Impact and Optimization of the Conditions of Extraction of Phenolic Compounds and Antioxidant Activity of Olive Leaves (Moroccan picholine) Using Response Surface Methodology
Abstract
:1. Introduction
2. Materials and Methods
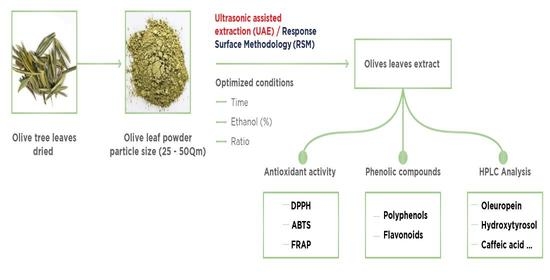
2.1. Preparation of the Powder
2.2. Chemicals
2.3. Experimental Design and Statistical Analysis
2.4. Ultrasound-Assisted Extraction of Bioactive Compounds
2.5. Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
2.6. In Vitro Antioxidant Activity
2.6.1. DPPH Radical Reduction Test
2.6.2. ABTS Radical Test
2.6.3. Ferric Reducing Antioxidant Power (FRAP) Test
2.7. Model Verification
2.8. Qualitative and Quantitative Analysis by HPLC-MS
2.9. Statistical Analysis
3. Results and Discussion
3.1. Evaluation and Optimization of Extraction Conditions
3.2. Fitting the Model and Analysis of Variance
- ▪
- The linear effect of extraction time (X1) was significant for TFC and DPPH;
- ▪
- The solvent/solid ratio (X2) was significant for TPC;
- ▪
- The concentration (X3) was significant for the TPC;
- ▪
- The quadratic effect of solvent concentration (X3) and ratio (X2) was significant for all responses except DPPH;
- ▪
- The X1 X2 interaction effect also significantly impacted TFC, DPPH, and ABTS;
- ▪
- The X1 X3 interaction was significant for ABTS.
3.3. Development of Second Order Polynomial Models
3.4. Effect of Process Variables
3.4.1. Effect of Extraction Time
3.4.2. Effect of Solid–Liquid Ratio
3.4.3. Effect of Solvent Concentration
3.5. Determination and Validation of Optimal Conditions
3.6. HPLC-MS Analysis
3.7. Scanning Electron Microscopy
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prakash Maran, J.; Manikandan, S.; Thirugnanasambandham, K.; Vigna Nivetha, C.; Dinesh, R. Box–Behnken Design Based Statistical Modeling for Ultrasound-Assisted Extraction of Corn Silk Polysaccharide. Carbohydr. Polym. 2013, 92, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Benavente-García, O.; Castillo, J.; Lorente, J.; Ortuño, A.; Del Rio, J.A. Antioxidant Activity of Phenolics Extracted from Olea Europaea L. Leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Ghomari, O.; Sounni, F.; Massaoudi, Y.; Ghanam, J.; Drissi Kaitouni, L.B.; Merzouki, M.; Benlemlih, M. Phenolic Profile (HPLC-UV) of Olive Leaves According to Extraction Procedure and Assessment of Antibacterial Activity. Biotechnol. Rep. 2019, 23, e00347. [Google Scholar] [CrossRef] [PubMed]
- Jemai, H.; Bouaziz, M.; Fki, I.; El Feki, A.; Sayadi, S. Hypolipidimic and Antioxidant Activities of Oleuropein and Its Hydrolysis Derivative-Rich Extracts from Chemlali Olive Leaves. Chem.-Biol. Interact. 2008, 176, 88–98. [Google Scholar] [CrossRef]
- Niaounakis, M.; Halvadakis, C.P. Waste Management Series; Elsevier: Amsterdam, The Netherlands, 2006; Volume 5, ISBN 978-0-08-044851-0. [Google Scholar]
- Fares, R.; Bazzi, S.; Baydoun, S.E.; Abdel-Massih, R.M. The Antioxidant and Anti-Proliferative Activity of the Lebanese Olea Europaea Extract. Plant Foods Hum. Nutr. 2011, 66, 58–63. [Google Scholar] [CrossRef]
- Vian, M.A.; Fernandez, X.; Visinoni, F.; Chemat, F. Microwave Hydrodiffusion and Gravity, a New Technique for Extraction of Essential Oils. J. Chromatogr. A 2008, 1190, 14–17. [Google Scholar] [CrossRef]
- Sirichan, T.; Kijpatanasilp, I.; Asadatorn, N.; Assatarakul, K. Optimization of Ultrasound Extraction of Functional Compound from Makiang Seed by Response Surface Methodology and Antimicrobial Activity of Optimized Extract with Its Application in Orange Juice. Ultrason. Sonochem. 2022, 83, 105916. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Dzah, C.S.; Zandile, M.; Duan, Y.; Ma, H.; Luo, X. Advances in Ultrasound Assisted Extraction of Bioactive Compounds from Cash Crops—A Review. Ultrason. Sonochem. 2018, 48, 538–549. [Google Scholar] [CrossRef]
- Saifullah, M.; McCullum, R.; McCluskey, A.; Vuong, Q. Comparison of Conventional Extraction Technique with Ultrasound Assisted Extraction on Recovery of Phenolic Compounds from Lemon Scented Tea Tree (Leptospermum Petersonii) Leaves. Heliyon 2020, 6, e03666. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Ponmurugan, K.; Maran Jeganathan, P. Development and Validation of Ultrasound-Assisted Solid-Liquid Extraction of Phenolic Compounds from Waste Spent Coffee Grounds. Ultrason. Sonochem. 2017, 34, 206–213. [Google Scholar] [CrossRef]
- Baş, D.; Boyacı, İ.H. Modeling and Optimization I: Usability of Response Surface Methodology. J. Food Eng. 2007, 78, 836–845. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response Surface Methodology (RSM) as a Tool for Optimization in Analytical Chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Tang, S.; Guo, X.; Wang, L.; Liu, X.; Lu, X.; Guo, Y. Preparation and Application of Guanidyl-Functionalized Graphene Oxide-Grafted Silica for Efficient Extraction of Acidic Herbicides by Box-Behnken Design. J. Chromatogr. A 2018, 1571, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Mourjane, A.; Hanine, H.; El Adnany, E.M.; Ouhammou, M.; Hidar, N.; Nabil, B.; Boumendjel, A.; Bitar, K.; Mahrouz, M. Energetic Bio-Activation of Some Organic Molecules and Their Antioxidant Activity in the Pulp of the Moroccan Argan Tree «Argania spinosa L.». Molecules 2022, 27, 3329. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. IJMS 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [Green Version]
- Clary, J.J. The Toxicology of Methanol; John Wiley & Sons: Hoboken, NJ, USA, 2013; ISBN 0-470-31759-0. [Google Scholar]
- Ramić, M.; Vidović, S.; Zeković, Z.; Vladić, J.; Cvejin, A.; Pavlić, B. Modeling and Optimization of Ultrasound-Assisted Extraction of Polyphenolic Compounds from Aronia Melanocarpa by-Products from Filter-Tea Factory. Ultrason. Sonochem. 2015, 23, 360–368. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total Phenol Analysis: Automation and Comparison with Manual Methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar] [CrossRef]
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Maamri, S.; Djireb, F.; Stocker, P. Phenolic Extracts from Various Algerian Plants as Strong Inhibitors of Porcine Liver Carboxylesterase. J. Enzym. Inhib. Med. Chem. 2006, 21, 719–726. [Google Scholar] [CrossRef]
- Abdel-Hameed, E.-S.S.; Nagaty, M.A.; Salman, M.S.; Bazaid, S.A. Phytochemicals, Nutritionals and Antioxidant Properties of Two Prickly Pear Cactus Cultivars (Opuntia Ficus Indica Mill.) Growing in Taif, KSA. Food Chem. 2014, 160, 31–38. [Google Scholar] [CrossRef]
- Aadesariya, M.K.; Ram, V.R.; Dave, P.N. Evaluation of Antioxidant Activities by Use of Various Extracts from Abutilon Pannosum and Grewia Tenax Leaves in the Kachchh Region. MOJ Food Process. Technol. 2017, 17, 359. [Google Scholar]
- Oyaizu, M. Studies on Products of Browning Reaction Antioxidative Activities of Products of Browning Reaction Prepared from Glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Soltani, Y.; Ali-Bouzidi, M.; Toumi, F.; Benyamina, A. Activités Antioxydantes Des Extraits de Trois Organes de Juniperus Phoenicea L. de l’Ouest Algérien. Phytothérapie 2017, 16, 142–148. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Puigventós, L.; Navarro, M.; Alechaga, É.; Núñez, O.; Saurina, J.; Hernández-Cassou, S.; Puignou, L. Determination of Polyphenolic Profiles by Liquid Chromatography-Electrospray-Tandem Mass Spectrometry for the Authentication of Fruit Extracts. Anal. Bioanal. Chem. 2015, 407, 597–608. [Google Scholar] [CrossRef] [Green Version]
- Harborne, A. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1998; ISBN 0-412-57270-2. [Google Scholar]
- Atkinson, A.C.; Donev, A.N. Optimum Experimental Designs; Clarendon Press: Oxford, UK, 1992; Volume 5. [Google Scholar]
- Chakraborty, S.; Uppaluri, R.; Das, C. Optimization of Ultrasound-Assisted Extraction (UAE) Process for the Recovery of Bioactive Compounds from Bitter Gourd Using Response Surface Methodology (RSM). Food Bioprod. Process. 2020, 120, 114–122. [Google Scholar] [CrossRef]
- Tiwari, B.K.; O’Donnell, C.P.; Cullen, P.J. Effect of Non Thermal Processing Technologies on the Anthocyanin Content of Fruit Juices. Trends Food Sci. Technol. 2009, 20, 137–145. [Google Scholar] [CrossRef]
- Jovanović, A.A.; Đorđević, V.B.; Zdunić, G.M.; Pljevljakušić, D.S.; Šavikin, K.P.; Gođevac, D.M.; Bugarski, B.M. Optimization of the Extraction Process of Polyphenols from Thymus Serpyllum L. Herb Using Maceration, Heat- and Ultrasound-Assisted Techniques. Sep. Purif. Technol. 2017, 179, 369–380. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Raza, A.; Wang, Y.-W.; Xu, X.-Q.; Chen, G.-H. Optimization of Surfactant-Mediated, Ultrasonic-Assisted Extraction of Antioxidant Polyphenols from Rattan Tea (Ampelopsis grossedentata) Using Response Surface Methodology. Phcog. Mag. 2017, 13, 446. [Google Scholar] [CrossRef] [Green Version]
- Madrona, G.S.; Terra, N.M.; Coutinho Filho, U.; de Santana Magalhães, F.; Cardoso, V.L.; Reis, M.H.M. Purification of Phenolic Compounds from Genipap (Genipa americana L.) Extract by the Ultrasound Assisted Ultrafiltration Process. Acta Scientiarum. Technol. 2019, 41, e35571. [Google Scholar] [CrossRef] [Green Version]
- Rocha, J.D.C.G.; Procopio, F.R.; Mendonca, A.C.; Vieira, L.M.; Perrone, I.T.; Barros, F.A.R.D.; Stringheta, P.C. Optimization of Ultrasound-Assisted Extraction of Phenolic Compounds from Jussara (Euterpe edulis M.) and Blueberry (Vaccinium myrtillus) Fruits. Food Sci. Technol. 2017, 38, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Saci, F.; Benchikh, Y.; Louaileche, H.; Bey, M.B. Optimization of Ultrasound-Assisted Extraction of Phenolic Compounds and Antioxidant Activity from Carob Pulp (Ceratonia siliqua l.) by Using Response Surface Methodology. Ann. Univ. Dunarea Jos Galati. Fascicle VI-Food Technol. 2018, 42, 26–39. [Google Scholar]
- Xiao, W.; Han, L.; Shi, B. Microwave-Assisted Extraction of Flavonoids from Radix Astragali. Sep. Purif. Technol. 2008, 62, 614–618. [Google Scholar] [CrossRef]
- Zakaria, F.; Tan, J.-K.; Mohd Faudzi, S.M.; Abdul Rahman, M.B.; Ashari, S.E. Ultrasound-Assisted Extraction Conditions Optimisation Using Response Surface Methodology from Mitragyna Speciosa (Korth.) Havil Leaves. Ultrason. Sonochem. 2021, 81, 105851. [Google Scholar] [CrossRef] [PubMed]
- Tubtimdee, C.; Shotipruk, A. Extraction of Phenolics from Terminalia Chebula Retz with Water–Ethanol and Water–Propylene Glycol and Sugaring-out Concentration of Extracts. Sep. Purif. Technol. 2011, 77, 339–346. [Google Scholar] [CrossRef]
- He, B.; Zhang, L.-L.; Yue, X.-Y.; Liang, J.; Jiang, J.; Gao, X.-L.; Yue, P.-X. Optimization of Ultrasound-Assisted Extraction of Phenolic Compounds and Anthocyanins from Blueberry (Vaccinium ashei) Wine Pomace. Food Chem. 2016, 204, 70–76. [Google Scholar] [CrossRef]
- Noroozi, F.; Bimakr, M.; Ganjloo, A.; Aminzare, M. A Short Time Bioactive Compounds Extraction from Cucurbita Pepo Seed Using Continuous Ultrasound-assisted Extraction. Food Meas. 2021, 15, 2135–2145. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound Assisted Extraction (UAE) of Bioactive Compounds from Fruit and Vegetable Processing by-Products: A Review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Caldas, T.W.; Mazza, K.E.L.; Teles, A.S.C.; Mattos, G.N.; Brígida, A.I.S.; Conte-Junior, C.A.; Borguini, R.G.; Godoy, R.L.O.; Cabral, L.M.C.; Tonon, R.V. Phenolic Compounds Recovery from Grape Skin Using Conventional and Non-Conventional Extraction Methods. Ind. Crops Prod. 2018, 111, 86–91. [Google Scholar] [CrossRef]
- Savic, I.M.; Savic Gajic, I.M. Optimization of Ultrasound-Assisted Extraction of Polyphenols from Wheatgrass (Triticum aestivum L.). J. Food Sci. Technol. 2020, 57, 2809–2818. [Google Scholar] [CrossRef]
- Rodrigues, S.; Fernandes, F.A.N.; de Brito, E.S.; Sousa, A.D.; Narain, N. Ultrasound Extraction of Phenolics and Anthocyanins from Jabuticaba Peel. Ind. Crops Prod. 2015, 69, 400–407. [Google Scholar] [CrossRef]
- Zvicevičius, G.; Liaudanskas, M.; Viškelis, P. Phenolic Compound Quantification and Antioxidant Activity Determination in Ethanol Extracts of Apple Pulp and Peels. 2014. Available online: https://lsmu.lt/cris/handle/20.500.12512/15459 (accessed on 14 April 2023).
- Kaderides, K.; Papaoikonomou, L.; Serafim, M.; Goula, A.M. Microwave-Assisted Extraction of Phenolics from Pomegranate Peels: Optimization, Kinetics, and Comparison with Ultrasounds Extraction. Chem. Eng. Process.-Process Intensif. 2019, 137, 1–11. [Google Scholar] [CrossRef]
- Li, H.; Pordesimo, L.; Weiss, J. High Intensity Ultrasound-Assisted Extraction of Oil from Soybeans. Food Res. Int. 2004, 37, 731–738. [Google Scholar] [CrossRef]








| Code Symbols | Independent Variables | Level | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| X1 | Time (min) | 30 | 45 | 60 |
| X2 | Ratio (mL/g) | 5 | 12.5 | 20 |
| X3 | Ethanol (%) | 20 | 60 | 100 |
| N°Exp | Time (min; X1) | Ratio (mL/g; X2) | Concentration (%; X3) | TPC (mg EAG/g DM; Y1) | TFC (mg EC/g DM; Y2) | DPPH (%; Y3) | ABTS (%; Y4) | FRAP (%; Y5) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reel | Predicts | Reel | Predicts | Reel | Predicts | Reel | Predicts | Reel | Predicts | ||||
| 1 | 30(−1) | 5(−1) | 60(0) | 59.23 | 58.65 | 11.12 | 11.14 | 76.12 | 74.97 | 76.34 | 76.89 | 73.65 | 72.21 |
| 2 | 60(1) | 5(−1) | 60(0) | 66.36 | 63.65 | 13.96 | 14.05 | 85.69 | 84.59 | 85.41 | 84.05 | 76.55 | 79.15 |
| 3 | 30(−1) | 20(1) | 60(0) | 52.98 | 55.69 | 11.85 | 11.77 | 76.25 | 77.35 | 79.69 | 81.05 | 82.65 | 80.05 |
| 4 | 60(1) | 20(1) | 60(0) | 53.31 | 53.90 | 11.96 | 11.95 | 75.36 | 76.51 | 76.23 | 75.68 | 73.76 | 75.20 |
| 5 | 30(−1) | 12.5(0) | 20(−1) | 56.96 | 56.02 | 11.08 | 10.88 | 77.36 | 76.46 | 75.66 | 74.58 | 72.36 | 74.92 |
| 6 | 60(1) | 12.5(0) | 20(−1) | 55.25 | 56.43 | 12.15 | 11.88 | 78.85 | 81.84 | 79.84 | 81.22 | 76.02 | 74.53 |
| 7 | 30(−1) | 12.5(0) | 100(1) | 48.69 | 47.51 | 10.69 | 10.96 | 70.95 | 80.99 | 83.46 | 82.08 | 74.12 | 75.61 |
| 8 | 60(1) | 12.5(0) | 100(1) | 49.36 | 50.31 | 12.85 | 13.05 | 78.36 | 79.26 | 77.24 | 77.77 | 80.65 | 78.09 |
| 9 | 45(0) | 5(−1) | 20(−1) | 60.26 | 61.79 | 10.96 | 11.15 | 75.28 | 77.34 | 76.08 | 76.06 | 75.14 | 74.03 |
| 10 | 45(0) | 20(1) | 20(−1) | 56.45 | 54.69 | 10.45 | 10.74 | 76.52 | 76.33 | 74.67 | 73.84 | 71.92 | 71.96 |
| 11 | 45(0) | 5(−1) | 100(1) | 51.96 | 50.31 | 12.39 | 12.10 | 77.39 | 79.58 | 76.87 | 77.70 | 72.18 | 72.14 |
| 12 | 45(0) | 20(1) | 100(1) | 49.65 | 48.12 | 11.23 | 11.04 | 74.96 | 72.90 | 75.69 | 75.71 | 76.98 | 78.10 |
| 13 | 45(0) | 12.5(0) | 60(0) | 72.98 | 72.41 | 15.99 | 16.34 | 82.25 | 81.65 | 82.96 | 82.65 | 86.95 | 86.28 |
| 14 | 45(0) | 12.5(0) | 60(0) | 72.26 | 72.41 | 16.98 | 16.34 | 81.12 | 81.65 | 83.23 | 82.65 | 86.45 | 86.28 |
| 15 | 45(0) | 12.5(0) | 60(0) | 71.99 | 72.41 | 16.06 | 16.34 | 81.59 | 76.99 | 81.75 | 82.65 | 85.45 | 86.28 |
| Source | Sum of Squares | Estimation of Coefficients | Degree of Freedom | Medium Square | Value F | Value p | Remarks |
|---|---|---|---|---|---|---|---|
| TPC | |||||||
| Model | 990.43 | 9 | 11005 | 17.53 | 0.0028 | significant | |
| Intercept, X0 | 72.41 * | ||||||
| Linear | |||||||
| X1 | 5.15 | 0.8025 | 1 | 5.15 | 0.8209 | 0.4065 | |
| X2 | 80.77 | −3.18 * | 1 | 80.77 | 12.87 | 0.0157 | |
| X3 | 107.02 | −3.66 * | 1 | 107.02 | 17.05 | 0.0091 | |
| Interaction | |||||||
| X1 X2 | 11.56 | −1.70 | 1 | 11.56 | 1.84 | 0.2328 | |
| X1 X3 | 1.42 | 0.5950 | 1 | 1.42 | 0.2256 | 0.6548 | |
| X2 X3 | 0.5625 | 0.3750 | 1 | 0.5625 | 0.0896 | 0.7767 | |
| Quadratic | |||||||
| X21 | 249.94 | −8.23 * | 1 | 249.94 | 39.83 | 0.0015 | |
| X22 | 142.51 | −6.21 * | 1 | 142.51 | 22.71 | 0.0050 | |
| X23 | 498.34 | −11.62 * | 1 | 498.34 | 79.40 | 0.0003 | |
| Residual | 31.38 | 5 | 6.28 | ||||
| Lack of fit | 30.86 | 3 | 10.29 | 39.27 | 0.0249 | significant | |
| Error | 0.5238 | 2 | 0.2619 | ||||
| Total | 1021.81 | 14 | |||||
| Accuracy Adequacy | 12.17 | ||||||
| CV% | 4.28 | ||||||
| R2 | 0.969 | ||||||
| R2Ajust | 0.91 | ||||||
| Average | 58.51 | ||||||
| TFC | |||||||
| Model | 61.99 | 9 | 6.89 | 31.55 | 0.0007 | significant | |
| Intercept, X0 | 16.34 * | ||||||
| Linear | |||||||
| X1 | 4.77 | 0.7725 * | 1 | 4.77 | 21.87 | 0.0055 | |
| X2 | 1.08 | −0.3675 | 1 | 1.08 | 4.95 | 0.0767 | |
| X3 | 0.7938 | 0.3150 | 1 | 0.7938 | 3.64 | 0.1148 | |
| Interaction | |||||||
| X1 X2 | 1.86 | −0.6825 * | 1 | 1.86 | 8.54 | 0.0330 | |
| X1 X3 | 0.2970 | 0.2725 | 1 | 0.2970 | 1.36 | 0.2960 | |
| X2 X3 | 0.1056 | −0.1625 | 1 | 0.1056 | 0.4838 | 0.5177 | |
| Quadratic | |||||||
| X21 | 12.54 | −1.84 * | 1 | 12.54 | 57.44 | 0.0006 | |
| X22 | 19.16 | −2.28 * | 1 | 19.16 | 87.76 | 0.0002 | |
| X23 | 29.11 | −2.81 * | 1 | 29.11 | 133.35 | <0.0001 | |
| Residual | 1.09 | 5 | 0.2183 | ||||
| Lack of fit | 0.4811 | 3 | 0.1604 | 0.5253 | 0.7074 | Not significant | |
| Error | 0.6105 | 2 | 0.3052 | ||||
| Total | 63.08 | 14 | |||||
| Accuracy Adequacy | 14.69 | ||||||
| CV% | 3.69 | ||||||
| R2 | 0.98 | ||||||
| R2Ajust | 0.95 | ||||||
| Average | 12.65 | ||||||
| DPPH | |||||||
| Model | 165.56 | 9 | 18.40 | 5.19 | 0.0423 | significant | |
| Intercept, X0 | 81.65 * | ||||||
| Linear | |||||||
| X1 | 38.63 | 2.20 * | 1 | 38.63 | 10.89 | 0.0215 | |
| X2 | 16.22 | −1.42 | 1 | 16.22 | 4.57 | 0.0855 | |
| X3 | 5.04 | −0.7937 | 1 | 5.04 | 1.42 | 0.2867 | |
| Interaction | |||||||
| X1 X2 | 27.35 | −2.61 * | 1 | 27.35 | 7.71 | 0.0390 | |
| X1 X3 | 8.76 | 1.48 | 1 | 8.76 | 2.47 | 0.1768 | |
| X2 X3 | 3.37 | −0.9175 | 1 | 3.37 | 0.9493 | 0.3747 | |
| Quadratic | |||||||
| X21 | 8.06 | −1.48 | 1 | 8.06 | 2.27 | 0.1920 | |
| X22 | 12.24 | −1.82 | 1 | 12.24 | 3.45 | 0.1224 | |
| X23 | 53.19 | −3.80 * | 1 | 53.19 | 14.99 | 0.0117 | |
| Residual | 17.74 | 5 | 3.55 | ||||
| Lack of fit | 17.09 | 3 | 5.70 | 17.68 | 0.0540 | Not significant | |
| Error | 0.6445 | 2 | 0.3222 | ||||
| Total | 183.30 | 14 | |||||
| Accuracy Adequacy | 8.2473 | ||||||
| CV% | 2.42 | ||||||
| R2 | 0.9032 | ||||||
| R2Ajust | 0.73 | ||||||
| Average | 77.87 | ||||||
| ABTS | |||||||
| Model | 163.76 | 9 | 18.20 | 8.05 | 0.0168 | significant | |
| Intercept, X0 | 82.65 * | ||||||
| Linear | |||||||
| X1 | 1.59 | 0.4462 | 1 | 1.59 | 0.7045 | 0.4395 | |
| X2 | 8.86 | −1.05 | 1 | 8.86 | 3.92 | 0.1046 | |
| X3 | 6.14 | 0.8762 | 1 | 6.14 | 2.72 | 0.1602 | |
| Interaction | |||||||
| X1 X2 | 39.25 | −3.13 * | 1 | 39.25 | 17.36 | 0.0088 | |
| X1 X3 | 27.04 | −2.60 * | 1 | 27.04 | 11.96 | 0.0181 | |
| X2 X3 | 0.0132 | 0.0575 | 1 | 0.0132 | 0.0058 | 0.9420 | |
| Quadratic | |||||||
| X21 | 0.0000 | −0.0033 | 1 | 0.0000 | 0.0000 | 0.9968 | |
| X22 | 38.42 | −3.23 * | 1 | 38.42 | 16.99 | 0.0092 | |
| X23 | 47.68 | −3.59 * | 1 | 47.68 | 21.08 | 0.0059 | |
| Residual | 11.31 | 5 | 2.26 | ||||
| Lack of fit | 10.06 | 3 | 3.35 | 5.40 | 0.1602 | Not significant | |
| Error | 1.24 | 2 | 0.6212 | ||||
| Total | 175.07 | 14 | |||||
| Accuracy Adequacy | 8.31 | ||||||
| CV% | 1.90 | ||||||
| R2 | 0.94 | ||||||
| R2Ajust | 0.82 | ||||||
| Average | 79.01 | ||||||
| FRAP | |||||||
| Model | 364.85 | 9 | 40.54 | 5.21 | 0.0418 | significant | |
| Intercept, X0 | 86.28 * | ||||||
| Linear | |||||||
| X1 | 2.20 | 0.5250 | 1 | 2.20 | 0.2836 | 0.6171 | |
| X2 | 7.59 | 0.9737 | 1 | 7.59 | 0.9757 | 0.3686 | |
| X3 | 9.01 | 1.06 | 1 | 9.01 | 1.16 | 0.3309 | |
| Interaction | 0.5250 | ||||||
| X1 X2 | 34.75 | −2.95 | 1 | 34.75 | 4.47 | 0.0881 | |
| X1 X3 | 2.06 | 0.7175 | 1 | 2.06 | 0.2649 | 0.6287 | |
| X2 X3 | 16.08 | 2.00 | 1 | 16.08 | 2.07 | 0.2099 | |
| Quadratic | |||||||
| X21 | 98.21 | −3.95 * | 1 | 57.58 | 7.41 | 0.0417 | |
| X22 | 275.63 | −5.68 * | 1 | 119.19 | 15.33 | 0.0112 | |
| X23 | 101.96 | −6.55 * | 1 | 158.25 | 20.36 | 0.0063 | |
| Residual | 57.58 | 5 | 7.77 | ||||
| Lack of fit | 119.19 | 3 | 12.57 | 21.55 | 0.0447 | significant | |
| Error | 158.25 | 2 | 0.5833 | ||||
| Total | 38.87 | 14 | |||||
| Accuracy Adequacy | 6.29 | ||||||
| CV% | 3.59 | ||||||
| R2 | 0.90 | ||||||
| R2Ajust | 0.73 | ||||||
| Average | 77.79 | ||||||
| The Optimal Conditions | The Answers | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X1 (min) | X2 (mL/g) | X3 (%) | TPC (mg GAE/g DM) | TFC (mg EC/g DM) | DPPH (%) | ABTS (%) | FRAP (%) | |||||
| 53.5 | 9.83 | 59.7 | Reel | Predict | Reel | Predict | Reel | Predict | Reel | Predict | Reel | Predict |
| Objective | Maximum | Maximum | Maximum | Maximum | Maximum | |||||||
| Optimized values | 74.45 ± 1.22 | 72.40 | 17.08 ± 1.85 | 16.42 | 83.45 ± 0.89 | 82.58 | 82.85 ± 1.52 | 83.06 | 87.01 ± 2.35 | 85.79 | ||
| N° PIC | TR (min) | Phenolic Compounds | Chemical Formulas | Concentration of the First Extraction (mg/g) | Concentration of Second Extraction (mg/g) |
|---|---|---|---|---|---|
| 1 | 7.09 | Hydroxytyrosol | C8H10O3 | 45.40 ± 1.2 | 10.02 ± 2.12 |
| 2 | 10.52 | Catechin | C15H14O6 | 12.90 ± 1.35 | 2.21 ± 1.36 |
| 3 | 12.72 | Caffeic acid | C9H8O4 | 79.50 ± 1.25 | 15.32 ± 3.36 |
| 4 | 15.23 | Vanillin | C8H8O3 | 12.70 ± 1.36 | 0.98 ± 2.36 |
| 5 | 20.52 | Naringin | C9H8O3 | 45.40 ± 2.45 | 32.23 ± 1.25 |
| 6 | 21.30 | Oleuropein | C25H32O13 | 114.10 ± 3.42 | 40.23 ± 2.78 |
| 7 | 26.48 | Quercetin | C15H10O7 | 23.00 ± 2.38 | 12.21 ± 1.45 |
| 8 | 27.44 | Kaempferol | C15H10O6 | 29.00 ± 1.96 | 9.36 ± 1.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Adnany, E.M.; Elhadiri, N.; Mourjane, A.; Ouhammou, M.; Hidar, N.; Jaouad, A.; Bitar, K.; Mahrouz, M. Impact and Optimization of the Conditions of Extraction of Phenolic Compounds and Antioxidant Activity of Olive Leaves (Moroccan picholine) Using Response Surface Methodology. Separations 2023, 10, 326. https://doi.org/10.3390/separations10060326
El Adnany EM, Elhadiri N, Mourjane A, Ouhammou M, Hidar N, Jaouad A, Bitar K, Mahrouz M. Impact and Optimization of the Conditions of Extraction of Phenolic Compounds and Antioxidant Activity of Olive Leaves (Moroccan picholine) Using Response Surface Methodology. Separations. 2023; 10(6):326. https://doi.org/10.3390/separations10060326
Chicago/Turabian StyleEl Adnany, El Mustapha, Najat Elhadiri, Ayoub Mourjane, Mourad Ouhammou, Nadia Hidar, Abderrahim Jaouad, Khalid Bitar, and Mostafa Mahrouz. 2023. "Impact and Optimization of the Conditions of Extraction of Phenolic Compounds and Antioxidant Activity of Olive Leaves (Moroccan picholine) Using Response Surface Methodology" Separations 10, no. 6: 326. https://doi.org/10.3390/separations10060326
APA StyleEl Adnany, E. M., Elhadiri, N., Mourjane, A., Ouhammou, M., Hidar, N., Jaouad, A., Bitar, K., & Mahrouz, M. (2023). Impact and Optimization of the Conditions of Extraction of Phenolic Compounds and Antioxidant Activity of Olive Leaves (Moroccan picholine) Using Response Surface Methodology. Separations, 10(6), 326. https://doi.org/10.3390/separations10060326