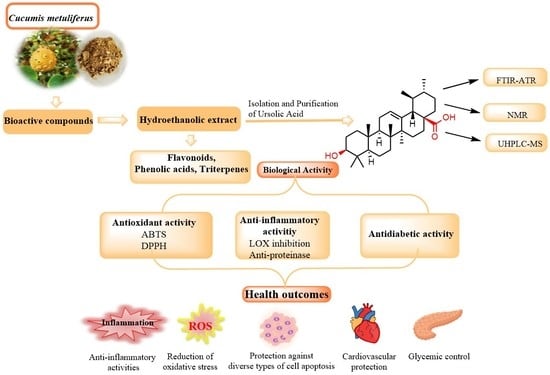
Cucumis metuliferus L. Fruits Extract with Antioxidant, Anti-Inflammatory, and Antidiabetic Properties as Source of Ursolic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents, Plant Materials, and Apparatus
2.2. Preparation of Crude Exact of C. metuliferus
2.3. HPLC-DAD Analysis of the Extract
2.4. Isolation and Purification of Ursolic Acid from C. metuliferus Extract
2.5. Structure Elucidation and Identification of the Isolated Compound
2.5.1. Attenuated Total Reflection Fourier Transform Infrared Spectroscopy (FTIR-ATR) Analysis
2.5.2. Analysis of Fraction by UHPLC-MS
2.5.3. NMR Analysis
2.6. Antioxidant Assay
2.6.1. DPPH Scavenging Activity
2.6.2. ABTS Radical Cation Decolorization Assay
2.7. In Vitro Anti-Inflammatory Activities
2.7.1. Anti-Lipoxygenase Activity
2.7.2. Anti-Proteinase Action
2.8. In Vitro Evaluation of the Antidiabetic Activity
2.9. Statistical Analysis
3. Results and Discussion
3.1. Preparation of Crude Exact of C. metuliferus
3.2. Identification of Bioactive Compounds by HPLC
3.3. Analysis and Identification of Ursolic Acid
3.3.1. FT-IR Analysis
3.3.2. Sample Analysis by UPLC-MS
3.3.3. NMR Analysis
3.4. Evaluation of the Antioxidant Activity
3.5. Evaluation of the in Vitro Anti-Inflammatory Activities
3.6. In Vitro Evaluation of the Antidiabetic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fischer, N.M.; Pallazola, V.A.; Xun, H.; Cainzos-Achirica, M.; Michos, E.D. The evolution of the heart-healthy Ddet for vascular health: A walk through Time. Vasc. Med. 2020, 25, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Dzah, C.S.; Asante-Donyinah, D.; Letsyo, E.; Dzikunoo, J.; Adams, Z.S. Dietary olyphenols and obesity: A Review of polyphenol effects on lipid and glucose metabolism, mitochondrial homeostasis, and starch digestibility and absorption. Plant Foods Hum. Nutr. 2023, 78, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lutz, M. Healthy sustainable food patterns and systems: A planetary urgency. Medwave 2021, 21, e8436. [Google Scholar] [CrossRef] [PubMed]
- Dinu, M.; Pagliai, G.; Sofi, F. A heart-healthy diet: Recent insights and practical recommendations. Curr. Cardiol. Rep. 2017, 19, 95. [Google Scholar] [CrossRef]
- Van Raaij, J.; Hendriksen, M.; Verhagen, H. Potential for improvement of population diet through reformulation of commonly eaten foods. Public Health Nutr. 2009, 12, 325–330. [Google Scholar] [CrossRef]
- Herforth, A.; Arimond, M.; Álvarez-Sánchez, C.; Coates, J.; Christianson, K.; Muehlhoff, E. A global review of food-based dietary guidelines. Adv. Nutr. 2019, 10, 590–605. [Google Scholar] [CrossRef]
- Ekstrand, B.; Scheers, N.; Rasmussen, M.K.; Young, J.F.; Ross, A.B.; Landberg, R. Brain Foods—The role of diet in brain performance and health. Nutr. Rev. 2021, 79, 693–708. [Google Scholar] [CrossRef]
- Mozaffarian, D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef]
- Pistollato, F.; Iglesias, R.C.; Ruiz, R.; Aparicio, S.; Crespo, J.; Lopez, L.D.; Manna, P.P.; Giampieri, F.; Battino, M. Nutritional patterns associated with the maintenance of neurocognitive functions and the risk of dementia and Alzheimer’s disease: A focus on human studies. Pharmacol. Res. 2018, 131, 32–43. [Google Scholar] [CrossRef]
- Opie, R.S.; Itsiopoulos, C.; Parletta, N.; Sanchez-Villegas, A.; Akbaraly, T.N.; Ruusunen, A.; Jacka, F.N. Dietary Recommendations for the prevention of depression. Nutr. Neurosci. 2017, 20, 161–171. [Google Scholar] [CrossRef]
- Wang, X. Healthy diet during pregnancy—Navigating the double-ddged Sword. Am. J. Clin. Nutr. 2021, 114, 414–415. [Google Scholar] [CrossRef] [PubMed]
- Omokhua-Uyi, A.G.; Van Staden, J. Phytomedicinal relevance of South African Cucurbitaceae species and their safety assessment: A review. J. Ethnopharmacol. 2020, 259, 112967. [Google Scholar] [CrossRef] [PubMed]
- Busuioc, A.C.; Botezatu, A.V.D.; Furdui, B.; Vinatoru, C.; Maggi, F.; Caprioli, G.; Dinica, R.M. Comparative study of the chemical compositions and antioxidant activities of fresh juices from Romanian Cucurbitaceae varieties. Molecules 2020, 25, 5468. [Google Scholar] [CrossRef] [PubMed]
- Murthy, H.N.; Paek, K.-Y. Bioactive Compounds in Underutilized Vegetables and Legumes; Murthy, H.N., Paek, K.Y., Eds.; Reference Series in Phytochemistry; Springer International Publishing: Cham, Switzerland, 2021; ISBN 978-3-030-57414-7. [Google Scholar]
- Usman, J.G.; Sodipo, O.A.; Kwaghe, A.V.; Sandabe, U.K. Uses of Cucumis metuliferus: A review. Cancer Biol. 2015, 5, 24–34. [Google Scholar]
- Bisognin, D.A. Origin and evolution of cultivated Cucurbits. Ciência Rural 2002, 32, 715–723. [Google Scholar] [CrossRef]
- Hartman, J.L.; Wehner, T.C.; Ma, G.; Perkins-Veazie, P. Citrulline and arginine content of taxa of Cucurbitaceae. Horticulturae 2019, 5, 22. [Google Scholar] [CrossRef]
- Mallick, M.F.R.; Masui, M. Origin, Distribution and taxonomy of melons. Sci. Hortic. 1986, 28, 251–261. [Google Scholar] [CrossRef]
- González, R.; Ballester, I.; López-Posadas, R.; Suárez, M.D.; Zarzuelo, A.; Martínez-Augustin, O.; Sánchez de Medina, F. Effects of flavonoids and other polyphenols on inflammation. Crit. Rev. Food Sci. Nutr. 2011, 51, 331–362. [Google Scholar] [CrossRef]
- Innih, S.O.; Eze, I.G.; Omage, K. Cardiovascular benefits of Momordica charantia in cholesterol-fed wistar rats. Clin. Phytosci. 2021, 7, 65. [Google Scholar] [CrossRef]
- De Melo, M.L.S.; Narain, N.; Bora, P.S. Characterisation of some nutritional constituents of melon (Cucumis melo hybrid AF-522) seeds. Food Chem. 2000, 68, 411–414. [Google Scholar] [CrossRef]
- Šovljanski, O.; Šeregelj, V.; Pezo, L.; Šaponjac, V.T.; Vulić, J.; Cvanić, T.; Markov, S.; Ćetković, G.; Čanadanović-Brunet, J. Horned melon pulp, peel, and seed: New insight into phytochemical and biological properties. Antioxidants 2022, 11, 825. [Google Scholar] [CrossRef] [PubMed]
- Šeregelj, V.; Šovljanski, O.; Šaponjac, V.T.; Vulić, J.; Ćetković, G.; Markov, S.; Čanadanović-Brunet, J. Horned melon (Cucumis metuliferus E. Meyer Ex. Naudin)—Current knowledge on its phytochemicals, biological benefits, and potential applications. Processes 2022, 10, 94. [Google Scholar] [CrossRef]
- Ferrara, L. The dietary importance of a tropical fruit: The kiwano. Ingred. Aliment. 2006, 5, 14–17. [Google Scholar]
- Anyanwu, A.; Jimam, N.; Wannang, N. Assessment of the effects of Cucumis metuliferus fruits alkaloids against Newcastle disease virus-LaSota. Environ. Dis. 2016, 1, 130. [Google Scholar] [CrossRef]
- Seo, D.Y.; Lee, S.R.; Heo, J.W.; No, M.H.; Rhee, B.D.; Ko, K.S.; Kwak, H.B.; Han, J. Ursolic acid in health and disease. Korean J. Physiol. Pharmacol. 2018, 22, 235–248. [Google Scholar] [CrossRef]
- Ramos-Hryb, A.B.; Pazini, F.L.; Kaster, M.P.; Rodrigues, A.L.S. Therapeutic potential of ursolic acid to manage neurodegenerative and psychiatric diseases. CNS Drugs 2017, 31, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Abbasi, B.A.; Ahmad, R.; Mahmood, T.; Kanwal, S.; Ali, B.; Khalil, A.T.; Shah, S.A.; Alam, M.M.; Badshah, H. Ursolic acid a promising candidate in the therapeutics of breast cancer: Current status and future implications. Biomed. Pharmacother. 2018, 108, 752–756. [Google Scholar] [CrossRef]
- Venugopal, R.; Liu, R.H. Phytochemicals in diets for breast cancer prevention: The importance of resveratrol and ursolic acid. Food Sci. Hum. Wellness 2012, 1, 1–13. [Google Scholar] [CrossRef]
- Ramirez, C.N.; Li, W.; Zhang, C.; Wu, R.; Su, S.; Wang, C.; Gao, L.; Yin, R.; Kong, A.N.T. In vitro-in vivo dose response of ursolic acid, sulforaphane, PEITC, and curcumin in cancer prevention. AAPS J. 2018, 20, 19. [Google Scholar] [CrossRef]
- Gu, W.; Hao, Y.; Zhang, G.; Wang, S.F.; Miao, T.T.; Zhang, K.P. Synthesis, in vitro antimicrobial and cytotoxic activities of new carbazole derivatives of ursolic acid. Bioorg. Med. Chem. Lett. 2015, 25, 554–557. [Google Scholar] [CrossRef]
- Shao, J.W.; Dai, Y.C.; Xue, J.P.; Wang, J.C.; Lin, F.P.; Guo, Y.H. In vitro and in vivo anticancer activity evaluation of ursolic acid derivatives. Eur. J. Med. Chem. 2011, 46, 2652–2661. [Google Scholar] [CrossRef]
- Mlala, S.; Oyedeji, A.O.; Gondwe, M.; Oyedeji, O.O. Ursolic acid and its derivatives as bioactive agents. Molecules 2019, 24, 2751. [Google Scholar] [CrossRef]
- Wu, P.P.; Zhang, K.; Lu, Y.J.; He, P.; Zhao, S.Q. In vitro and in vivo evaluation of the antidiabetic activity of ursolic acid derivatives. Eur. J. Med. Chem. 2014, 80, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Li, Y.; Liu, X.; Jiang, S. Determination of free isomeric oleanolic acid and ursolic acid in Pterocephalus hookeri by capillary zone electrophoresis. J. Pharm. Biomed. Anal. 2007, 43, 1331–1334. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, L.; Qiu, H.; Zhang, X.; Guo, W.; Chen, W.; Tian, Y.; Fu, L.; Shi, D.; Cheng, J.; et al. Ursolic acid simultaneously targets multiple signaling pathways to suppress proliferation and induce apoptosis in colon cancer cells. PLoS ONE 2013, 8, e63872. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.H.; Su, Q.; Zang, Z.H. Simultaneous determination of oleanolic acid and ursolic acid by RP-HPLC in the leaves of Eriobotrya japonica Lindl. J. Pharm. Anal. 2012, 2, 238–240. [Google Scholar] [CrossRef]
- Shanmuga Sundaram, R.; Ramanathan, M.; Rajesh, R.; Satheesh, B.; Saravanan, D. LC-MS quantification of rosmarinic acid and ursolic acid in the Ocimum sanctum Linn. leaf extract (Holy Basil, Tulsi). J. Liq. Chromatogr. Relat. Technol. 2012, 35, 634–650. [Google Scholar] [CrossRef]
- Fan, J.P.; Kong, T.; Zhang, L.; Tong, S.; Tian, Z.Y.; Duan, Y.H.; Zhang, X.H. Solubilities of ursolic acid and oleanolic acid in four solvents from (283.2 to 329.7) K. J. Chem. Eng. Data 2011, 56, 2723–2725. [Google Scholar] [CrossRef]
- Fan, J.P.; Liao, D.D.; Zhang, X.H. Ultrasonic assisted extraction of ursolic acid from apple pomace: A novel and facile technique. Sep. Sci. Technol. 2016, 51, 1344–1350. [Google Scholar] [CrossRef]
- Wang, X.; Sun, W.; Cao, J.; Qu, H.; Bi, X.; Zhao, Y. Structures of new triterpenoids and cytotoxicity activities of the isolated major compounds from the fruit of Momordica charantia L. J. Agric. Food Chem. 2012, 60, 3927–3933. [Google Scholar] [CrossRef]
- Tian, S.; Shi, Y.; Yu, Q.; Upur, H. Determination of oleanolic acid and ursolic acid contents in Ziziphora clinopodioides Lam. by HPLC method. Pharmacogn. Mag. 2010, 6, 116–119. [Google Scholar] [CrossRef]
- Wandjou, J.G.N.; Mevi, S.; Sagratini, G.; Vittori, S.; Dall’acqua, S.; Caprioli, G.; Lupidi, G.; Mombelli, G.; Arpini, S.; Allegrini, P.; et al. Antioxidant and enzyme inhibitory properties of the polyphenolic-rich extract from an ancient apple variety of central Italy (Mela rosa dei Monti Sibillini). Plants 2020, 9, 9. [Google Scholar] [CrossRef]
- Verma, S.C.; Jain, C.L.; Kumari, A.; Padhi, M.M.; Devalla, R.B. Microwave-assisted extraction and rapid isolation of ursolic acid from the leaves of eucalyptus × Hybrida maiden and its quantification using HPLC-diode array Technique. J. Sep. Sci. 2013, 36, 1255–1262. [Google Scholar] [CrossRef]
- Kaur, P.; Gupta, R.C.; Dey, A.; Kumar Pandey, D. Simultaneous quantification of oleanolic acid, ursolic acid, betulinic acid and lupeol in different populations of five Swertia species by using HPTLC-densitometry: Comparison of different extraction methods and solvent selection. Ind. Crops Prod. 2019, 130, 537–546. [Google Scholar] [CrossRef]
- Zongo, E.; Busuioc, A.; Meda, R.N.-T.; Botezatu, A.V.; Mihaila, M.D.; Mocanu, A.-M.; Avramescu, S.M.; Koama, B.K.; Kam, S.E.; Belem, H.; et al. Exploration of the antioxidant and anti-inflammatory potential of Cassia sieberiana DC and Piliostigma thonningii (Schumach.) Milne-Redh, traditionally used in the treatment of hepatitis in the Hauts-Bassins region of Burkina Faso. Pharmaceuticals 2023, 16, 133. [Google Scholar] [CrossRef] [PubMed]
- Costea, L.; Chițescu, C.L.; Boscencu, R.; Ghica, M.; Lupuliasa, D.; Mihai, D.P.; Deculescu-ioniță, T.; Duțu, L.E.; Popescu, M.L.; Luță, E.A.; et al. The polyphenolic profile and antioxidant activity of five vegetal extracts with hepatoprotective potential. Plants 2022, 11, 1680. [Google Scholar] [CrossRef]
- Fouedjou, R.T.; Nguelefack, E.P.; Ponou, B.K.; Nguelefack, T.B.; Barboni, L.; Tapondjou, L.A. Antioxidant activities and chemical constituents of extracts from Cordyline fruticosa (L.) A. Chev. (Agavaceae) and Eriobotrya japonica (Thunb) Lindl, (Rosaceae). Pharmacologia 2016, 7, 103–113. [Google Scholar] [CrossRef]
- Kaur, N.; Chahal, K.; Singh, R.U. Phytochemical screening and antioxidant activity of Anethum graveolens L. seed extracts. Pharma Innov. J. 2018, 7, 324–329. [Google Scholar]
- Matsusaka, Y.; Kawabata, J. Evaluation of antioxidant capacity of non-edible parts of some selected tropical fruits. Food Sci. Technol. Res. 2010, 16, 467–472. [Google Scholar] [CrossRef]
- Sirivibulkovit, K.; Nouanthavong, S.; Sameenoi, Y. Paper-Based DPPH assay for antioxidant activity analysis. Anal. Sci. 2018, 34, 795–800. [Google Scholar] [CrossRef]
- Dinica, R.M.; Sandu, C.; Botezatu, A.V.D.; Busuioc, A.C.; Balanescu, F.; Mihaila, M.D.I.; Dumitru, C.N.; Furdui, B.; Iancu, A.V. Allantoin from valuable Romanian animal and plant sources with promising anti-inflammatory activity as a nutricosmetic ingredient. Sustainability 2021, 13, 170. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Park, S.J.; Mariadoss, A.V.A.; Sathiyaseelan, A.; Veeraraghavan, V.P.; Kim, S.J.; Wang, M.H. Chemical composition, cntioxidant, and anti-diabetic activities of ethyl acetate fraction of Stachys riederi Var. Japonica (Miq.) in streptozotocin-induced Type 2 diabetic mice. Food Chem. Toxicol. 2021, 155, 112374. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Bălănescu, F.; Botezatu, A.V.; Marques, F.; Busuioc, A.; Marincaş, O.; Vînătoru, C.; Cârâc, G.; Furdui, B.; Dinica, R.M. Bridging the chemical profile and biological activities of a new variety of Agastache foeniculum (Pursh) Kuntze extracts and essential oil. Int. J. Mol. Sci. 2023, 24, 828. [Google Scholar] [CrossRef]
- Okpoko, C.; Ezenyi, I.; Adzu, B.; Salawu, O. Evaluation of two medicinal plants used for arthritis in Northern Nigeria with focus on Terminalia avicennioides Guill. & Perr. and its mechanism of action. Sci. African 2020, 8, e00357. [Google Scholar] [CrossRef]
- Fofana, S.; Gnoula, C.; Draogo, M.O.E.; Eacute, E.P.; Eacute, R.H.N.E.B.; Nikiema, J.B.; Guissou, I.P.; Simpore, J. DPPH radical scavenging and lipoxygenase inhibitory effects in extracts from Erythrina senegalensis (Fabaceae) DC. Afr. J. Pharm. Pharmacol. 2016, 10, 185–191. [Google Scholar] [CrossRef]
- Naz, R.; Ayub, H.; Nawaz, S.; Islam, Z.U.; Yasmin, T.; Bano, A.; Wakeel, A.; Zia, S.; Roberts, T.H. Antimicrobial activity, toxicity and anti-inflammatory potential of methanolic extracts of four ethnomedicinal plant species from Punjab, Pakistan. BMC Complement. Altern. Med. 2017, 17, 302. [Google Scholar] [CrossRef]
- Oyedepo, O.O.; Femurewa, A.J. Anti-protease and membrane stabilizing activities of extracts of Fagra zanthoxiloides, Olax subscorpioides and Tetrapleura tetraptera. Int. J. Pharm. 1995, 33, 65–69. [Google Scholar] [CrossRef]
- Wan, L.S.; Chen, C.P.; Xiao, Z.Q.; Wang, Y.L.; Min, Q.X.; Yue, Y.D.; Chen, J.C. In vitro and in vivo anti-diabetic activity of Swertia kouitchensis extract. J. Ethnopharmacol. 2013, 147, 622–630. [Google Scholar] [CrossRef]
- Sales, P.M.; Souza, P.M.; Simeoni, L.A.; Magalhães, P.O.; Silveira, D. α-Amylase inhibitors: A Review of raw material and isolated compounds from plant source. J. Pharm. Pharm. Sci. 2012, 15, 141. [Google Scholar] [CrossRef]
- Ngenge Tamfu, A.; Mfifen Munvera, A.; Veronica Dediu Botezatu, A.; Talla, E.; Ceylan, O.; Tagatsing Fotsing, M.; Tanyi Mbafor, J.; Shaheen, F.; Mihaela Dinica, R. Synthesis of benzoyl esters of β-amyrin and lupeol and evaluation of their antibiofilm and antidiabetic activities. Results Chem. 2022, 4, 100322. [Google Scholar] [CrossRef]
- Vieira, E.F.; Podlasiak, M.; Moreira, M.M.; Grosso, C.; Rodrigues, F.; Fernandes, V.C.; Delerue-Matos, C. New insights of phytochemical profile and in vitro antioxidant and neuroprotective activities from optimized extract of horned melon fruit. J. Food Meas. Charact. 2022, 16, 1847–1858. [Google Scholar] [CrossRef]
- Maluleke, M.K.; Moja, S.J.; Nyathi, M.; Modise, D.M. Nutrient concentration of African horned cucumber (Cucumis metuliferus L.) fruit under different soil types, environments, and varying irrigation water levels. Horticulturae 2021, 7, 76. [Google Scholar] [CrossRef]
- Bogopa, J. P2 Exploring the functional components of the African horned cucumber (Cucumis metuliferus), Mokapana (Tswana)—Characterisation by phenolic content and antioxidant activity in Botswana. Biochem. Pharmacol. 2017, 139, 125. [Google Scholar] [CrossRef]
- Ani, O.N.; Achikanu, C.E.; Onyishi, C.K. Comparative analysis of minerals, heavy metals and amino acids compositions of the seeds and juice of Cucumis metuliferus. Asian J. Res. Biochem. 2020, 54, 31–42. [Google Scholar] [CrossRef]
- Pai, S.R.; Upadhya, V.; Hegde, H.V.; Joshi, R.K.; Kholkute, S.D. Determination of betulinic acid, oleanolic acid and ursolic acid from Achyranthes aspera L. using RP-UFLC-DAD analysis and evaluation of various parameters for their optimum Yield. Indian J. Exp. Biol. 2016, 54, 196–202. [Google Scholar]
- Zhou, Z.; Tong, H.H.Y.; Li, L.; Shek, F.L.Y.; Lv, Y.; Zheng, Y. Synthesis, characterization and thermal analysis of ursolic acid solid forms. Cryst. Res. Technol. 2015, 50, 538–548. [Google Scholar] [CrossRef]
- Gómez-Pulido, L.D.M.; González-Cano, R.C.; Domínguez, E.; Heredia, A. Structure determination of oleanolic and ursolic acids: A combined density functional theory/vibrational spectroscopy methodology. R. Soc. Open Sci. 2021, 8, 210162. [Google Scholar] [CrossRef]
- Samsonowicz, M.; Kalinowska, M.; Gryko, K. Enhanced Antioxidant activity of ursolic acid by complexation with copper (II): Experimental and theoretical study. Materials 2021, 14, 264. [Google Scholar] [CrossRef]
- Sang, S.; Lapsley, K.; Rosen, R.T.; Ho, C.T. New prenylated benzoic acid and other constituents from almond hulls (Prunus amygdalus Batsch). J. Agric. Food Chem. 2002, 50, 607–609. [Google Scholar] [CrossRef]
- Somantri, A.D.; Kurnia, D.; Zainuddin, A.; Dharsono, H.D.; Satari, M.H. Action mode of ursolic acid as a natural antioxidant and inhibitor of superoxide dismutase: In vitro and in silico study. J. Adv. Pharm. Technol. Res. 2021, 12, 389–394. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A Review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011, 89, 217–233. [Google Scholar] [CrossRef]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef]
- Jedinák, A.; Mučková, M.; Košt’álová, D.; Maliar, T.; Mašterová, I. Antiprotease and antimetastatic activity of ursolic acid isolated from Salvia officinalis. Z. Fur Nat.-Sect. C J. Biosci. 2006, 61, 777–782. [Google Scholar] [CrossRef]
- Checker, R.; Sandur, S.K.; Sharma, D.; Patwardhan, R.S.; Jayakumar, S.; Kohli, V.; Sethi, G.; Aggarwal, B.B.; Sainis, K.B. Potent anti-inflammatory activity of ursolic acid, a triterpenoid antioxidant, is mediated through suppression of NF-ΚB, AP-1 and NF-AT. PLoS ONE 2012, 7, e31318. [Google Scholar] [CrossRef]
- Son, J.; Lee, S.Y. Therapeutic potential of ursonic acid: Comparison with ursolic acid. Biomolecules 2020, 10, 1505. [Google Scholar] [CrossRef]
- Jabeen, M.; Ahmad, S.; Shahid, K.; Sadiq, A.; Rashid, U. Ursolic acid hydrazide based organometallic complexes: Synthesis, characterization, antibacterial, antioxidant, and docking studies. Front. Chem. 2018, 6, 55. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.; Prasad, N.R. Effect of ursolic acid, a triterpenoid antioxidant, on ultraviolet-B radiation-induced cytotoxicity, lipid peroxidation and DNA damage in human lymphocytes. Chem. Biol. Interact. 2008, 176, 99–107. [Google Scholar] [CrossRef]
- Pradhan, B.; Patra, S.; Behera, C.; Nayak, R.; Jit, B.P.; Ragusa, A.; Jena, M. Preliminary investigation of the antioxidant, anti-diabetic, and anti-inflammatory activity of Enteromorpha intestinalis extracts. Molecules 2021, 26, 1171. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, Ł.; Skąpska, S.; Marszałek, K. Ursolic acid—A pentacyclic triterpenoid with a wide spectrum of pharmacological activities. Molecules 2015, 20, 20614–20641. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Houghton, P.J.; Soumyanath, A. α-Amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. J. Ethnopharmacol. 2006, 107, 449–455. [Google Scholar] [CrossRef] [PubMed]


| Compound | HPLC-DAD | UHPLC-MS | |||||
|---|---|---|---|---|---|---|---|
| RT * | λ, nm | μg/g of Extract | SD ** | RSD % | Exact Mass | [M-H]− Ion (m/z) | |
| Hydroxybenzoic acids | |||||||
| Gallic acid | 5.9 | 272.00 | 401.39 | 1.46 | 0.36 | 170.02152 | 169.01302 |
| Catechin | 17.6 | 280.00 | 6576.76 | 3.86 | 0.06 | 290.07904 | 289.071 |
| Epicatechin | 23.9 | 280.00 | 2016.71 | 3.59 | 0.18 | 302.04265 | 289.071 |
| Procianidin B2 | 24.3 | 230.00 | 35.88 | 0.22 | 0.61 | 578.14242 | 577.1558 |
| Procianidin A2 | 29.9 | 230.00 | 756.10 | 1.86 | 0.25 | 578.14242 | 577.1558 |
| Flavonols | |||||||
| Rutin | 31.4 | 265.00 | 39.42 | 0.23 | 0.60 | 610.15338 | 609.14613 |
| Quercetin | 35.8 | 365.00 | 5.98 | 0.56 | 9.43 | 302.04265 | 301.23813 |
| Quercetin-3-D-galactoside | 32.0 | 265.00 | 11.62 | 0.28 | 2.45 | 464.09548 | 463.0876 |
| Kampferol-3-glucoside | 33.6 | 265.00 | 21.90 | 0.70 | 3.20 | 448.10056 | 447.09331 |
| Kampferol | 37.8 | 365.00 | 5.14 | 1.49 | 28.88 | 286.04774 | 285.13422 |
| Hydrocinnamic acids | |||||||
| Neochlorogenic acid | 10.5 | 325.00 | 22.89 | 0.53 | 2.31 | ||
| Chlorogenic acid | 22.3 | 325.00 | 77.39 | 0.15 | 0.19 | 354.09508 | 354.09508 |
| Caffeic acid | 22.9 | 325.00 | 58.65 | 0.57 | 0.96 | 180.04226 | 179.03501 |
| p-Coumaric acid | 28.9 | 325.00 | 116.11 | 1.46 | 1.26 | 164.04734 | 163.03954 |
| trans-Ferulic acid | 30.5 | 325.00 | 34.27 | 1.86 | 5.42 | 194.05791 | 193.05066 |
| Triterpenes | |||||||
| Oleanolic acid | 45.8 | 210.00 | 551.83 | 3.25 | 0.59 | 456.36034 | 455.35309 |
| Ursolic acid | 45.9 | 210.00 | 577.31 | 3.19 | 0.55 | 456.36034 | 455.35309 |
| Physical Properties | Ursolic Acid Fraction |
|---|---|
| Color | white |
| Melting Point | 291–293 °C |
| Solubility | soluble in ethanol, DMSO |
| Rf Value Solvent system: chloroform: methanol (95:5, v/v) | 0.41 |
| Assignments | ATR-IR Bands (cm−1) | |
|---|---|---|
| Standard Commercial UA | Isolated UA from C. metuliferus | |
| νass (OH) w | 3523.33 | 3523.22 |
| ν (O–H) w | 2965.41 | 2966.61 |
| ν (CH) m | 2953.90; 2918.75 | 2954.34; 2918.32 |
| νass (C=O) s | 1714.66 | 1715.54 |
| νas (COO−) w | 1553.56 | 1543.80 |
| βas (OH) m | 1453.78 | 1454.54 |
| νs (COO−; C=O) w | 1405.15 | 1406.03 |
| δs (CH3) w | 1385.72 | 1386.45 |
| βs (OH) m | 1374.72 | 1375.79 |
| ν(C–OH) m | 1029.87 | 1030.96 |
| γ (−C=C−, CH) m | 999.56 | 999.59 |
| ν (C–C, C–O, C–H) w | 971.08 | 972.12 |
| δs (CH3) w | 807.15 | 808.14 |
| Samples | IC50 DPPH (µg/mL) | IC50 ABTS (µg/mL) |
|---|---|---|
| Fruit extract | 32.74 ± 0.022 d | 11.37 ± 0.071 b |
| Isolated ursolic acid | 4.27 ± 0.009 a | 6.96 ± 0.014 a |
| Trolox | 10.57 ± 0.002 b | 32.56 ± 0.002 c |
| Ascorbic acid | 20.34 ± 0.034 c | 13.76 ± 0.044 b |
| Samples | Anti-Lipoxygenase Activity IC50 (µg/mL) * | Anti-Proteinase Action IC50 (µg/mL) * | α-amylase Inhibition IC50 (µg/mL) * | β-glucosidase Inhibition IC50 (µg/mL) * |
|---|---|---|---|---|
| Hydroethanolic extract | 32.90 ± 0.045 b | 16.34 ± 0.067 b | 429.541 ± 0.252 c | 385.685 ± 0.758 c |
| Ursolic acid fraction | 18.61 ± 0.086 a | 12.53 ± 0.044 a | 394.264 ± 0.143 b | 322.412 ± 0.517 b |
| Aspirin | 160.20 ± 0.020c | |||
| Indomethacin | 45.12 ± 0.014 c | |||
| Acarbose | 341.577 ± 0.398 a | 308.474 ± 0.296 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Busuioc, A.C.; Costea, G.V.; Botezatu, A.V.D.; Furdui, B.; Dinica, R.M. Cucumis metuliferus L. Fruits Extract with Antioxidant, Anti-Inflammatory, and Antidiabetic Properties as Source of Ursolic Acid. Separations 2023, 10, 274. https://doi.org/10.3390/separations10050274
Busuioc AC, Costea GV, Botezatu AVD, Furdui B, Dinica RM. Cucumis metuliferus L. Fruits Extract with Antioxidant, Anti-Inflammatory, and Antidiabetic Properties as Source of Ursolic Acid. Separations. 2023; 10(5):274. https://doi.org/10.3390/separations10050274
Chicago/Turabian StyleBusuioc, Anna Cazanevscaia, Giorgiana Valentina Costea, Andreea Veronica Dediu Botezatu, Bianca Furdui, and Rodica Mihaela Dinica. 2023. "Cucumis metuliferus L. Fruits Extract with Antioxidant, Anti-Inflammatory, and Antidiabetic Properties as Source of Ursolic Acid" Separations 10, no. 5: 274. https://doi.org/10.3390/separations10050274
APA StyleBusuioc, A. C., Costea, G. V., Botezatu, A. V. D., Furdui, B., & Dinica, R. M. (2023). Cucumis metuliferus L. Fruits Extract with Antioxidant, Anti-Inflammatory, and Antidiabetic Properties as Source of Ursolic Acid. Separations, 10(5), 274. https://doi.org/10.3390/separations10050274