 58, 2009–2016. [Google Scholar] [CrossRef]
- Basu, M.; Guha, A.K.; Ray, L. Adsorption of Lead on Lentil Husk in Fixed Bed Column Bioreactor. Bioresour. Technol. 2019, 283, 86–95. [Google Scholar] [CrossRef]
- Chatterjee, S.; Mondal, S.; De, S. Design and Scaling up of Fixed Bed Adsorption Columns for Lead Removal by Treated Laterite. J. Clean. Prod. 2018, 177, 760–774. [Google Scholar] [CrossRef]
- Luo, X.; Yuan, J.; Liu, Y.; Liu, C.; Zhu, X.; Dai, X.; Ma, Z.; Wang, F. Improved Solid-Phase Synthesis of Phosphorylated Cellulose Microsphere Adsorbents for Highly Effective Pb2+ Removal from Water: Batch and Fixed-Bed Column Performance and Adsorption Mechanism. ACS Sustain. Chem. Eng. 2017, 5, 5108–5117. [Google Scholar] [CrossRef]
- Kutty, S.R.M.; Almahbashi, N.M.Y.; Nazrin, A.A.M.; Malek, M.A.; Noor, A.; Baloo, L.; Ghaleb, A.A.S. Adsorption Kinetics of Colour Removal from Palm Oil Mill Effluent Using Wastewater Sludge Carbon in Column Studies. Heliyon 2019, 5, e02439. [Google Scholar] [CrossRef] [PubMed]
- Jha, D.; Haider, M.B.; Kumar, R.; Shim, W.G.; Marriyappan Sivagnanam, B. Batch and Continuous Adsorptive Desulfurization of Model Diesel Fuels Using Graphene Nanoplatelets. J. Chem. Eng. Data 2020, 65, 2120–2132. [Google Scholar] [CrossRef]











| Citation | Adsorbent Material (Pellets or Beads) | Contaminant | Capacity (mg/g) | Year |
|---|---|---|---|---|
| Jung et al. [20] | Alginate/Biochar | Phosphate | 158 | 2016 |
| Solgi et al. [13] | Chitosan/Ca-Chitosan | Sulfate | 47 | 2020 |
| Wang et al. [21] * | Alginate/Corncob Char/Bacteria | Nitrate/phenol/Mn(II) | 95/73/94 * | 2022 |
| Gubitosa et al. [22] | Kiwi Peel | Ciprofloxacin | 40 | 2022 |
| Hamidon et al. [23] | Nanocellulose Beads | 4-Chlorophenol | 65 | 2022 |
| Mohamed et al. [24] | Agro-Waste/Chitosan | Lead | 1.9 | 2022 |
| Steiger et al. [25] | Agro-Waste/Chitosan | Methylene Blue | 135 | 2023 |
| Sample ID | Young’s Modulus (E, MPa; Dry) | Young’s Modulus (E, MPa; Wet) | Density (ρ, g/cm3) | Moisture Uptake (wt.%) |
|---|---|---|---|---|
| WS60 | 1447 ± 64 | 102 ± 10 | 1.29 ± 0.03 | 340 ± 9 |
| # | C0 (mg/L) | Z (cm) | Q (mL/min) | ts (min) | qe (mg/g) |
|---|---|---|---|---|---|
| 1 | 500 | 40 | 2 | 1020 | 26.1 |
| 2 | 1100 | 40 | 2 | 740 | 38.7 |
| 3 | 2100 | 40 | 2 | 540 | 39.5 |
| 4 | 1100 | 20 | 2 | 620 | 45.7 |
| 5 | 1100 | 30 | 2 | 780 | 38.0 |
| 6 | 1100 | 30 | 5 | 550 | 60.2 |
| 7 | 1100 | 30 | 8 | 360 | 59.0 |
| C0 (mg/L) | Z (cm) | Q (mL/min) | Thomas Model | Yoon–Nelson Model | ||||
|---|---|---|---|---|---|---|---|---|
| kTH (mL g−1 min−1) | qTH (mg/g) | R2 | kYN (min−1) | τ (min) | R2 | |||
| 500 | 40 | 2 | 0.0119 | 26.1 | 0.989 | 0.0059 | 522.4 | 0.989 |
| 1100 | 40 | 2 | 0.0058 | 35.6 | 0.980 | 0.0064 | 307.7 | 0.981 |
| 2100 | 40 | 2 | 0.0044 | 36.6 | 0.976 | 0.0092 | 144.7 | 0.976 |
| 1100 | 20 | 2 | 0.0077 | 42.0 | 0.990 | 0.0085 | 190.9 | 0.990 |
| 1100 | 30 | 2 | 0.0060 | 46.1 | 0.978 | 0.0071 | 263.4 | 0.978 |
| 1100 | 30 | 5 | 0.0068 | 57.4 | 0.973 | 0.0083 | 212.7 | 0.977 |
| 1100 | 30 | 8 | 0.0120 | 54.3 | 0.950 | 0.0140 | 61.5 | 0.960 |
| C0 (mg/L) | Z (cm) | Q (mL/min) | Thomas Model | Yoon–Nelson Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| kTH (mL g−1 min−1) | qTH (mg/g) | R2 | SSE | χ2 | kYN (min−1) | τ (min) | R2 | SSE | χ2 | |||
| 500 | 40 | 2 | 0.0119 | 26.1 | 0.989 | 0.03 | 0.001 | 0.0059 | 522.4 | 0.989 | 0.03 | 0.001 |
| 1100 | 40 | 2 | 0.0058 | 35.6 | 0.980 | 0.04 | 0.002 | 0.0064 | 307.7 | 0.981 | 0.04 | 0.002 |
| 2100 | 40 | 2 | 0.0044 | 36.6 | 0.976 | 0.03 | 0.002 | 0.0092 | 144.7 | 0.976 | 0.03 | 0.002 |
| 1100 | 20 | 2 | 0.0077 | 42.0 | 0.990 | 0.03 | 0.001 | 0.0085 | 190.9 | 0.990 | 0.02 | 0.001 |
| 1100 | 30 | 2 | 0.0060 | 46.1 | 0.978 | 0.05 | 0.02 | 0.0071 | 263.4 | 0.978 | 0.05 | 0.003 |
| 1100 | 30 | 5 | 0.0068 | 57.4 | 0.973 | 0.04 | 0.03 | 0.0083 | 212.7 | 0.977 | 0.03 | 0.002 |
| 1100 | 30 | 8 | 0.0120 | 54.3 | 0.950 | 0.04 | 0.03 | 0.0140 | 61.5 | 0.960 | 0.03 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solgi, M.; Steiger, B.G.K.; Wilson, L.D. A Fixed-Bed Column with an Agro-Waste Biomass Composite for Controlled Separation of Sulfate from Aqueous Media. Separations 2023, 10, 262. https://doi.org/10.3390/separations10040262
Solgi M, Steiger BGK, Wilson LD. A Fixed-Bed Column with an Agro-Waste Biomass Composite for Controlled Separation of Sulfate from Aqueous Media. Separations. 2023; 10(4):262. https://doi.org/10.3390/separations10040262
Chicago/Turabian StyleSolgi, Mostafa, Bernd G. K. Steiger, and Lee D. Wilson. 2023. "A Fixed-Bed Column with an Agro-Waste Biomass Composite for Controlled Separation of Sulfate from Aqueous Media" Separations 10, no. 4: 262. https://doi.org/10.3390/separations10040262
APA StyleSolgi, M., Steiger, B. G. K., & Wilson, L. D. (2023). A Fixed-Bed Column with an Agro-Waste Biomass Composite for Controlled Separation of Sulfate from Aqueous Media. Separations, 10(4), 262. https://doi.org/10.3390/separations10040262